Abstract
Cyanobacterial blooms in water have been extensively studied as they produce bioactive and toxic metabolites, commonly known as cyanotoxins. Additionally, the presence of cyanobacteria and, consequently, the production of cyanotoxins, have increased in extent and frequency worldwide. Therefore, the risk associated with the presence of these microorganisms and their toxins has become a matter of great concern. On the other hand, conventional processes for water treatment are inefficient for their elimination and/or degradation, so their presence in water persists at trace and ultra-trace concentrations. In this regard, it is important to develop alternatives to monitor cyanotoxins and allow their detection at low levels in water supply and purification systems, in order to ensure water of good quality for human consumption. In this work, different methodologies, implemented both at laboratory scale and in situ in aqueous bodies, are described. Among these methodologies, traditional and passive techniques are highlighted. Appropriate analytical and sample preparation methods used in the detection and quantification of cyanotoxins are also addressed. It was found that the use of passive samplers is a convenient and a cost-effective method of identifying the presence of these toxins in water at concentrations in the order of µg/L and ng/L. Moreover, studying the by-products generated from the degradation of natural toxins in aquatic environments and evaluating their possible adverse effects is crucial in terms of the management and control of cyanobacteria and cyanotoxin pollution in water.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Cyanobacteria, also called blue-green algae, are prokaryotic, unicellular, photosynthetic organisms present in various aquatic ecosystems (Gaysina et al., 2019). They are found in lentic environments, in both fresh and brackish water, including reservoirs, lakes, lagoons and coastal water. They are present particularly in tropical areas, where their growth is promoted by high temperatures and abundance of nutrients (O’Neil et al., 2012). Consequently, climate change, together with the eutrophication of water bodies, has led to a disproportionate increase in harmful cyanobacterial algal blooms (cyanoHAB) around the globe (Paerl & Otten, 2013; Wells et al., 2015). This poses a risk to human health, especially when these water bodies are used for irrigation, recreation or supplying the population without efficient prior removal treatment being carried out (Paerl et al., 2018; Sedan & Andrinolo, 2011; Singh & Babele, 2020). In fact, conventional purification processes are inefficient for their elimination (Dehghani, 2016); since they can involve the retention of cyanobacterial cells, in general terms, but not the effective degradation of cyanotoxins (He et al., 2016). On the other hand, the use of disinfectants, such as chlorine, compromises the integrity of the cells without guaranteeing their elimination, causing cell lysis and, consequently, the release of intracellular toxins (Merel, et al., 2013a). Furthermore, conventional disinfection processes can generate disinfection by-products that represent a serious threat to human health as they are toxic and difficult to be degraded after their formation (Wang et al., 2021). For this reason, several physical, chemical and biological processes have currently been proposed to control their proliferation in water bodies (Cobo, 2015). These include nutrient load reduction, algaecides, sediment coating agents, activated carbon adsorption, ultrafiltration, wetlands, aeration or mixing, and ultrasound (Choi et al., 2022; Liu et al., 2017; Purcell et al., 2012; Zhang et al., 2021). It is important to note that, even though these techniques work to some degree, each one has certain limitations. However, ultrasound has recently attracted attention due to its simple operation, low impact on ecosystems (Yao et al., 2020) and, specifically for its efficiency in the cyanobacteria control (Munoz et al., 2021; Park et al., 2017).
The biosynthesis of cyanotoxins is a complex process that is influenced by environmental conditions and varies according to the strains present. Not all genera of cyanobacteria produce toxins, making it difficult to implement predictive models to identify relationships between toxin production and environmental and water quality factors (Christensen et al., 2021). Additionally, the level of toxicity of a sample containing a mixture of these toxins depends on the presence of variants that have specific toxicity mechanisms in vertebrates (Huisman et al., 2018). Hepatotoxins, for instance, affect the liver, and are considered to be probably the most common cyanotoxins. They represent a group of structurally different compounds that includes more than 90 microcystin (MC) variants, 9 nodularin (NOD) variants, and 3 cylindrospermopsin (CYN) variants (Merel et al., 2013b). Globally, the MC and NOD families are the cyanobacterial toxins most frequently found in fresh and brackish water blooms. MC are commonly produced by Microcystis spp., Oscillatoria spp., Nostoc spp., Anabaena spp. and Anabaenopsis spp.; while NOD are produced mainly by Nodularia spumigena in brackish waters and CYN, by Cylindrospermopsis raciborskii in freshwater (Kaur, 2019). Both MC and NOD are water-soluble molecules (Merel et al., 2010) and their cyclic structure provides them with resistance to physical and chemical factors (Malik et al., 2020). On the other hand, neurotoxins affect the nervous system and include 20 saxitoxin (STX) variants and 6 anatoxin (ANTX) variants (Merel et al., 2013b). Finally, dermatotoxins induce irritation of the skin and mucous membranes, as well as allergic reactions, and include apliasiatoxin (APTX) and lingbiatoxin (LTX), which as yet have been detected only in seawater (Merel et al., 2013a).
Microcystin-LR (MC-LR) is the most toxic and widely distributed variant (Caramés, 2016). In addition to its high toxicity, its bioaccumulation capacity (Umehara et al., 2017) and biomagnification (Crettaz-Minaglia et al., 2017) should be highlighted. For drinking water, the World Health Organization (WHO) has recommended a provisional guideline value of 1 μg/L total MC-LR, suggesting a tolerable daily MC-LR intake of 0.04 μg/kg body weight (WHO, 2011). Nevertheless, concentrations in water bodies with potentially toxic strains sometimes exceed this value. Even stricter limits (0.1–0.3 μg/L) have been implemented by some regulatory agencies when adopting their own rules on the safety of drinking water based on WHO guidelines; this is the case of the United States Environmental Protection Agency (U.S. EPA) who adopted that limit for sensitive population and children under 6 years old (Buratti et al., 2017). Long-term compliance with these limits requires the application of a monitoring system for cyanobacteria and cyanotoxins, in order to optimize and facilitate their effective removal during the drinking water treatment operation (He et al., 2016; Westrick et al., 2010). Therefore, there is an ongoing effort to develop rapid, simple, sensitive and cost-effective methods that allow time-integrated monitoring of cyanobacteria and their toxins (Gaget et al., 2017a; Marcé et al., 2016).
During the last decades, the presence of cyanoHAB has increased in extent and frequency worldwide (Brooks et al., 2016), largely due to the lack of preventive actions aimed at reducing discharges to water sources, and obviously, the impact of global warming, which favor optimal conditions for the growth of these microorganisms (Paerl et al., 2016). A pronounced increase has been found in Asia and Africa, mainly in developing countries, given the intensification of human activities and dependence on agricultural fertilizers (Hou et al., 2022). In this regard, a recent study carried out in China systematically analyzed the reports of MC presence in lakes and reservoirs throughout the country and pointed out the need to strengthen the monitoring and control of MC in water, since they are detected in 100% from 59 lakes and 84% from 37 reservoirs (Wei et al., 2022). Colombia is not the exception. The presence of cyanobacterial blooms and MC have recently been recurrently identified in different reservoirs in the country (Caly et al., 2022; León & Peñuela, 2019; Loaiza-González et al., 2021; Palacio Gómez et al., 2019). Nevertheless, the presence of these substances in water is not yet addressed by the Colombian environmental legislation (Munoz et al., 2021), since there is not a systematic record of cyanobacterial blooms, despite the great diversity of aquatic ecosystems in the country (Salomón et al., 2020), leaving the risk due to the presence of cyanotoxins unnoticed.
Given the situation described above, in this work, techniques for monitoring cyanobacterial toxins in water reservoirs are described, addressing different alternatives that have been applied to date. Among these, the use of passive samplers is proposed as a convenient and profitable method for their detection and quantification at trace and ultra-trace concentrations. The objective of detecting and monitoring cyanotoxins is to allow the implementation of prevention and control strategies, to reduce their presence and manage the risk they represent to human and ecosystem health.
2 Cyanotoxins: Characteristics and Adverse Effects
Cyanobacteria are the oldest photosynthetic gram-negative microorganisms that exist in fresh, brackish and marine water, as well as in terrestrial environments (Kulabhusan & Campbell, 2021). They come in various forms, unicellular, colonial or multicellular filamentous, and inhabit all possible environments, even under precarious conditions of light and nutrients (Vidal et al., 2021). Microcystis spp. is one of the most widespread cyanobacteria in freshwater ecosystems. It survives winter season in the benthic zone and rises to the epilimnion during the summer, where it accumulates and forms blooms on the water surface (Caramés, 2016).
An algal bloom is a phenomenon in which biomass is produced significantly during a short period of time, being simultaneously linked to a decrease in phytoplankton diversity (You et al., 2022). Apart from the production of toxins, an algal bloom increases the turbidity of water and can locally increase water temperature. Moreover, when dying, blooms increase the release of harmful substances and the amount of organic matter, whose decomposition leads to anoxia and hypoxia situations (Nowicka-Krawczyk et al., 2022). Even though the potential risk of many cyanobacterial metabolites remains largely unknown (Janssen, 2019), the presence of cyanobacteria in reservoirs is so common that has even developed a series of ecological mechanisms to deal with biotic and abiotic stress (Hu & Rzymski, 2019). Phytoplankton lives a few days; nevertheless, when blooms are formed, they can last for weeks, threatening ecological stability and the integrity of aquatic ecosystems. In this regard, the life cycle of cyanobacteria plays an important role. Two-stages can be considered in the life cycle, one growing and nitrogen-fixing stage and one stage that combines the resting, germinating and vegetative stages (Hense & Beckmann, 2010). Benthic resting stages of phytoplankton may contain a large amount of biomass, which can contribute significantly to bloom generation, as well as their magnitude and distribution patterns (Kremp, 2000). Therefore, life cycle processes can control timing and duration of blooms and, subsequently, the species succession and dominance.
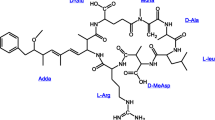
These algal blooms are complex communities. With them, toxic and non-toxic strains coexist and can only be distinguished by genetic methods. These methods allow the identification of toxigenic cyanobacteria within a bloom that simultaneously contain several of the most widespread cyanotoxins (Casero et al., 2019). Such mixtures of cyanotoxins are common in freshwater bodies and could have antagonistic and synergistic effects on organisms (Christensen et al., 2021). Among these, the hepatotoxic and tumor-promoting MC are the most common, and are considered one of the most dangerous groups. Among the MC congeners, MC-LR is the most toxic and abundant. For this reason, it has been widely studied, followed by MC-RR and MC-YR (Li et al., 2017). These are a family of cyclic heptapeptide compounds formed by a common structure of 5 fixed amino acids and another 2 changing amino acids, which characterize each variant. To date, more than 100 structural variants have been characterized and are named according to the amino acids incorporated at positions 2 and 4; MC-LR, for example, contains leucine (L) at position 2 and arginine (R) at position 4.
Globally, the accumulation of toxins is due to the massive proliferation of cyanoHAB, which have a negative impact on the environment (Pham & Utsumi, 2018). Although the majority of harmful cyanobacterial products are confined within cells, they are eventually released into the surrounding water environment after cell death, whether naturally or artificially induced. According to the WHO, human exposure to cyanobacteria and their biologically active products has effects of varying severity (WHO, 2011). The specific effects depend on the type of cyanotoxin, amount and route of exposure (Trevino-Garrison et al., 2015). The last of these can be direct ingestion, dermal contact or dialysis. By inhibiting protein phosphatases and generating oxidative stress in eukaryotic cells (Su et al., 2020), MC seriously poison various animals and plants, and endanger human life, especially through drinking water supply and /or accumulation in the food chain (Li et al., 2017).
Recently, Feng et al. (2020) confirmed that low-dose MC-LR exposure can promote the proliferation of human hepatocellular carcinoma cells. In this regard, for most chemicals that may be present in water, including cyanotoxins, a threshold dose is established to represent an estimate of the amount that can be ingested daily without an appreciable risk to health. For MC-LR, this threshold has a value of 0.04 μg/kg, as previously indicated (WHO, 2011). However, apart from its chronicity, many terrestrial and aquatic animal deaths, including in humans, have been reported due to the occurrence of acute exposure to MC.
Another critical scenario of exposure occurs through irrigation or consumption systems supplied by natural water bodies such as ponds, reservoirs and lakes in which the cyanobacterial toxins are present. These may affect the growth and development of crops, or may contaminate drinking water or animals or plants eaten by humans, thereby entering the body through the digestive tract and causing harm to health or even death through diarrhea, nerve paralysis, liver damage and poisoning (Zhou et al., 2021). Such an emergency occurred in 2014 in Toledo, OH (Lake Erie, USA) causing authorities to shut down the city's water supply due to the threat to public health posed by elevated MC levels (Davis et al., 2019).
Given the risks to human health and events described above, the monitoring of cyanotoxins in water bodies have become challenge for a long time.
3 Sampling of Natural Water Bodies with the Presence of Cyanotoxins
In Colombia, in 2011, the National Institute of Health (INS, by its acronym in Spanish) published a manual for the collection, preservation and transport of samples in municipal distribution systems intended for the human consumption. The purpose of this is to evaluate the quality of water supplied to the population, in compliance with Decree 1575 of 2007 and its complementary resolutions (INS, 2011). Nevertheless, while mention is made of multiple compounds with recognized adverse effects on human health, such as heavy metals, pesticides, bacteria, parasites, viruses, and even emerging contaminants, cyanobacteria and their toxic metabolites are completed omitted, despite their negative impact on humans, animals and ecosystems, and the possibility of sampling these in natural water through some existing methodologies (Fig. 1).
3.1 Traditional Cyanotoxin Sampling Techniques
Traditional monitoring programs for cyanobacteria and their toxins are based on the collection of specific samples at specific times. In these programs, the sampling strategy significantly influences the selection of sampling points at an adequate frequency, both spatially and temporally (Pobel et al., 2011). Surface water samples are commonly collected manually. For this purpose, it is commonly recommended to use amber glass containers, in order to avoid possible adsorption on plastic bottles (Kamp et al., 2016) and to minimize exposure to sunlight (Kurtz et al., 2021), which could otherwise trigger photochemical reactions that could alter the physicochemical conditions of the sample. Therefore, adequate collection, preservation and storage of the samples are essential in order to guarantee the accuracy of the analysis carried out.
The determination of phytoplankton establishes the quality and level of eutrophication of the water bodies. In general, surface water samples are collected at depths between 0.3 and 1 m, while biomass samples are generally taken from the surface using phytoplankton nets (IDEAM & INVEMAR, 2017). Therefore, the sample can be obtained using two mechanisms: a Kemmerer bottle (point sampling) or a 20 µm conical net (volumetric sampling) (ICONTEC, 2016, 2020). The sampling of phytoplanktonic algae can be quantitative or qualitative. In the first case, an approximate volume of water of 250 mL is collected and a preservative is added to this; the sample should not be filtered, since it is intended to express the biomass of algae in cells per milliliter or cell density (ICONTEC, 2018). For qualitative samples, a record of the morphotypes present in the water body is carried out, enabling the identification of the presence of potentially toxic organisms, although the organisms are only reported as presence/absence (IDEAM & INVEMAR, 2017). In this regard, the greater the number of trawls, the greater the representation of the species present.
Subsequently, to carry out the analysis of extracellular cyanotoxins present in the water bodies, the samples are filtered with a pore size of 0.45 µm to separate the biomass that contains intracellular toxins. The samples must be refrigerated in dark conditions in order to avoid the degradation of the toxins. During this process, it is essential not to exceed the minimum storage time of 24 h. When prolonged storage is required, samples can be frozen at a temperature of -20 °C (ICONTEC, 2018). However, freezing of samples can release toxins from damaged cells. Storage time should not exceed 6 months. Additionally, it is recommended to add conservation reagents to the sampling containers, using trizma as a buffer (pH equal to 7), 2-chloroacetamide as antimicrobial, and ethylenediaminetetraacetic acid to inhibit the binding of analytes to metals (EPA/600/R-17 /344).
It should be noted that these active/random sampling techniques have several drawbacks, such as the need to sample large volumes to recover a sufficient mass of toxins. Additionally, it is necessary to carry out a lengthy cleaning process before performing the instrumental analyzes for the detection and quantification of cyanotoxins in the water samples taken (León & Peñuela, 2019). Furthermore, MC concentrations can vary over time, and episodes of high concentration peaks can be missed in the traditional monitoring scheme. An increase in sampling frequency or the installation of automatic sampling systems may provide a solution, but this can often be difficult, especially in remote areas (Kohoutek et al., 2008). Therefore, it is necessary to incorporate sensitive and representative alternatives such as passive sampling.
3.2 Passive Cyanotoxin Sampling Techniques
Passive sampling is a technique that can be used effectively for sensitive and integrated monitoring of chemicals in the aquatic environment (Kudela, 2017). It offers an attractive alternative to traditional sampling methods, as it helps avoid many of the problems described above. This technique has recently been used to study the occurrence, seasonal dynamics, and spatial distribution of MC in the environment (Wang et al., 2022; Wiltsie et al., 2018). It has been shown that passive samplers based on polar organic chemical integrative sampler (POCIS), in addition to being used to monitor certain anthropogenic pollutants, can sequester MC in the environment, allowing their time-weighted average (TWA) concentrations to be estimated over an extended period of time ranging from several days to several weeks (Kohoutek et al., 2008, 2010). POCIS consist of a solid sequestration medium (sorbent) enclosed within a hydrophilic microporous membrane. The membrane may be semi-permeable, allowing chemicals of interest to pass through and accumulate on the sorbent, while excluding particulate and biological matter and other interfering substances. POCIS were originally designed to mimic the exposure of aquatic organisms to dissolved chemicals (Alvarez et al., 2004).
The first application of passive samplers for MC was carried out at pilot scale by Kohoutek et al. (2008). They demonstrated the suitability of this tool for monitoring cyanotoxins in water bodies, using commercially available POCIS passive samplers, which effectively accumulated MC after 7 d of field exposure. To find a more efficient, sensitive and selective configuration for MC sequestration, Kohoutek et al. (2008) conducted experiments at laboratory scale where they evaluated 4 different porous membranes (polycarbonate, polyester, polyethersulfone and nylon) and 2 sorbents (Oasis HLB and Bondesil-LMS), exposing samplers of different configurations to MC (MC-RR and MC-LR) for 14 d under stable conditions. They observed differences in the sampling rates and amount of MC accumulated depending on the sampler configurations (membranes and sorbent materials). Finally, samplers built with the polycarbonate membrane and Oasis HLB sorbent (sorbent loading, 2.75 mg cm−2) provided the highest sampling rates (0.022 L d−1 for MC-RR and 0.017 L d−1 for MC-LR) (Kohoutek et al., 2008).
Kohoutek et al. (2010) optimized the design of the samplers, using the physical compression method to seal the membranes instead of other approaches that use adhesives, heat, etc. This design ensured ideal stretching of the membrane and uniform distribution of sorbent material, as well as providing a very good seal. The polyethylene support was made of hollow screws and nuts and had dimensions of 30.0 and 40.4 mm internal and external diameter, respectively. This allowed the use of 47 or 90 mm commercially available membranes. The total exchange surface area of the membrane (counting both sides) was 14.1 cm2 (Kohoutek et al., 2010). Subsequently, they validated the sampler configuration, obtaining the MC sampling rates for two different exposure scenarios (Rs = L d−1 in static conditions and in turbulent conditions). The Rs values are used to calculate the time-weighted average concentrations in natural water bodies. The calibration of the passive sampler carried out under variable conditions and different concentrations of MC revealed linearity of the sampling up to 4 weeks. This time is considered sufficient, concerning the high temporal and spatial variability of the cyanobacterial blooms (Kohoutek et al., 2010).
3.2.1 Long-Term Sampling and Linear Absorption Period Estimation for Cyanotoxins
The operating time profile of passive sampling devices is gradual and consists of three regimes. The first of these is the kinetic regime, where the absorption of target compounds in the sampler is linear. The second is the intermediate phase, which is characterized by a decrease in the sampling rate and curvilinear absorption kinetics. The third is known as the near equilibrium phase, where the sampler reaches capacity and the sampling rate is close to zero. A first-order model is often used to fit experimental measurements. The absorption process can be generalized and described by Eq. (1).
where \({C}_{sample}(t)\) is the concentration of the compound of interest in the sampler as a function of time \((t)\), \({C}_{medium}\) refers to the concentration of the compound in the environment, and \(k1\) and \(k2\) are the absorption and elimination rate constants, respectively.
During the linear absorption regime (relatively short sampling periods),\({C}_{medium}\) can be deduced from the measured amount of target compound in the passive sampler ( \({C}_{sample}(t)\)) as a function of the sampling rate (Rs); that is, the total volume of medium released from analyte per day. The linear absorption period of MC obtained by Kohoutek et al. (2008) of approximately 28 d, seems to be shorter than that described by Alvarez et al. (2004) who studied passive sampling of polar pharmaceuticals and pesticides. This could be explained by the designed sampler having a higher sampling rate than commercial POCIS, resulting in a lower effective thickness of the sample. This was achieved by using a smaller load of the solid sorbent (i.e., a lower ratio of sorbent mass per surface area) leading to a higher rate of diffusion and a faster response of the passive sampler to concentration of contaminant in the medium (Kohoutek et al., 2008).
3.2.2 Monitoring of Cyanotoxins in Continental Water
A relevant aspect of passive water samplers is that, by design, they can only measure MC in dissolved phase, which is considerably lower during cyanobacteria bloom periods (Wang et al., 2022). Wang et al. (2022) jointly implemented two different passive samplers. A first device was developed from organic diffusive gradients in thin films (o-DGT) based on diffusive polyacrylamide gel and hydrophilic-lipophilic balance (HLB) junction gel for MC in water bodies (Yao et al., 2019). Additionally, Wang et al. (2022) developed a solid phase adsorption toxin tracking (SPATT) device to estimate MC levels in three lakes in California, USA. Weighted average MC concentrations in time by o-DGT were lower than random water samples, probably because active sampling techniques measure both dissolved phases and MC contained within the cyanobacteria. Total concentrations of MC were up to 10.9 μg L−1, with MC-LR being the main variant, with a maximum concentration of 2.74 μg L−1. It is noteworthy that o-DGT showed a higher correlation with manual sampling compared to that evidenced with the use of SPATT. Both o-DGT and random samples gave comparable results for three MC variants (-LR, -RR and -YR) at levels below 0.1 μg L−1. The mass accumulation of MC was linear over 10 d (R2 ≥ 0.98); while sampling rates (2.68–3.22 mL d −1) and diffusion coefficients (0.90–1.08 × 10−6 cm2 s−1) of the three MC were obtained at 20 °C (Wang et al., 2022). According to Nyoni et al. (2017) the accumulation rate of most of the tested MC compounds depends on the temperature and flow rate.
Meanwhile, Jaša et al. (2019) used calibrated adsorption-based passive samplers (Fig. 2) to monitor MC over time, at three large-scale potable water treatment plants (PWTP) in the Czech Republic during two upwelling seasons, in parallel with traditional discrete sampling. Concentrations estimated by passive samplers were correlated with measurements of extracellular MC from discrete sampling of drinking water reservoirs. Notably, extracellular MC represent the fraction of toxins available for sequestration by passive sampling (Kohoutek et al., 2008, 2010), while discrete sampling involves the concentrations of extracellular and total MC. MC were detected in the epilimnion water samples at concentrations up to 14 μg/L in raw water, but their levels in PWTP were less than 1 μg/L (Jaša et al., 2019), which is the value established by the WHO for drinking water, as indicated above (WHO, 2011).
Under normal ecophysiological conditions, most MC are present within cyanobacterial cells, and the fraction of extracellular toxins in the soluble phase generally represents less than 30% of the total MC (Buratti et al., 2017), which is an important factor for their elimination in drinking water treatment. Conventional treatment technologies, such as coagulation/filtration, remove cyanobacteria and intracellular toxins, but have limited efficacy for extracellular toxins (He et al., 2016; Merel et al., 2013a). As such, it is common to detect MC in the final treated effluent distributed to the population. That situation was detected by Jaša et al. (2019); however, they found that the concentrations of MC in the effluents of the PWAP were below the limit of quantification of discrete sampling (< 25 ng/L).
Passive samplers, in combination with LC–MS/MS analysis, provide excellent sensitivity in the detection of time-weighted average concentrations of MC in the range 20–200 pg/L after 14 d. Thus, passive samplers adequately reflect the spatio-temporal variations of the MC, their removal efficiency in the POWs and the concentrations of toxins in the treated water (Jaša et al., 2019). In this regard, this type of samplers can be used for the evaluation and management of health risks of MC during the operation of PWTP.
Similarly, and problematically, wastewater treatment plants (WWTP) are prone to the proliferation of cyanobacterial species, which are favored by the long residence times of the water and the nutrient-rich environments (Romanis et al., 2021). Dense blooms often interrupt or hamper treatment processes due to the production of extracellular polymeric substances, which hinder microleakage. In this situation, cyanoHAB also represent an important challenge for the authorities since the released toxins can affect humans and animals exposed to contaminated water and, similarly, the implementation of special treatments is required to eliminate residual toxic cells and soluble toxins (Sukenik & Kaplan, 2021). Therefore, the distribution of wastewater contaminated with cyanobacteria and their toxins represents a significant risk (Romanis et al., 2021).
3.2.3 Monitoring of Cyanotoxins in Coastal Water
During the last decade, there has been an increase in the commercial cultivation and exploitation of shellfish, and contamination of these with biotoxins from microalgae is a public health problem worldwide. Therefore, MacKenzie et al. (2004) developed a simple and sensitive in situ method to monitor the occurrence of toxic algal blooms and shellfish contamination events. These authors introduced the idea of detecting various algal toxins by passively adsorbing them directly from seawater using solid phase adsorbents. These toxin tracking devices, SPATT, consist of sewn polyester mesh bags containing activated polystyrenedivinylbenzene resin, and are capable of adsorbing lipophilic algae toxins dissolved in seawater. Thus, SPATT bags provide a more convenient means of time-averaged sampling before or during algal blooms than shellfish or phytoplankton analysis, which is much more time-consuming and difficult to extract (MacKenzie et al., 2004).
Rundberget et al. (2009) developed passive sampling discs (Fig. 3) according to the SPATT method of MacKenzie et al. (2004), for application in field studies to monitor the dynamics of seaweed toxins in situ during mixed algal blooms in Norway. The authors obtained a convenient and cost-effective method (Rundberget et al., 2009). Likewise, Zendong et al. (2016) employed SPATT passive samplers in their study and were able to demonstrate the presence of algal toxins in Nigerian coastal water, despite the unfavorable environmental conditions caused by low salinities.
Assays of various adsorption substrates have been carried out to select the best candidates for the lipophilic marine biotoxin groups; however, it is necessary to study the selection of adequate substrates to retain the solubility in water of the most polar compounds, such as domoic acid and saxitoxins (MacKenzie, 2010). Notably, this method provides reliable, sensitive, and time-integrated sampling to monitor the occurrence of toxic blooms, and has several significant advantages over current phytoplankton and shellfish monitoring methods, including simplicity and low cost. Likewise, the matrices are relatively clean, which simplifies the extraction process and provides information on the dynamics of the toxins (Picardo et al., 2019).
Additionally, Lance et al. (2021) analyzed intracellular (in phytoplankton) and extracellular (dissolved in water) MC over a two-year period at five stations along a river near the coast of Brittany, France, using two types of samplers, bivalves and SPATT. SPATTs integrate extracellular MC, even at low ambient concentrations (0.2 µg/L) (Lance et al., 2021). Both samplers provided an accurate assessment of contamination level and total MC content (intra and extracellular), demonstrating the need to include cyanotoxins in the monitoring of seafood from estuarine areas.
3.2.4 Monitoring of Cyanotoxins in Sediments
The bloom-forming species have a competitive advantage over other phytoplankton species since they regulate their buoyancy and position in the water column through gas vacuoles (Cobo, 2015). Sampling of these is generally superficial; nonetheless, it has been verified that they accumulate in sediments and constitute a source and/or sink with high resuspension potential (Bormans et al., 2020). This indicates that water quality monitoring programs should consider benthic cyanobacteria as a potential source of toxins (Gaget et al., 2017b). Furthermore, the sampling and monitoring techniques commonly used to study planktonic species are not necessarily applicable to benthic forms. Conventional benthic sampling consists of collecting sediment core samples (Karlson et al., 2012; Savichtcheva et al., 2011). While collecting sediment core samples provides a relatively realistic picture of the biodiversity present, it is limited in rocky areas, where benthic mats appear to thrive in epilithic biofilms (Gaget et al., 2020).
In view of the above, Gaget et al. (2020) developed and validated a new sampling device for routine monitoring of benthic mats. The sampling device they designed (Fig. 4) worked effectively in the field and provided a new solution to monitor this complex matrix. Three types of artificial substrates (wood, sandpaper and tile) were used to facilitate the development of microbial biofilms. Each substrate rectangle covered a total area of 45 cm2. Additionally, to achieve adequate colonization, the substrates were placed as close as possible to the sediment surface. This was done by means of rectangular frames (144 cm × 67 cm) made of PVC pipes covered with a mesh, which served as a support structure for the substrates, being able to anchor and rest on the sediment, thereby limiting movement (Gaget et al., 2020).
Substrates used to collect samples should be incubated for at least 6–8 weeks prior to the first sampling, in order to allow biofilm to colonize the surface. Additionally, a minimum of 3 replicate samples should be collected to adequately assess the microbial community. Wood substrates allow for the collection of a larger biomass than the other two materials and exhibit less variability between replicates in biomass. This is likely due to the more porous nature of wood, which could provide better anchorage for the biofilm, providing better resistance against physical disturbances (Gaget et al., 2020).
Despite decades of research on the growth, control and production of toxins by pelagic cyanobacteria, and the advances made in this area, there are currently relatively few studies evaluating the risk associated with benthic forms (Umehara et al., 2017). Casero et al. (2019) provide novel data on the presence of picocyanobacteria and benthic taxa that potentially produce ANTX neurotoxins (eg, Phormidium sp.) in thermally stratified deep-water bodies. Therefore, further studies on the use of passive samplers, as well as the sampling of benthic cyanotoxins, are necessary.
In view of the above, once the sampling is carried out, which can be by random or integrated techniques, depending on the relevant requirement and/or available resources, analysis must be performed to detect and quantify the type and concentration of the cyanotoxins. The purpose of this analysis is to provide information on health risks associated with their presence in such diverse environments.
4 Cyanotoxin Detection and Quantification Techniques
An appropriate analytical method is also important for the accurate measurement of MC. To date, great progress has been made in the detection of cyanotoxins using various analytical techniques. Consequently, there are a variety of methods that allow routine monitoring of these bioactive substances, and deciding which technique to use can be challenging. Some of the assays available for their detection in environmental samples include ELISA (enzyme-linked immunosorbent assay), PPIA (protein phosphatase inhibition assay), PSI (protein synthesis inhibition), gene sequence PCR (polymerase chain reaction) and chemical analysis by high-performance liquid chromatography (HPLC) coupled to mass spectrometer (LC–MS). The last of these is one of the most accurate but also expensive methods (Gaget, et al., 2017a; Picardo et al., 2019). Moreover, there are a number of extraction and cleaning strategies for the preparation of the sample prior to assay, the most used of which include liquid–liquid extraction (LLE), solid phase microextraction (SPME), lyophilization and solid phase extraction (SPE). These show recoveries greater than 85% for some cyanotoxins such as MC (Picardo et al., 2019). It should be noted that, although there is generally a good correlation between the presence of potentially toxigenic cyanobacteria and the detection of the toxin, the number of cyanobacterial cells and the concentrations of toxins do not necessarily correlate. Therefore, counting the cells with the assistance of a microscope is not the best indicator of actual exposure to toxins (Gaget, et al., 2017a).
In this regard, the detection of cyanotoxins in natural water and treated effluents requires efficient extraction methodologies and analytical techniques with a high degree of sensitivity and selectivity. However, most techniques have low sensitivity and selectivity to distinguish among structurally similar congeners, along with the lack of reference standards (Janssen, 2019). Currently, there is a standardized method for their identification and quantification—EPA Method 544 “Determination of microcystins and nodularin in drinking water by solid phase extraction and liquid chromatography/tandem mass spectrometry (LC/MS/MS)”—(U.S. EPA, 2017), where the operating conditions are specified. This method combines liquid chromatography as a separation technique, and mass spectrometry for the detection, identification and quantification of the separated compounds, offering a powerful analytical technique. It is based on the detection of the analytes by the charge mass ratio, the precursor ion detection and the fragmentation characteristic of each compound that is previously determined for each cyanotoxin. Therefore, this technique allows the unambiguous detection of the desired molecules in the water samples even for low limits of quantification (León & Peñuela, 2019).
Furthermore, real-time toxin monitoring and detection is a challenging task, due to the heterogeneous nature of cyanobacterial blooms, the presence of different cyanotoxin variants and the low molecular weighted cyanotoxin congeners. Detection techniques are generally performed in a centralized laboratory and are not suitable for on-site detection. In view of this situation, Kulabhusan and Campbell (2021) manufactured lateral flow immunoassay (LFIA) reagent strips consisting of four components, a sample pad, a conjugate pad, a nitrocellulose (NC) membrane and an absorbent pad, all laminated to a plastic card. The LFIA uses poly- or monoclonal antibodies as a recognition probe; the principle of which lies in the movement of the target analyte and the binding to the recognition probe on the NC membrane. This methodology has gained attention due to its application in the field, sensitive detection, speed and cost-effectiveness (Kulabhusan & Campbell, 2021).
Finally, important research has been done in recent decades to obtain robust and sensitive analytical methods to determine and control the presence of cyanotoxins in the environment. These methods range from immunochemistry to analytical methods based on gas chromatography or liquid chromatography coupled to mass spectrometry analyzers (Picardo et al., 2019). Likewise, new and improved designs have been made of passive sampling tools for the efficient monitoring of biotoxins that can affect freshwater and coastal environments, drinking water reservoirs and supplies.
5 Conclusions
CyanoHAB in aquatic ecosystems represent a major challenge for health authorities since their presence extends to diverse environments both fresh and brackish water, including reservoirs, lakes, lagoons, and coastal water bodies; and both the pelagic and benthic stages. These organisms constitute a serious environmental problem, affecting human and animal health due to their production of toxic metabolites. Moreover, their production has increased in extent and frequency worldwide. Therefore, the risk associated with the presence of cyanotoxins has become a matter of great concern, and their detection and quantification is important in natural water bodies of all kinds, including residual water, water for consumption, irrigation and recreation, as well as in sediments.
Various studies in recent years have shown that the detection and quantification of cyanotoxins can be achieved with passive samplers, which can be used effectively for time-integrated measurements of trace and ultra-trace concentrations of these hazardous pollutants. Passive samplers thus represent a valuable tool for monitoring the quality of drinking water, assessing the risk posed by the presence of MC, and managing the situation. The techniques used in the preparation of samples and their analysis for the detection and quantification of cyanotoxins are diverse, being liquid and gas chromatography coupled to mass spectrometry, as well as immunochemistry, techniques of great importance.
Furthermore, studying the by-products generated from the degradation of natural toxins in aquatic environments and evaluating their possible adverse effects, both for the health of humans and that of other living beings, are crucial aspects in the management of cyanoHAB in water and control of cyanotoxin pollution.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Alvarez, D. A., Petty, J. D., Huckins, J. N., Jones-Lepp, T. L., Getting, D. T., Goddard, J. P., & Manahan, S. E. (2004). Development of a passive, in situ, integrative sampler for hydrophilic organic contaminants in aquatic environments. Environmental Toxicology and Chemistry, 23(7), 1640–1648. https://doi.org/10.1897/03-603
Bormans, M., Savar, V., Legrand, B., Mineaud, E., Robert, E., Lance, E., & Amzil, Z. (2020). Cyanobacteria and cyanotoxins in estuarine water and sediment. Aquatic Ecology, 0123456789. https://doi.org/10.1007/s10452-020-09764-y
Brooks, B. W., Lazorchak, J. M., Howard, M. D. A., Johnson, M. V. V., Morton, S. L., Perkins, D. A. K., Reavie, E. D., Scott, G. I., Smith, S. A., & Steevens, J. A. (2016). Are harmful algal blooms becoming the greatest inland water quality threat to public health and aquatic ecosystems? Environmental Toxicology and Chemistry, 35(1), 6–13. https://doi.org/10.1002/etc.3220
Buratti, F. M., Manganelli, M., Vichi, S., Stefanelli, M., Scardala, S., Testai, E., & Funari, E. (2017). Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Archives of Toxicology, 91(3), 1049–1130. https://doi.org/10.1007/s00204-016-1913-6
Caly, L. F., Rodríguez, D. C., & Peñuela, G. A. (2022). Monitoring of cyanobacteria and cyanotoxins in a Colombian tropical reservoir. Environmental Science and Pollution Research, 29(35), 52775–52787. https://doi.org/10.1007/s11356-022-19216-9
Caramés, D. M. M. (2016). Tecnologías de control de floraciones de cianobacterias y algas nocivas en cuerpos de agua, con énfasis en el uso de irradiación por ultrasonido. Innotec, 12(12), 54–61. http://ojs.latu.org.uy/index.php/INNOTEC/article/view/367
Casero, M. C., Velázquez, D., Medina-Cobo, M., Quesada, A., & Cirés, S. (2019). Unmasking the identity of toxigenic cyanobacteria driving a multi-toxin bloom by high-throughput sequencing of cyanotoxins genes and 16S rRNA metabarcoding. Science of the Total Environment, 665, 367–378. https://doi.org/10.1016/j.scitotenv.2019.02.083
Choi, J. S., Park, Y. H., Kim, S., Son, J., Park, J., & Choi, Y. E. (2022). Strategies to control the growth of cyanobacteria and recovery using adsorption and desorption. Bioresource Technology, 365, 128133. https://doi.org/10.1016/j.biortech.2022.128133
Christensen, V. G., Stelzer, E. A., Eikenberry, B. C., Olds, H. T., LeDuc, J. F., Maki, R. P., Saley, A. M., Norland, J., & Khan, E. (2021). Cyanotoxin mixture models: Relating environmental variables and toxin co-occurrence to human exposure risk. Journal of Hazardous Materials, 415, 125560. https://doi.org/10.1016/j.jhazmat.2021.125560
Cobo, F. (2015). Métodos de control de las floraciones de cianobacterias en agues continentales. Limnetica, 34(1), 247–268. http://www.limnetica.net/Limnetica/Limne34/L34a247_Control_cianobacterias_aguas_continentales.pdf
Crettaz-Minaglia, M., Sedan, D., & Giannuzzi, L. (2017). Bioacumulacion y biomagnificacion de cianotoxinas en organismos acuaticos de agua dulce. In Cianobacterias como determinantes ambientales de la salud (pp. 171–186). Ministerio de Salud de la Nación, departamento de Salud Ambiental. http://sedici.unlp.edu.ar/bitstream/handle/10915/72653/Documento_completo.pdf-PDFA.pdf?sequence=1&isAllowed=y
Davis, T. W., Stumpf, R., Bullerjahn, G. S., McKay, R. M. L., Chaffin, J. D., Bridgeman, T. B., & Winslow, C. (2019). Science meets policy: A framework for determining impairment designation criteria for large waterbodies affected by cyanobacterial harmful algal blooms. Harmful Algae, 81, 59–64. https://doi.org/10.1016/j.hal.2018.11.016
Dehghani, M. H. (2016). Removal of cyanobacterial and algal cells from water by ultrasonic waves — A review. Journal of Molecular Liquids, 222, 1109–1114. https://doi.org/10.1016/j.molliq.2016.08.010
Feng, Y., Chen, X., Ding, W., Ma, J., Zhang, B., & Li, X. (2020). MicroRNA-16 participates in the cell cycle alteration of HepG2 cells induced by MC-LR. Ecotoxicology and Environmental Safety, 192, 110295. https://doi.org/10.1016/j.ecoenv.2020.110295
Gaget, V., Hobson, P., Keulen, A., Newton, K., Monis, P., Humpage, A. R., Weyrich, L. S., & Brookes, J. D. (2020). Toolbox for the sampling and monitoring of benthic cyanobacteria. Water Research, 169, 115222. https://doi.org/10.1016/j.watres.2019.115222
Gaget, V., Humpage, A. R., Huang, Q., Monis, P., & Brookes, J. D. (2017a). Benthic cyanobacteria: A source of cylindrospermopsin and microcystin in Australian drinking water reservoirs. Water Research, 124, 454–464. https://doi.org/10.1016/j.watres.2017.07.073
Gaget, V., Lau, M., Sendall, B., Froscio, S., & Humpage, A. R. (2017b). Cyanotoxins: Which detection technique for an optimum risk assessment? Water Research, 118, 227–238. https://doi.org/10.1016/j.watres.2017.04.025
Gaysina, L. A., Saraf, A., & Singh, P. (2019). Cyanobacteria in diverse habitats. In Cyanobacteria: From Basic Science to Applications (pp. 1–28). Elsevier Inc. https://doi.org/10.1016/B978-0-12-814667-5.00001-5
He, X., Liu, Y. L., Conklin, A., Westrick, J., Weavers, L. K., Dionysiou, D. D., Lenhart, J. J., Mouser, P. J., Szlag, D., & Walker, H. W. (2016). Toxic cyanobacteria and drinking water: Impacts, detection, and treatment. Harmful Algae, 54, 174–193. https://doi.org/10.1016/j.hal.2016.01.001
Hense, I., & Beckmann, A. (2010). The representation of cyanobacteria life cycle processes in aquatic ecosystem models. Ecological Modelling, 221(19), 2330–2338. https://doi.org/10.1016/j.ecolmodel.2010.06.014
Hou, X., Feng, L., Dai, Y., Hu, C., Gibson, L., Tang, J., Lee, Z., Wang, Y., Cai, X., Liu, J., Zheng, Y., & Zheng, C. (2022). Global mapping reveals increase in lacustrine algal blooms over the past decade. Nature Geoscience, 15(2), 130–134. https://doi.org/10.1038/s41561-021-00887-x
Hu, C., & Rzymski, P. (2019). Programmed cell death-like and accompanying release of microcystin in freshwater bloom-forming cyanobacterium microcystis: From identification to ecological relevance. Toxins, 11(12), 1–19. https://doi.org/10.3390/toxins11120706
Huisman, J., Codd, G. A., Paerl, H. W., Ibelings, B. W., Verspagen, J. M. H., & Visser, P. M. (2018). Cyanobacterial blooms. Nature Reviews Microbiology, 16(8), 471–483. https://doi.org/10.1038/s41579-018-0040-1
ICONTEC. (2016). NTC-ISO 5667–4 Gestión ambiental. Calidad del agua. Muestreo. Guía para el muestreo de lagos naturales y artificiales. In Norma Técnica Colombiana.
ICONTEC. (2018). NTC-ISO 5667–3 Calidad del agua. Muestreo. Directrices para la preservación y manejo de las muestras. In Norma Tecnica Colombiana.
ICONTEC. (2020). NTC-ISO 5667–2 Gestión ambiental. Calidad del agua. Muestreo. Técnicas generales de muestreo. In Norma Técnica Colombiana.
IDEAM, & INVEMAR. (2017). Protocolo de Monitoreo del Agua - Colombia. http://documentacion.ideam.gov.co/openbiblio/bvirtual/023773/PROTOCOLO_MONITOREO_AGUA_IDEAM.pdf
INS. (2011). Manual de instrucciones para la toma, preservación y transporte de muestras de agua de consumo humano para análisis de laboratorio.
Janssen, E. M. L. (2019). Cyanobacterial peptides beyond microcystins – A review on co-occurrence, toxicity, and challenges for risk assessment. Water Research, 151, 488–499. https://doi.org/10.1016/j.watres.2018.12.048
Jaša, L., Sadílek, J., Kohoutek, J., Straková, L., Maršálek, B., & Babica, P. (2019). Application of passive sampling for sensitive time-integrative monitoring of cyanobacterial toxins microcystins in drinking water treatment plants. Water Research, 153, 108–120. https://doi.org/10.1016/j.watres.2018.12.059
Kamp, L., Church, J. L., Carpino, J., Faltin-Mara, E., & Rubio, F. (2016). The effects of water sample treatment, preparation, and storage prior to cyanotoxin analysis for cylindrospermopsin, microcystin and saxitoxin. Chemico-Biological Interactions, 246, 45–51. https://doi.org/10.1016/j.cbi.2015.12.016
Karlson, A. M. L., Nascimento, F. J. A., Suikkanen, S., & Elmgren, R. (2012). Benthic fauna affects recruitment from sediments of the harmful cyanobacterium Nodularia spumigena. Harmful Algae, 20, 126–131. https://doi.org/10.1016/j.hal.2012.09.001
Kaur, G. (2019). Freshwater Cyanotoxins. In Biomarkers in Toxicology (pp. 601–613). Elsevier Inc. https://doi.org/10.1016/b978-0-12-814655-2.00035-9
Kohoutek, J., Babica, P., Bláha, L., & Maršálek, B. (2008). A novel approach for monitoring of cyanobacterial toxins: Development and evaluation of the passive sampler for microcystins. Analytical and Bioanalytical Chemistry, 390(4), 1167–1172. https://doi.org/10.1007/s00216-007-1785-y
Kohoutek, J., Maršálek, B., & Bláha, L. (2010). Evaluation of the novel passive sampler for cyanobacterial toxins microcystins under various conditions including field sampling. Analytical and Bioanalytical Chemistry, 397(2), 823–828. https://doi.org/10.1007/s00216-010-3578-y
Kremp, A. (2000). Distribution, dynamics and in situ seeding potential of Scrippsiella hangoei (dinophyceae) cyst populations from the Baltic Sea. Journal of Plankton Research, 22(11), 2155–2169. https://doi.org/10.1093/plankt/22.11.2155
Kudela, R. M. (2017). Passive Sampling for Freshwater and Marine Algal Toxins. In Comprehensive Analytical Chemistry (Vol. 78). Elsevier Ltd. https://doi.org/10.1016/bs.coac.2017.08.006
Kulabhusan, P. K., & Campbell, K. (2021). Recent trends in the detection of freshwater cyanotoxins with a critical note on their occurrence in Asia. Trends in Environmental Analytical Chemistry, 32, e00150. https://doi.org/10.1016/j.teac.2021.e00150
Kurtz, T., Zeng, T., & Rosario-Ortiz, F. L. (2021). Photodegradation of cyanotoxins in surface waters. Water Research, 192, 116804. https://doi.org/10.1016/j.watres.2021.116804
Lance, E., Lepoutre, A., Savar, V., Robert, E., Bormans, M., & Zouher, A. (2021). In situ use of bivalves and passive samplers to reveal water contamination by microcystins along a freshwater-marine continuum in France. Water Research, 204, 117620. https://doi.org/10.1016/j.watres.2021.117620
León, C., & Peñuela, G. A. (2019). Detected cyanotoxins by UHPLC MS/MS technique in tropical reservoirs of northeastern Colombia. Toxicon, 167, 38–48. https://doi.org/10.1016/j.toxicon.2019.06.010
Li, J., Li, R., & Li, J. (2017). Current research scenario for microcystins biodegradation – A review on fundamental knowledge, application prospects and challenges. Science of the Total Environment, 595, 615–632. https://doi.org/10.1016/j.scitotenv.2017.03.285
Liu, B., Qu, F., Liang, H., Van der Bruggen, B., Cheng, X., Yu, H., Xu, G., & Li, G. (2017). Microcystis aeruginosa-laden surface water treatment using ultrafiltration: Membrane fouling, cell integrity and extracellular organic matter rejection. Water Research, 112, 83–92. https://doi.org/10.1016/j.watres.2017.01.033
Loaiza-González, J. M., Rubio-Clemente, A., León-Salazar, M. C., Rodríguez Loaiza, D. C., & Peñuela Mesa, G. A. (2021). Recurrencia de toxinas cianobacterianas en cuerpos de agua eutrofizados. In Prácticas y herramientas de sostenibilidad (pp. 153–181). Tecnológico de Antioquia Institución Universitaria.
MacKenzie, L. A. (2010). In situ passive solid-phase adsorption of micro-algal biotoxins as a monitoring tool. Current Opinion in Biotechnology, 21(3), 326–331. https://doi.org/10.1016/j.copbio.2010.01.013
MacKenzie, L., Beuzenberg, V., Holland, P., McNabb, P., & Selwood, A. (2004). Solid phase adsorption toxin tracking (SPATT): A new monitoring tool that simulates the biotoxin contamination of filter feeding bivalves. Toxicon, 44(8), 901–918. https://doi.org/10.1016/j.toxicon.2004.08.020
Malik, J. K., Bharti, V. K., Rahal, A., Kumar, D., & Gupta, R. C. (2020). Cyanobacterial (blue-green algae) toxins. In Handbook of Toxicology of Chemical Warfare Agents (pp. 467–478). INC. https://doi.org/10.1016/b978-0-12-819090-6.00031-3
Marcé, R., George, G., Buscarinu, P., Deidda, M., Dunalska, J., De Eyto, E., Flaim, G., Grossart, H. P., Istvanovics, V., Lenhardt, M., Moreno-Ostos, E., Obrador, B., Ostrovsky, I., Pierson, D. C., Potužák, J., Poikane, S., Rinke, K., Rodríguez-Mozaz, S., Staehr, P. A., … Jennings, E. (2016). Automatic high frequency monitoring for improved lake and reservoir management. Environmental Science and Technology, 50(20), 10780–10794. https://doi.org/10.1021/acs.est.6b01604
Merel, S., Clément, M., & Thomas, O. (2010). State of the art on cyanotoxins in water and their behaviour towards chlorine. Toxicon, 55(4), 677–691. https://doi.org/10.1016/j.toxicon.2009.10.028
Merel, S., Villarín, M. C., Chung, K., & Snyder, S. (2013a). Spatial and thematic distribution of research on cyanotoxins. Toxicon, 76, 118–131. https://doi.org/10.1016/j.toxicon.2013.09.008
Merel, S., Walker, D., Chicana, R., Snyder, S., Baurès, E., & Thomas, O. (2013b). State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environment International, 59, 303–327. https://doi.org/10.1016/j.envint.2013.06.013
Munoz, M., Cirés, S., de Pedro, Z. M., Colina, J. Á., Velásquez-Figueroa, Y., Carmona-Jiménez, J., Caro-Borrero, A., Salazar, A., Santa María Fuster, M. C., Contreras, D., Perona, E., Quesada, A., & Casas, J. A. (2021). Overview of toxic cyanobacteria and cyanotoxins in Ibero-American freshwaters: Challenges for risk management and opportunities for removal by advanced technologies. Science of the Total Environment, 761, 143197 https://doi.org/10.1016/j.scitotenv.2020.143197
Nowicka-Krawczyk, P., Żelazna-Wieczorek, J., Skrobek, I., Ziułkiewicz, M., Adamski, M., Kaminski, A., & Żmudzki, P. (2022). Persistent Cyanobacteria Blooms in Artificial Water Bodies—An Effect of Environmental Conditions or the Result of Anthropogenic Change. International Journal of Environmental Research and Public Health, 19(12), 6990. https://doi.org/10.3390/ijerph19126990
Nyoni, H., Mamba, B. B., & Msagati, T. A. M. (2017). Development of a silicone-membrane passive sampler for monitoring cylindrospermopsin and microcystin LR-YR-RR in natural waters. Physics and Chemistry of the Earth, 100, 189–200. https://doi.org/10.1016/j.pce.2016.10.010
O’Neil, J. M., Davis, T. W., Burford, M. A., & Gobler, C. J. (2012). The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae, 14, 313–334. https://doi.org/10.1016/j.hal.2011.10.027
Paerl, H. W., Gardner, W. S., Havens, K. E., Joyner, A. R., McCarthy, M. J., Newell, S. E., Qin, B., & Scott, J. T. (2016). Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae, 54, 213–222. https://doi.org/10.1016/j.hal.2015.09.009
Paerl, H. W., & Otten, T. G. (2013). Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microbial Ecology, 65(4), 995–1010. https://doi.org/10.1007/s00248-012-0159-y
Paerl, H. W., Otten, T. G., & Kudela, R. (2018). Mitigating the Expansion of Harmful Algal Blooms Across the Freshwater-to-Marine Continuum [News]. Environmental Science and Technology, 52(10), 5519–5529. https://doi.org/10.1021/acs.est.7b05950
Palacio Gómez, K., Hernández Atilano, E., Peñuela Mesa, G., Aguirre Ramírez, N., & Vélez Macías, F. (2019). Características morfológicas de las cianobacterias y fitoplancton dominante en tres embalses de Antioquia: un enfoque basado en la forma y el biovolumen. Revista U.D.C.A Actualidad & Divulgación Científica, 22(2), e1306. https://doi.org/10.31910/rudca.v22.n2.2019.1306
Park, J., Church, J., Son, Y., Kim, K. T., & Lee, W. H. (2017). Recent advances in ultrasonic treatment: Challenges and field applications for controlling harmful algal blooms (HABs). Ultrasonics Sonochemistry, 38, 326–334. https://doi.org/10.1016/j.ultsonch.2017.03.003
Pham, T. L., & Utsumi, M. (2018). An overview of the accumulation of microcystins in aquatic ecosystems. Journal of Environmental Management, 213, 520–529. https://doi.org/10.1016/j.jenvman.2018.01.077
Picardo, M., Filatova, D., Nuñez, O., & Farré, M. (2019). Recent advances in the detection of natural toxins in freshwater environments. TrAC - Trends in Analytical Chemistry, 112, 75–86. https://doi.org/10.1016/j.trac.2018.12.017
Pobel, D., Robin, J., & Humbert, J. F. (2011). Influence of sampling strategies on the monitoring of cyanobacteria in shallow lakes: Lessons from a case study in France. Water Research, 45(3), 1005–1014. https://doi.org/10.1016/j.watres.2010.10.011
Purcell, D., Parsons, S. A., Jefferson, B., Holden, S., Campbell, A., Wallen, A., Chipps, M., Holden, B., & Ellingham, A. (2012). Experiences of algal bloom control using green solutions barley straw and ultrasound, an industry perspective. Water and Environment Journal, 27(2), 148–156. https://doi.org/10.1111/j.1747-6593.2012.00338.x
Romanis, C. S., Pearson, L. A., & Neilan, B. A. (2021). Cyanobacterial blooms in wastewater treatment facilities: Significance and emerging monitoring strategies. Journal of Microbiological Methods, 180, 106123. https://doi.org/10.1016/j.mimet.2020.106123
Rundberget, T., Gustad, E., Samdal, I. A., Sandvik, M., & Miles, C. O. (2009). A convenient and cost-effective method for monitoring marine algal toxins with passive samplers. Toxicon, 53(5), 543–550. https://doi.org/10.1016/j.toxicon.2009.01.010
Salomón, S., Rivera-Rondón, C. A., & Zapata, Á. M. (2020). Cyanobacterial blooms in Colombia: State of knowledge and research needs in the context of climate global change. Revista de La Academia Colombiana de Ciencias Exactas, Fisicas y Naturales, 44(171), 376–391. https://doi.org/10.18257/raccefyn.1050
Savichtcheva, O., Debroas, D., Kurmayer, R., Villar, C., Jenny, J. P., Arnaud, F., Perga, M. E., & Domaizon, I. (2011). Quantitative PCR enumeration of total/toxic Planktothrix rubescens and total cyanobacteria in preserved DNA isolated from lake sediments. Applied and Environmental Microbiology, 77(24), 8744–8753. https://doi.org/10.1128/AEM.06106-11
Sedan, D., & Andrinolo, D. (2011). Cianobacterias y cianotoxinas: Efectos en la salud humana. Casos informados y primeros acercamientos al estudio epidemiológico. In Cianobacterias como determinantes ambientales de la salud (pp. 67–78). http://sedici.unlp.edu.ar/handle/10915/91471
Singh, A., & Babele, P. K. (2020). Dynamics of harmful cyanobacterial blooms and their toxins: environmental and human health perspectives and management strategies. In Advances in Cyanobacterial Biology. INC. https://doi.org/10.1016/b978-0-12-819311-2.00020-6
Su, R. C., Lad, A., Breidenbach, J. D., Kleinhenz, A. L., Modyanov, N., Malhotra, D., Haller, S. T., & Kennedy, D. J. (2020). Assessment of diagnostic biomarkers of liver injury in the setting of microcystin-LR (MC-LR) hepatotoxicity. Chemosphere, 257, 127111. https://doi.org/10.1016/j.chemosphere.2020.127111
Sukenik, A., & Kaplan, A. (2021). Cyanobacterial harmful algal blooms in aquatic ecosystems: A comprehensive outlook on current and emerging mitigation and control approaches. Microorganisms, 9(7), 1472. https://doi.org/10.3390/microorganisms9071472
Trevino-Garrison, I., Dement, J., Ahmed, F. S., Haines-Lieber, P., Langer, T., Ménager, H., Neff, J., Van Der Merwe, D., & Carney, E. (2015). Human illnesses and animal deaths associated with freshwater harmful algal blooms—Kansas. Toxins, 7(2), 353–366. https://doi.org/10.3390/toxins7020353
U.S. EPA (United States Environmental Protection Agency). (2017). Single Laboratory Validated Method for Determination of Microcystins and Nodularin in Ambient Freshwaters by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS).
Umehara, A., Takahashi, T., Komorita, T., Orita, R., Choi, J. W., Takenaka, R., Mabuchi, R., Park, H. D., & Tsutsumi, H. (2017). Widespread dispersal and bio-accumulation of toxic microcystins in benthic marine ecosystems. Chemosphere, 167, 492–500. https://doi.org/10.1016/j.chemosphere.2016.10.029
Vidal, L., Ballot, A., Azevedo, S. M. F. O., Padisák, J., & Welker, M. (2021). Introduction to cyanobacteria. In Toxic Cyanobacteria in Water (pp. 163–211). https://doi.org/10.1201/9781003081449-3
Wang, P., Du, B., Smith, J., Lao, W., Wong, C. S., & Zeng, E. Y. (2022). Development and field evaluation of the organic-diffusive gradients in thin-films (o-DGT) passive water sampler for microcystins. Chemosphere, 287(P1), 132079. https://doi.org/10.1016/j.chemosphere.2021.132079
Wang, Y., Li, F., Du, J., Shi, X., Tang, A., Fu, M. L., Sun, W., & Yuan, B. (2021). Formation of nitrosamines during chloramination of two algae species in source water—Microcystis aeruginosa and Cyclotella meneghiniana. Science of the Total Environment, 798, 149210. https://doi.org/10.1016/j.scitotenv.2021.149210
Wei, H., Jia, Y., & Wang, Z. (2022). Microcystin pollution in lakes and reservoirs: A nationwide meta-analysis and assessment in China. Environmental Pollution, 309, 119791. https://doi.org/10.1016/j.envpol.2022.119791
Wells, M. L., Trainer, V. L., Smayda, T. J., Karlson, B. S. O., Trick, C. G., Kudela, R. M., Ishikawa, A., Bernard, S., Wulff, A., Anderson, D. M., & Cochlan, W. P. (2015). Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae, 49, 68–93. https://doi.org/10.1016/j.hal.2015.07.009
Westrick, J. A., Szlag, D. C., Southwell, B. J., & Sinclair, J. (2010). A review of cyanobacteria and cyanotoxins removal/inactivation in drinking water treatment. Analytical and Bioanalytical Chemistry, 397(5), 1705–1714. https://doi.org/10.1007/s00216-010-3709-5
WHO. (2011). Guidelines for drinking-water quality, 4th edition. The World Health Organization. https://www.who.int/publications/i/item/9789241548151
Wiltsie, D., Schnetzer, A., Green, J., Borgh, M. V., & Fensin, E. (2018). Algal blooms and cyanotoxins in Jordan Lake. North Carolina. Toxins, 10(2), 92. https://doi.org/10.3390/toxins10020092
Yao, L., Steinman, A. D., Wan, X., Shu, X., & Xie, L. (2019). A new method based on diffusive gradients in thin films for in situ monitoring microcystin-LR in waters. Scientific Reports, 9(1), 1–8. https://doi.org/10.1038/s41598-019-53835-6
Yao, Y., Pan, Y., & Liu, S. (2020). Power ultrasound and its applications: A state-of-the-art review. Ultrasonics Sonochemistry, 62, 104722. https://doi.org/10.1016/j.ultsonch.2019.104722
You, L., Tong, X., Te, S. H., Tran, N. H., bte Sukarji, N. H., He, Y., & Gin, K. Y. H. (2022). Multi-class secondary metabolites in cyanobacterial blooms from a tropical water body: Distribution patterns and real-time prediction. Water Research, 212, 118129 https://doi.org/10.1016/j.watres.2022.118129
Zendong, Z., Kadiri, M., Herrenknecht, C., Nézan, E., Mazzeo, A., & Hess, P. (2016). Algal toxin profiles in Nigerian coastal waters (Gulf of Guinea) using passive sampling and liquid chromatography coupled to mass spectrometry. Toxicon, 114, 16–27. https://doi.org/10.1016/j.toxicon.2016.02.011
Zhang, L., Yang, J., Liu, L., Wang, N., Sun, Y., Huang, Y., & Yang, Z. (2021). Simultaneous removal of colonial Microcystis and microcystins by protozoa grazing coupled with ultrasound treatment. Journal of Hazardous Materials, 420, 126616. https://doi.org/10.1016/j.jhazmat.2021.126616
Zhou, C., Chen, H., Zhao, H., & Wang, Q. (2021). Microcystin biosynthesis and toxic effects. Algal Research, 55, 102277. https://doi.org/10.1016/j.algal.2021.102277
Funding
Open Access funding provided by Colombia Consortium.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Loaiza-González, J.M., Rubio-Clemente, A. & Peñuela, G.A. Cyanotoxin Monitoring and Detection Using Passive Sampling Application. Water Air Soil Pollut 235, 423 (2024). https://doi.org/10.1007/s11270-024-07195-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07195-1