Abstract
Phthalic acid esters (PAEs) are dialkyl or alkyl/aryl ester derivatives of phthalic acid. PAEs are colorless, odorless, and flavorless oily liquids. PAEs are the main plasticizers used in industry and households. DEHP (di-(2-ethyl hexyl) phthalate) is the main plasticizer used in the polymer industry, whereas DMP (dimethyl phthalate) and DEP (diethyl phthalate) are used mainly as solvents or fixatives in cosmetics and personal care products. PAEs are synthetic organic compounds poorly soluble in water but soluble in organic solvents. Into the environment, they are introduced during the production, use and degradation, packaging, and transportation of plastic products. In the environment, PAEs are degraded in three ways: by hydrolysis, photodegradation, and microbial degradation, whereas the biodegradation and hydrolysis of PAEs in the environment are very slow. PAEs are pollutants of soil, water, groundwater, river water, marine water, air, sediments, vegetables, and biota. Due to the great interest in the subject of environmental pollution by PAEs and the emergence of new information in this area, it is extremely important to systematically review the current knowledge. In the presented paper, the occurrence of PAEs in different environmental matrices was reviewed. The toxicity to plants, animals, and humans was also described.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
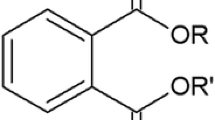
Phthalic acid esters (PAEs) (otherwise known as phthalates, phthalate esters, or dialkyl phthalate esters) are composed of one benzene ring and two ester groups (Sablayrolles et al., 2005) which are located in the ortho position (F. Chen et al., 2021; Erythropel et al., 2014; Przybylińska & Wyszkowski, 2016; Xiong & Pei, 2021). PAEs are a group of 1,2-benzene dicarboxylate esters (N. Chen et al., 2017; Ghosh & Sahu, 2022; Liang et al., 2008). Phthalates are dialkyl or alkyl/aryl ester derivatives of phthalic acid (benzene-1,2-dicarboxylic acid) (Fig. 1, Table 1) (Gani et al., 2017; Liang et al., 2008; Vasseghian et al., 2023; Ventrice et al., 2013; J. Zhao et al., 2018).
Phthalic anhydrite and alcohols (mainly aliphatic) are used for the catalytic synthesis of PAEs (Holland, 2018; Puri et al., 2023; Sharma & Kaur, 2020; Staples, 2003; Tuan Tran et al. 2022,b; Xiong & Pei, 2021). PAEs are colorless (D.-W. Gao & Wen, 2016; Vasseghian et al., 2023), odorless and flavorless (Tuan Tran et al. 2022,b) oily liquids (Julinová & Slavík, 2012; Sharma & Kaur, 2020; Staples, 2003). According to the length of the hydrocarbon chain, PAEs can be divided into two groups low molecular weight (LMW) and high molecular weight (HMW) (Ventrice et al., 2013; Xiong & Pei, 2021). To LMW PAEs are classified: butyl benzyl phthalate (BBP), dibutyl phthalate (DBP), diethyl phthalate (DEP), and dimethyl phthalate (DMP). di-(2-ethyl hexyl) phthalate (DEHP), diheptyl phthalate (DHpP), diisodecyl phthalate (DIDP), and diisononyl phthalate (DINP) are considered as HMW (Guo et al., 2024; Poopal et al., 2020; Xiong & Pei, 2021).
PAEs are the main plasticizers used in industry and households (L. Chen et al. 2024b; Holland, 2018; Müller & Kördel, 1993). They are used as plasticizers in the production of polyvinyl chloride (PVC) to increase its flexibility (Wang, Li, et al., 2023), and also in styrene, rubber, and cellulose-based products. DEHP, which accounts for 50% of all plasticizers used in PVC, may constitute up to 40-50% of the weight of the PVC and from 10% to 60% of other polymers (X. Cheng et al., 2015; Erythropel et al., 2014; Holland, 2018; Li, Chen, et al., 2016; J. Wang et al., 2013; Wang, Chen, et al., 2015; Lijun Wang et al., 2018; F. Zhao et al., 2022; J. Zhao et al., 2018). The other applications of PAEs include industrial (like in the paint or petrochemical industry), but also domestic (like toys, and cosmetics) or specific (like medical, agricultural e.g. pesticides) (Bodzek et al., 2004; F. Chen et al., 2021; N. Chen et al., 2017; Z. Cheng et al., 2021; D. Gao et al., 2014; D.-W. Gao & Wen, 2016; Giuliani et al., 2020; Godwin n.d.; Heudorf et al., 2007; Julinová & Slavík, 2012; Latini et al., 2004; Przybylińska & Wyszkowski, 2016; Rudel & Perovich, 2009; Sablayrolles et al., 2005; Saeidnia & Abdollahi, 2013; Sathyanarayana, 2008; Sharma & Kaur, 2020; Tuan Tran et al. 2022,b; J. Wang et al., 2014; X. Wang et al., 2021; L. Wei et al., 2020; X. Wei et al., 2016; Wu et al., 2019; G. C. C. Yang et al., 2016; J. Zhao et al., 2018; Zhou et al., 2021). The worldwide production of PAEs is about 8 million tons per year (Naveen et al., 2022). China is the largest producer of PAEs in the world. In 2017, 32% of produced phthalates was produced by China (Bai et al., 2024). Additionally, China is the largest importer of phthalates in the world, with a market share of 7.7% in 2015 (Vasseghian et al., 2023).
LMW phthalates are commonly used in cosmetics and the main application of HMW PAEs is in the polymer industry as plasticizers. Up to 80% of the PAEs are produced for this purpose (Gani et al., 2017; D. Gao et al., 2014; Ghosh & Sahu, 2022; H. Liu et al., 2010; J. Wang et al., 2013; Lijun Wang et al., 2018; Zheng et al., 2014). As the solvents, DMP and DEP are mostly used, others such as DBP, DEHP, DIDP, and DINP are mainly used in the plastic industry as plasticizers (Godwin, n.d.; Edwards et al., 2022; Hou et al., 2021; Staples, 2003; Xiong & Pei, 2021; Zhou et al., 2021). DBP and DEHP are the two main PAEs present in agricultural films, accounting for more than 97.1% of the total PAEs applied in agricultural films. High applications result in very high emissions to the environment. It was estimated that in China up to 18.8 tons of DBP and 42.2 tons of DEHP in 2017 from mulching films and 5.94 tons of DBP and 24.5 tons of DEHP from greenhouse films were emitted (Zhang, Ma, et al., 2021).
PAEs are known to be hydrophobic synthetic organic compounds. Their water solubility is 4.0 × 102 mg/L for DEP to 2.6 × 10-3 mg/L for DEHP (Bodzek et al., 2004; Przybylińska & Wyszkowski, 2016; Zhang, Jiao, et al., 2021). The physicochemical properties like the hydrophobicity of PAEs depend on the length of the hydrocarbon chain. The shorter the chain, the less hydrophobic PAEs are (Ghosh & Sahu, 2022; B. C. Tran et al., 2015; Xiong & Pei, 2021), and LMW are better soluble in water and have a higher vapor pressure (Ghosh & Sahu, 2022; Staples, 2003; Tuan Tran et al. 2022,b; J. Zhao et al., 2018). HMW PAEs absorb more easily onto organic matter and particles (Ghosh & Sahu, 2022), thus they are present widely in the wastewater or sewage sludge from wastewater treatment plants (WWTPs) (B. C. Tran et al., 2015).
Such a wide use of phthalates makes them a common environmental pollutant. Additionally, these are compounds that cause various diseases in humans. Therefore, it is important to determine their concentration in various matrices and develop efficient methods of removing them from the environment. This review focuses on the results of research conducted in recent years, which examined: the concentration of phthalates in various environmental matrices and in food products, the fate of phthalates in the environment and methods of their removal. Additionally, the effects of phthalates on plants, animals and people were reviewed.
2 Occurrence and Environmental Fate of PAEs
In the environment, PAEs are degraded in three ways: by hydrolysis, photodegradation, and microbial degradation (Zhang, Jiao, et al., 2021) (Fig. 2). The biodegradation and hydrolysis of PAEs in the environment are very slow (D.-W. Gao & Wen, 2016). However, some microorganisms able to degrade PAEs were isolated from different matrices. The microbial removal of PAEs was evidenced using sewage sludge-derived Achromobacter strains. Similarly, bacteria extracted from soil such as Agromyces and Bacillus revealed a high PAEs degradation efficiency. PAEs degraded bacteria were found also in sediments (Curvibacter), compost (Providencia), bioreactors (Pseudoxanthomonas) (F. Chen et al., 2021), salty soil (Ren et al., 2023) or marine water (Ou et al., 2023). The rate of PAEs biodegradation is related to the conditions and it decreases in anaerobic, cold environments where microbes that degraded phthalates cannot live (D.-W. Gao & Wen, 2016; Liang et al., 2008). The rate of PAEs biodegradation depends on their bioavailability and HMW PAEs, due to their hydrophobicity, are less bioavailable and their biodegradation rates are lower (Staples, 2003). In a year experiment (Q. Sun et al., 2023), a PAEs periodical peak in winter and summer in the soil was noted stressing the impact of the seasonal changes in temperatures, humidity, sun operation, and activity of soil organisms.
PAEs with long side chains like DEHP, DHP (dihexyl phthalate), and DNOP (di-n-octyl phthalate) are also less biodegradable than phthalates with short side chains such as DMP, DEP, DBP, and BBP (Liang et al., 2008). DEHP, the representative of HMW phthalate, has a high octanol-water partitioning coefficient (log Kow = 7.5) and is more difficult to biodegrade than LMW phthalates (D.-W. Gao & Wen, 2016; Wang, Zhang, et al., 2015). Solubility in water, low log Kow, and weak interactions with organic matter in the soil are crucial parameters in the biodegradation of PAEs. DEP, which log Kow is 2.42 is prone to biodegradation (F. Chen et al., 2021; Zhou et al., 2021). The susceptibility of phthalates to degradation, e.g. by hydrolysis, is related to their structure. PAEs with long side chains underwent hydrolysis at a lower level than those with short side chains (F. Chen et al., 2021; Zhou et al., 2021). The steric effect of phthalates side ester chains affects hydrolysis. The side ester chains hinder the hydrolytic enzymes from binding to the phthalates and thereby inhibit their hydrolysis (Liang et al., 2008).
PAEs are biodegradable in the laboratory with pure cultures of bacteria and several environments with their natural communities of microflora (Keyser et al., 1976). DEHP is quickly degraded in the environment under aerobic conditions. Many bacteria can hydrolyze DEHP in aerobic environments (i.e., genomes Rhodococcus, Bacillus, Micrococcus, Enterococcus, Pseudomonas, Acinetobacter, Mycobacterium, Corynebacterium, Brevibacterium, Gordonia, etc.), but fungi can do the same (i.e., Penicillium lilacinum, Aspergillus Niger, Aspergillus puniceus, Actinomucor elegans, Phanerochaete chrysosporium, etc.) (Erythropel et al., 2014). There are also known species of fungi that can degrade phthalates under aerobic, anoxic, and anaerobic conditions and which are present in various environments such as rivers, marine waters, sediments, and soils (D.-W. Gao & Wen, 2016; Liang et al., 2008; Wang, Zhang, et al., 2015). Unfortunately, some of them can only transform PAEs into phthalate monoesters (PME) which are still toxic for animals (F. Chen et al., 2021; Erythropel et al., 2014). It is hard to find one microorganism that can completely PAEs biodegradation. The solution is to use various microorganism cultures (Liang et al., 2008).
The major route of decomposition of DEHP is the metabolic breakdown by microorganisms (Wang, Zhang, et al., 2015). The PAEs molecules are firstly “cut” into smaller monoesters and final phthalic acid (PA). The next step is the enzymatic (with esterase) decomposition of PA into benzoic acid (BA). The BA ring cleavage leads to the final products: CO2 and H2O (F. Chen et al., 2021; Heudorf et al., 2007; Liang et al., 2008; Sathyanarayana, 2008; Ventrice et al., 2013; Zhang, Jiao, et al., 2021).
PAEs have weak interactions with polymers via hydrogen bonds or by van der Waals forces (Hou et al., 2021). Such weak interactions are responsible for their wide presence in the environment as PAEs, at the proper temperature, pH, or other conditions, are simply leached from polymer (F. Chen et al., 2021; Z. Cheng et al., 2021; Dargnat et al., 2009; D. Gao et al., 2014; M. Gao et al., 2018; Giuliani et al., 2020; Heudorf et al., 2007; Julinová & Slavík, 2012; Liang et al., 2008; H. Liu et al., 2010; Przybylińska & Wyszkowski, 2016; Rudel & Perovich, 2009; Sathyanarayana, 2008; Wang, Zhang, et al., 2015; Xiong & Pei, 2021; G. C. C. Yang et al., 2016; Zhang, Ma, et al., 2021; F. Zhao et al., 2022; Zhou et al., 2021). This process is easier in outdoor products under natural conditions like rain, wind, frost, ultraviolet rays, insects, and bacteria (Bi et al., 2021). The main application of phthalates is plasticizers (Hui et al., 2021). Any processing of plastics (from production to disposal) is the source of PAEs in the environment (X. Cheng et al., 2015; Hui et al., 2021; Przybylińska & Wyszkowski, 2016; Sharma & Kaur, 2020; B. C. Tran et al., 2015; J. Wang et al., 2013; Wang, Chen, et al., 2015; Wang, Zhang, et al., 2015; Lijun Wang et al., 2018; Wu et al., 2019; Zheng et al., 2014). PAEs are present in all environmental matrices and are considered pollutants of soil, water, wastewater, and air (Table 2, Fig. 3A-B) (F. Chen et al., 2021; D.-W. Gao & Wen, 2016; Giuliani et al., 2020; Hui et al., 2021; Tuan Tran et al. 2022,b; Wang, Chen, et al., 2015; L. Wei et al., 2020; Wu et al., 2019; Xiong & Pei, 2021; G. C. C. Yang et al., 2016; Zhang, Ma, et al., 2021; J. Zhao et al., 2018; Zheng et al., 2014). They are found in the tissues and fluids of wildlife (X. Wei et al., 2016). PAEs which are more volatile such as DEP, DMP, DBP, and DIBP (diisobutyl phthalate) are more common in the gas phase, while DEHP and BBP are more prevalent in the dust phase. PAEs concentrations are linked with industrial activity and through it, they are present at higher levels in cities (Giuliani et al., 2020; Rudel & Perovich, 2009; Tuan Tran et al. 2022,b). And more PAEs are in the air in cities than in rural areas (Rudel & Perovich, 2009) the same relationship exists for river and sediment pollution (J. Wang et al., 2014). The type of water is also crucial when considering the fate of PAEs as biodilution or bioaccumulation may be observed (Gobbato et al., 2024). It was noted that total concentrations of 6 PAEs in algae and fish collected from a freshwater environment (Songhua River, China) were higher than in marine water (Bohai Bay, China) with DEHP and DBP being predominant (B. Liu et al., 2024).
PAEs are more common in the indoor environment, where their concentrations in the air are higher than concentrations in the outdoor air, and BBP is the main phthalate compound that is found in indoor air and dust in residences (Heudorf et al., 2007; X. Wang et al., 2021). The content of various PAEs in different parts of the city is correlated with local activities (X. Li et al., 2023). In residential areas, there is the highest concentration of DMP which is used in cosmetics. Near main routes there are high concentrations of BBP and DEP, these PAEs are present in car parts. In industrial areas, there are the most common DEHP, DBP, and DNOP which are used in many industrial processes (Lijun Wang et al., 2018). DEHP, DHP, DMEP (di(2-methoxyethyl) phthalate) (Heudorf et al., 2007; X. Wang et al., 2021). DMP, DEP, DBP, BBP, DEHP, and DNOP are the most frequently noted PAEs in surface water (seawater and freshwater), whereas DBP and DEHP are predominant in fresh and marine water (Giuliani et al., 2020). Comparing PAEs concentrations in suspended particles with PAEs concentrations in water and sediments it can be seen that the highest concentrations are in suspended particles the lowest concentrations are in the water. This is due to PAEs’ hydrophobic properties and the large specific surface area of the suspended particles and PAEs in water are degraded by hydrolysis (S. Sun et al., 2025; Zheng et al., 2014).
WWTPs effluent is considered the main source of PAEs in the aquatic environment (Chen, Niu, et al., 2024; D. Gao et al., 2014; Wu et al., 2019) as compounds from plastic products are washed back into wastewater (Sablayrolles et al., 2005). The main sources of PAEs in industrial wastewater are plastic and cosmetics plants (Gani et al., 2017). PAEs may be introduced into the environment also by landfill leachates (Liang et al., 2008). The PAEs concentrations in the sediments are correlated with the sediment organic matter (OM) and phosphorus contents but not with sediment pH (J. Wang et al., 2014). There are some HMW phthalates such as DEHP, DINP, and DIDP in the sludge, and they are strongly sorbed due to their high log Kow (n-octanol/water partition coefficient) for comparison DMP is not found in sludge because of their good solubility in water (B. C. Tran et al., 2015). DEHP may bioaccumulate in the soils and sediments (Saeidnia & Abdollahi, 2013). DBP and DEHP are the main soil pollutants it is the effect of atmospheric deposition and sewage sludge amendment (Giuliani et al., 2020). In general, DMP, DEP, DBP, BBP, DEHP, and DNOP, are considered priority pollutants. The sum of concentrations of these six PAEs makes up 64.8%, 72.9%, and 66.9% of all phthalates in water, sediments, and suspended particles respectively (Zheng et al., 2014). The lower the anthropogenic activity the lower the concentration of PAEs in sediments and soils. Therefore, due to this fact, they can be used to assess environmental pollution with PAEs in the vicinity of industrial plants (Lijun Wang et al., 2018; L. Wei et al., 2020; Zheng et al., 2014).
3 Toxicity of PAEs
3.1 PAEs Effect on Plants
Different crops can accumulate PAEs to different degrees (Hui et al., 2021). To reduce the amount of PAEs in the environment different plants should be planted in different years. Plants take up PAEs in two main ways, by leaves from the air and by roots from the soil (F. Zhao et al., 2022) (Fig. 4). Absorption from the air is the main route source for PAEs accumulation in plants growing in greenhouses (Grøn et al., 2000), and for plants from open fields, the main source of PAEs contamination is uptake from soil (F. Zhao et al., 2022). Plants take up PAEs through roots and may accumulate them in stems (D.-W. Gao & Wen, 2016). When the plant is exposed to air contaminated with PAEs, phthalates may be accumulated in the cuticular and wax layers in leaves, stems, and fruits, and most exposed to this are leafy vegetables (Zeng et al., 2020). PAEs concentration is lower in fruits than in leaves because the leaves have a higher surface area and are longer exposed to contamination (Wang, Chen, et al., 2015). This is the reason why the differences between the concentration of PAEs in the soil and the plants are observed (N. Chen et al., 2017; Saeidnia & Abdollahi, 2013; Sharma & Kaur, 2020; Zeng et al., 2020). Vegetables with high lipid content contain more PAEs and they should not be selected for cultivation in contaminated areas, such as around large cities (L. Wei et al., 2020). Sewage sludge containing PAEs used as a fertilizer may increase DEHP concentration in plants (Grøn et al., 2000).
The distribution of PAEs in plants results from their properties. PAEs with long side chains, such as DEHP are more difficult to transport from stems to leaves and their concentrations in stems are the highest (F. Zhao et al., 2022). PAEs may be known as an environmental stressor for plants by causing various physiological, morphological, cellular, and metabolic alterations in plants (Sharma & Kaur, 2020). PAEs in plants are responsible for the introduction of oxidative stress and the generation of reactive oxygen species and other intra- or extracellular free radicals affecting the metabolic activities of plants. As a result, a decrease in chlorophyll content, inhibition of growth and development, and damage to cell membranes are observed (Sharma & Kaur, 2020). PAEs may affect the amount of nutrients such as vitamins in plants, for example, DBP lowered the vitamin C and capsaicin contents of pepper (Wang, Chen, et al., 2015).
3.2 PAEs Effect on Animals
PAEs as compounds with a high Kow and low vapor pressure easily migrate in various water bodies and they very easily can enter into aquatic organisms where they cause a lot of damage (Zhang, Jiao, et al., 2021). PAEs that are introduced into the environment may bioaccumulate in animal tissues (Fig. 4) and the higher the trophic level the animal is, the more it is exposed to phthalates, thus, carnivorous fish are more prone to phthalates than herbivorous fish. However, the concentration in the body of high trophic level animals is often lower than that of lower trophic level. Responsible for it is the high metabolic capacity of high-trophic-level organisms (Z. Cheng et al., 2021; Staples, 2003; Zhang, Jiao, et al., 2021).
PAEs have potential carcinogenic, teratogenic, and mutagenic effects. Phthalates and their metabolites may affect metabolism and reproduction in fish and mammals (Dargnat et al., 2009; Domínguez-Morueco et al., 2014; Gani et al., 2017; Ghosh & Sahu, 2022; Liang et al., 2008; Tuan Tran et al. 2022.b; Wang, Chen, et al., 2015; J. Wang et al., 2014; G. C. C. Yang et al., 2016). PAEs are introduced into fish bodies mainly by gills and then are distributed throughout the body. The presence of PAEs in tissues may cause oxidative stress on organisms by accelerating the production of oxidation products (Poopal et al., 2020). DHpP may cause oxidative stress and genotoxic carcinogenic effects in the liver of rats (Poopal et al., 2020).
DBP, even at very small concentrations of 0.1, 0.5, 1.0, 5.0, and 10.0 mg/kg, affects the development of animals, which may result in a reduction in the number of different species around the world. Some studies indicate that DBP pollution is responsible for reducing the number of reptilian species (Przybylińska & Wyszkowski, 2016). In animals, phthalates affect the production of sex hormones which leads to reproductive defects. PAEs also can cause kidney, thyroid, and liver damage and contribute to liver and spleen cancer (Przybylińska & Wyszkowski, 2016).
PAEs are known to cause immune, metabolic, endocrine, and developmental toxicity and they have acute and chronic toxic effects on aquatic animals (Zhang, Jiao, et al., 2021). The lethal concentrations for some PAEs are known: LC50 96-h of DHpP to zebrafish is 500 mg/L and LC50 (96-h) of DIDP on zebrafish is 300 mg/L (Poopal et al., 2020). The potential ecological risk of PAEs in sediment was lower than that in water bodies (Wang, Liu, et al., 2023). PAEs present in the aquatic environment can enter plants and animals and pass along the food chain (Zhang, Jiao, et al., 2021). On the other hand, oysters, due to their shells, which have a high sorption capacity, can reduce the amount of PAEs in the water (Wofford et al., 1981).
3.3 Toxicity of PAEs on Humans
The toxicity of phthalates varies according to the structure of the compound and the organ in the body (Saeidnia & Abdollahi, 2013), and results from the presence of ester bonds (F. Chen et al., 2021). PAEs are dangerous because they bioaccumulate in living tissues and are poorly biodegradable. They have short-term effects like allergies and asthma and long-term like altered nervous and endocrine systems, cancer, lowered fertility, and disruptions in child development (Z. Cheng et al., 2021; Giuliani et al., 2020; Sablayrolles et al., 2005; Sathyanarayana, 2008; Staples, 2003; Tuan Tran et al. 2022,b; Ventrice et al., 2013; Xiong & Pei, 2021; Zhang, Ma, et al., 2021). PAEs have a strong influence on the fetus and the effects of this are seen in adults (Bodzek et al., 2004). PAEs released from the greenhouse film into the air may increase the risk of exposure to them by inhalation in people working in greenhouses (N. Chen et al., 2017; Saeidnia & Abdollahi, 2013; Zeng et al., 2020).
PAEs can be inhaled (from air contaminated with PAEs), ingested (food, plastic toys), and absorbed by the skin (personal care products, clothes) (Fig. 4) (Ghosh & Sahu, 2022; Heudorf et al., 2007; Sathyanarayana, 2008; Sharma & Kaur, 2020; Tuan Tran et al. 2022,b; Ventrice et al., 2013; Xiong & Pei, 2021; G. C. C. Yang et al., 2016; F. Zhao et al., 2022). In general, inhalation bioaccessibility and the lung cell penetration of PAEs (except for diethyl phthalate) under a healthy state was lower than that under the inflammatory condition (Huang et al., 2023).
The main source of human exposure to phthalates is the consumption of contaminated food products (Erythropel et al., 2014; Fierens et al., 2012; Ghosh & Sahu, 2022; Hou et al., 2021; Przybylińska & Wyszkowski, 2016; Staples, 2003; Zeng et al., 2020). Another route of human exposure to PAEs is dermal absorption of phthalates present in personal care products and children are at risk of ingestion of phthalates present in plastic toys (Przybylińska & Wyszkowski, 2016; Rudel & Perovich, 2009). Infants and young children are exposed to PAEs via breast milk, mouthing/sucking behaviors (Q. Sun et al., 2023), and dermally by personal care products but the main source of exposure is plastic toys (Heudorf et al., 2007; Latini et al., 2004; X. Li et al., 2023; Sathyanarayana, 2008; Tuan Tran et al. 2022,b).
There are correlations between levels of urinary metabolites of PAEs and indoor or personal air concentrations of the parent phthalate, this may suggest that indoor air contamination is one of the routes of human exposure (Rudel & Perovich, 2009). It was established that the endocrine-disrupting effects of PAEs in pregnant women were reduced by the simultaneous intake of glutamate, catechin, and folic acid (He et al., 2022). Because PAEs are hydrophobic they easily migrate and accumulate in lipids (Julinová & Slavík, 2012; Przybylińska & Wyszkowski, 2016). The exposure to PAEs is broad. More than 98% of the U.S. population had contact with PAEs which was confirmed by studying the biomarkers of phthalates exposures (Edwards et al., 2022). The presence of PAEs and their metabolites were confirmed in urine, blood, breast milk, or even in saliva and hair (Bi et al., 2021; Dargnat et al., 2009; Sathyanarayana, 2008; Sharma & Kaur, 2020; L. Wei et al., 2020; Xiong & Pei, 2021; F. Zhao et al., 2022).
The average daily human exposure to DEHP, according to the Agency for Toxic Substances and Disease Registry (2002) (ATSDR), was established at about 0.003–0.03 mg/kg/day (7.7–77 μmol/kg/day). It is worth stressing that children are prone to PAEs contamination (migration of PAEs into the placenta, excretion into breast milk, and toys (Giuliani et al., 2020)). HMW phthalates may cause irritation of sense organs like the eyes, nose, and throat (Gani et al., 2017).
The effect of PAEs on human health depends on the age of the person. They have a different impact on newborns, children, and adults. Early birth of low-weight of infants was noticed after prenatal exposure to PAEs which affects the future behavior of children. Children’s exposure to PAEs affects their intelligence quotient (IQ), metabolism (often noted obesity), and development of the nervous system. PAEs also may accelerate puberty in girls and delay puberty in boys. Infertility, sex hormones disturbances, and low semen quality are noted in adults exposed to PAEs (Ghosh & Sahu, 2022; Giuliani et al., 2020; Holland, 2018; Przybylińska & Wyszkowski, 2016; Rudel & Perovich, 2009; Saeidnia & Abdollahi, 2013; X. Wang et al., 2021; Xiong & Pei, 2021).
PAEs block the action of endogenous hormones by blocking their specific receptors and destroying their synthesis and metabolism (Ghosh & Sahu, 2022; Holland, 2018; B. C. Tran et al., 2015). PAEs are recognized as endocrine-disrupting substances (EDS) (Holland, 2018; X. Wei et al., 2016; H.-M. Zhao et al., 2015; Zheng et al., 2014) affecting human health (increasing the risk of cancer, developmental deformities, and mutations). PAEs are recognized recently as efficient inhibitory factors of human carboxylesterases (DEHP. DnOP, DPP) disturbing the endogenous substances catalyzed by CES1 (Gong et al., 2024). According to the United States Environmental Protection Agency (US EPA), DMP, DEP, DBP, BBP, DEHP, and DNOP were identified as pollutants requiring priority control whereas DEHP was classified as a probable human carcinogen (X. Cheng et al., 2015; Z. Cheng et al., 2021; Ghosh & Sahu, 2022; Hou et al., 2021; Hui et al., 2021; Li, Ma, et al., 2016; Saeidnia & Abdollahi, 2013; Sharma & Kaur, 2020; Wang, Chen, et al., 2015; Wang, Zhang, et al., 2015; Lijun Wang et al., 2018; X. Wang et al., 2021; Zhou et al., 2021). The carcinogenic and teratogenic properties of BBP and DEHP have been already confirmed (Hui et al., 2021; Lijun Wang et al., 2018). DIDP has an acceptable daily intake (ADI) estimated at 150 mg/kg bw/d. This phthalate may flow through the blood-brain barrier (BBB), and cause memory impairment, and oxidative stress (Poopal et al., 2020). PAEs induce the abnormal expression of peroxisome proliferator-activated, aryl hydrocarbon, and insulin receptors (Zhang, Yang, et al., 2023).
In general, PAEs demonstrate acute toxicity - their LD50 values reach 1 – 30 g/kg body weight (Tuan Tran et al. 2022,b). Minimal risk level (MRL) values were determined for some PAEs. MRI for DEHP via indirect exposure was established at 0.1 mg, whereas via chronic exposure - 0.06 mg/kg bw × day. Slightly lower hazard revealed DBP (0.5 mg/kg bw × day), DEP (acute oral exposure - 7 mg/ kg bw × day, chronic oral exposure - 5 mg/ kg bw × day), and DNOP (acute oral exposure - 3 mg/kg bw × day, indirect exposure - 0.4 mg/kg bw × day)) (Przybylińska & Wyszkowski, 2016). Similarly, tolerable daily intake (TDI) values were determined for: 0.02 mg/kg bw × day, 0.025 mg/ kg bw × day, or 0.044-0.05 mg/kg bw × day for DEHP (considering newborns aged up to 3 months and women of reproductive age, children aged 3-12 months, and other subjects, respectively); 0.37 mg/ kg bw × day for DNOP; 0.15 mg/ kg bw × day for DINP, and 0.25 mg/kg bw × day for DIDP (Przybylińska & Wyszkowski, 2016). As a result, no observable adverse effect levels (NOAEL) values for DEHP were set at 4.8-44 mg, and 750 mg/kg bw × day for DEP (Przybylińska & Wyszkowski, 2016), and TDI or a reference dose (RfD), both based on non-cancer effects, were used in various jurisdictions, e.g., 50 μg/kg bw in the EU, 44 μg/kg bw in Canada, and 20 μg/kg bw in the USA (Erythropel et al., 2014; Latini et al., 2004; Sharma & Kaur, 2020; Tuan Tran et al. 2022,b).
4 Food Contamination by PAEs
Contaminated food is the main route of human exposure to phthalates, and this makes it important to control the concentration of PAEs in different types of food (Wang, Ma, et al., 2023). Foodstuffs may be contaminated with phthalates during production, storage, and meal preparation. PAEs may enter vegetables and fruits during cultivation, because of soil and water contamination. Different types of food may be contaminated during production when machinery in factories contains PAEs like PVC pipes, especially in the dairy industry. And cooking may increase phthalate contamination when are used plastic dishes. Storing food can lead to its contamination with PAEs when the packages contain PAEs, e.g. polyethylene terephthalate (PET) bottles, phthalates may also migrate to food from lacquers and printing inks which are presented on the packages (Coltro et al., 2023;da Costa et al., 2023 ; Giuliani et al., 2020).
Food with higher fat content such as milk products, oils, and fatty meat have a higher concentration of PAEs, due to their hydrophobic properties (Edwards et al., 2022; Erythropel et al., 2014; Giuliani et al., 2020; Latini et al., 2004). Vegetables and fruits are irreplaceable sources of vitamins, fiber, and minerals for humans (da Costa et al., 2023). Vegetables growing on the ground contaminated with PAEs or in plastic greenhouses where the air is contaminated with PAEs are the main source of human exposure to PAEs (Zhou et al., 2021). As mentioned above contamination of vegetables with phthalates varies depending on the type of plant and where it grows. Leafy vegetables growing in greenhouses may absorb PAEs from the air and may have a higher concentration of PAEs than those grown in the open field. There are also differences between leafy vegetables and root vegetables. And also different parts of the same plant (Giuliani et al., 2020; Zhang, Ma, & Wang, 2023). Studies say that the phthalate content changes in sequence leafy vegetables > root vegetables > > fruits-vegetables > fungus > cauliflower vegetables > > beans (Zhang, Ma, & Wang, 2023). These differences are presented in Table 3.
PAEs contamination is regional due to their sources which are related to the type of crop (Zhou et al., 2021). The wastewater/sludge application in agriculture would be a significant source of PAEs in deep soils (X. Cheng et al., 2015). Other sources of soil contamination are fertilizers and pesticides which are used by farmers (Zhou et al., 2021), wastewater irrigation (Wang, Chen, et al., 2015), and plastic mulching films (Li, Chen, et al., 2016; J. Wang et al., 2013, 2014; L. Wei et al., 2020; H.-M. Zhao et al., 2015). Edible plants can be also contaminated with phthalates during the packaging, transport, and storage when plastic bags are used (Zhang, Ma, & Wang, 2023). Fruits and vegetables in shops may be contaminated with PAEs by labels and tapes. Phthalates may enter the peel and then into the pulp. This process begins immediately and is accelerated by the layer of wax on some fruits, e.g., apples (Hou et al., 2021).
Sludge organic fertilizers (SOFs) are recognized as the source of PAEs (Hui et al., 2021). Application of SOFs may lead to the accumulation of PAEs in soil increasing the environmental hazard. SOFs that contain up to 15.3 mg/kg of DEHP and up to 6.04 mg/kg of DNOP, were the main source of soil pollution by PAEs (>60% of the total content of Σ5PAEs in soil); the accumulation in soil was also favored due to the long persistence of DEHP and DNOP in the soil (T1/2 DEHP 30.8 d and DNOP 28.4 d) (Hui et al., 2021).
PAEs constitute up to 60% of the weight of the agricultural films that are currently used on a large scale in agriculture. As PAEs are not chemically bound to the film they can be easily washed out of this film and then accumulate in soil and plants as hazardous compounds (Li, Chen, et al., 2016, 2016; X. Wang et al., 2021; Zeng et al., 2020; Zhang, Ma, et al., 2021; F. Zhao et al., 2022). The longer use of agricultural films led to greater soil contamination with PAEs (Zeng et al., 2020). In China PAEs from agricultural films is the main source of soil PAEs contamination (Li, Chen, et al., 2016; Wang, Chen, et al., 2015; Zhang, Ma, et al., 2021; Zhou et al., 2021). DBP, DIBP, and DEHP are the three major PAEs present in agricultural films and they are also the major soil and air contaminants in plastic greenhouses (Li, Chen, et al., 2016; J. Wang et al., 2013; Zeng et al., 2020).
DEHP, DIBP, and DBP are the main PAEs present in the plastic film, reaching up to 15.9, 16.7, and 11.2 mg/kg, respectively. Higher phthalate contamination is caused by film aging and physicochemical processes (Zhou et al., 2021). Many investigations say that the concentration of PAEs in agricultural soils in various parts of China exceeded the level of mg/kg in many areas (Wang, Chen, et al., 2015; Zhou et al., 2021).
The variations in levels of PAEs in the soil in greenhouses may be related to the type and amount of agricultural film used, its age, and how long it was used (J. Wang et al., 2013). Therefore there are differences in the number of phthalates in plants (F. Zhao et al., 2022). PAEs from greenhouse films released into the interior air may be transported through diffusion into the soil and accumulated in the vegetables (Table 3) (Zeng et al., 2020). For instance, 60.9% of the DBP released from the greenhouse film, is degraded, while 96.3% of the DEHP is accumulated in plants (Zhang, Ma, et al., 2021). Soil contamination with phthalates leads to a reduction in soil fertility (Li, Ma, et al., 2016). The use of sludge as fertilizer may increase the content of PAEs, especially HMW phthalates in soils so to avoid their accumulation in the environment it is necessary to monitor them (Dargnat et al., 2009; D.-W. Gao & Wen, 2016; Sablayrolles et al., 2005; B. C. Tran et al., 2015).
The adsorption of PAEs is governed by soil organic matter (SOM) and minerals in the soil and any changes in the composition of SOM may affect PAEs adsorption, distribution, and degradation of PAEs by soil microorganisms. The content of PAEs at different soil depths varies due to the interactions with soil components, SOM quality and quantity, and microorganisms activity (Hui et al., 2021).
The concentration of PAEs in soil depends on its properties such as soil organic carbon (SOC), SOM content, pH, and TOC (total organic carbon). SOM controls the mobility of PAEs which are hydrophobic organic contaminants (HOCs) and the higher the level of SOM the higher the concentration of PAEs. The same correlation exists for an increase in pH, the higher pH the higher concentration of PAEs in soil (Li, Ma, et al., 2016; Zeng et al., 2020). But in many studies, there are no correlations observed between the TOC and PAEs and pH and PAEs concentration (Lijun Wang et al., 2018).
There are differences in the distribution of PAEs in topsoil. For example, PAEs concentrations are higher in cultivated fields than in uncultivated fields, and in addition, the amount of phthalates in the soil varies in fields with different crops due to the different consumption of fertilizers and pesticides (H. Liu et al., 2010). The concentrations of DEP, DBP, DIBP, and DEHP in soil decrease with the depth (Tuan Tran et al. 2022,b) due to the aerobic biodegradation in the top layers of soil, and these PAEs are sorbed in top layers. But at a depth of 31-110 cm contents of DBP, DIBP, and DEHP increase. At this depth, aerobic conditions changed into anaerobic conditions and biodegradation is more difficult. In the deeper layers of the soil more abundant are LMW phthalates like DMP and DEP which are not sorbed (H. Liu et al., 2010; B. C. Tran et al., 2015). PAEs with long side chains such as DEHP, DBP, and DIBP are absorbed by soil organic carbon and it is more difficult to degrade them than the PAEs with short side chains (e.g., DMP, DEP) resulting in higher concentrations of HMW PAEs in soil (Zeng et al., 2020).
The concentration of PAEs in the soil varies according to the season. It is related to the rate of biodegradation depending on the ambient temperature. Higher air temperatures in summer result in faster degradation of phthalates and their lower concentration in the soil (H. Liu et al., 2010). A similar relationship exists for air, where PAEs concentrations also are lower in hot seasons like summer than in cold like winter (D.-W. Gao & Wen, 2016). The amount of phthalates in the soil varies with the environment in sequence urban > agricultural > rural > forest with the results: in the urban soil 1089 ng/g dw, in the agricultural soil 407 ng/g dw, in the rural soil 154 ng/g dw, and in the forest soil 60 ng/g dw with DEHP as predominant (B. C. Tran et al., 2015).
Vegetable oils like olive oil, sunflower oil, and rapeseed oil may be easily contaminated with phthalates during production and especially during storage in plastic bottles. Raw materials such as olives, sunflower, or rapeseed may be contaminated during the harvest and transport to the mill. The PAEs concentration in oils varies depending on the type of plant, growing region, and number of production steps (Table 4). For example, oil obtained from a perennial plant e.g. olive oil has higher PAEs content than oil derived from annual crops like sunflower or rapeseed. This is due to the greater potential for bioaccumulation (da Costa et al., 2023; Giuliani et al., 2020; S. Y. Wang et al., 2022).
Milk and other dairy products have a high fat content by what they may be easily contaminated with phthalates which are hydrophobic compounds. A long production chain additionally increases the risk of contamination. Differences in phthalate contamination result from the type of product, its fat content, and type of packages (Table 5) (da Costa et al., 2023; Giuliani et al., 2020). The situation when cattle are fed contaminated feed and the method of milking process also have an impact on the concentration of phthalates in milk. The greatest contamination takes place during milk pasteurization where are used materials containing PAEs and high temperatures which may facilitate the migration of phthalates to milk (Giuliani et al., 2020).
Meat is the primary source of protein in the human diet. Like milk and dairy products meat has high fat content. That makes it important to control the contamination of meat and meat products with phthalates because they tend to concentrate in lipids. Meat may be contaminated with PAEs during production, storage, and cooking. Also, animals can eat contaminated feed and drink contaminated water. So, it is crucial to examine the phthalate concentration in feeds and water. By all these facts there are a lot of differences in PAEs concentration in different types of meat (Table 6) (da Costa et al., 2023; Giuliani et al., 2020).
A lot of phthalates find their way into the rivers and seas with sewage. There they may easily enter the water bodies like fish and seafood. Contamination of fish with PAEs is dangerous for people, especially in coastal countries where fish constitutes a crucial part of the diet. PAEs concentration in foods varies depending on the type of product (Table 7) (da Costa et al., 2023).
Mineral water and soft drinks are mostly sold in plastic bottles. PAEs can contaminate them by leaching from packages such as polyethylene terephthalate (PET) bottles (Heudorf et al., 2007; Xiong & Pei, 2021). The quality of the raw material for example: the use of recycled PET, the technology used in bottle production, the contamination of the water sources, and the cross-contamination in the factory can influence the phthalate content in mineral water and soft drinks. Another factor that affects PAEs contamination is storage time. A lot of studies show that the longer mineral water is stored in plastic bottles the greater the contamination it is. Storage in higher temperatures and exposure on sunlight also may increase PAEs content in mineral water. Soft drinks have higher phthalates concentrations than mineral water. The PAEs migration from PET bottles to soft drinks is 5 to 40 times higher than to mineral water. This is due to the fact that soft drinks contain a lot of preservatives and may have low pH. The type of used preservative also has an impact on PAEs level in soft drinks. The highest phthalate contamination is in soft drinks with K-sorbate (819.40 μg L−1), followed by one and a half times lower levels in drinks preserved with Na-benzoate and K-sorbate, seven times lower levels in drinks with Na-benzoate (116.93 μg L−1), and nine times lower in drinks preserved with orthophosphoric acid (91.67 μg L−1) (Giuliani et al., 2020). The concentration of PAEs in various types of mineral water and soft drinks and in various conditions shows Table 8.
In fast food samples, DBP (in 81% of samples) and DEHP (in 70% of samples) have been detected. BBP, BnOP, and DEP were also detected but at a lower concentration. It was established that fast food with meat has higher PAEs concentrations than that without meat (Edwards et al., 2022).
Food may be contaminated during preparation by gloves. The research showed that gloves used in restaurants contain up to 37% PAEs by weight (Edwards et al., 2022; Heudorf et al., 2007). The boiling of food products reduces the number of phthalates but frying and grilling may increase their concentration (Fierens et al., 2012).
5 Regulations
Physicochemical properties, especially water solubility and environmental persistence were the background for the recognition of PAEs as pollutants (F. Chen et al., 2021). Many countries set permissible concentrations of some phthalates. According to the Directive of the European Parliament and the Council of 2005, the use of DEHP, DBP, and BBP at concentrations higher than 0.1% relative to the weight of plasticized material in children's toys and childcare products was banned; similarly, the use of DINP, DIDP, and DNOP in children's toys and childcare products that may be placed in the child's mouth was limited (Domínguez-Morueco et al., 2014; Erythropel et al., 2014; Heudorf et al., 2007; Hou et al., 2021; Latini et al., 2004; Przybylińska & Wyszkowski, 2016; Zhang, Jiao, et al., 2021). In 2007, the Consumer Product Safety Improvement Act (CPSIA) in the United States regulated PAEs maximum content in children’s toys or childcare articles at up to 0.1% of 6 PAEs (D. Gao et al., 2014; Latini et al., 2004). At the same time, in Korea, the content of DBP, BBP, and DEHP in children’s stuff and DIDP, DINP, and DNOP for medical devices or cosmetics were regulated (Tuan Tran et al. 2022,b). Nowadays, child-care stuff, children’s toys containing DBP, BBP, DEHP, DINP, DNOP, and DIDP are not allowed to be produced in the European Union (EU) (Tuan Tran et al. 2022,b).
The reference values for selected airborne substances, including DEHP, DBP, DEP, and DMP, were set at 100 μg/m3 · h and 15 μg/m3 · year were limited in the Regulation of the Minister of Environment of Poland, 2010, (Przybylińska & Wyszkowski, 2016). In Poland, according to the Ministry of Health Regulations of 19 November 2002 the permissible concentration of DBP in drinking water is 20 μg/L (Bodzek et al., 2004). DEHP concentration of less than 8 μg/L is recommended for drinking water by the World Health Organization (WHO) (Domínguez-Morueco et al., 2014; Gani et al., 2017; Julinová & Slavík, 2012). The Directive of the European Parliament and the Council in 2013 established DEHP limits for surface waters at 1.3 μg/L (Dargnat et al., 2009; D. Gao et al., 2014; Przybylińska & Wyszkowski, 2016). The US Environmental Protection Agency (US EPA) has regulated a maximum admissible concentration of DEHP in water at 6 μg/L (Julinová & Slavík, 2012; H. Liu et al., 2010).
6 PAEs removal
PAEs from vegetables can be easily removed by washing and cooking (Fig. 5), approximately 31% ± 13% of PAEs were removed. The high solubility and vapor pressure of DMP and DEP make them prone to removal (39 ± 9% and 45 ± 22%, respectively) (N. Chen et al., 2017).
Adsorption and biodegradation are two main mechanisms for removing PAEs from environmental samples (D.-W. Gao & Wen, 2016; Julinová & Slavík, 2012) (Fig. 5). The efficiency of PAEs removal in WWTPs with microbial degradation is estimated at 81%, 90%, 93%, and 91% for DEHP, BBP, DMP, and DBP, respectively. Another study found that approximately 70%, 48%, 71%, 63%, and 61% of DEHP, BBP, DPP, DNOP, and DNP in the WWTPs were subjected to microbial degradation, respectively (D. Gao et al., 2014). Sorption to primary and secondary sludge increased DEHP removal efficiency to 94% (B. C. Tran et al., 2015). Cyclic-activated sludge technology (CAST) used in WWTPs enables a high removal efficiency for LMW PAEs with biotransformation as the main process. In WWTPs that used Anoxic/Oxic (A/O), and Anaerobic/Anoxic/Oxic (A/A/O) as treatment processes the PAEs removal efficiency is lower especially for HMW PAEs than in WWTPs CAST (D. Gao et al., 2014), but for DMP and DEP it gives the best results (Wu et al., 2019). Anoxic–oxic (A/O or AO) technology is the combined process of anaerobic hydrolysis technology and aerobic activated sludge method anaerobic–anoxic–oxic (A/A/O or A2O) technology added an anaerobic zone. These two technologies present high removal efficiencies for PAEs (D.-W. Gao & Wen, 2016). The aerated grit chamber where PAEs are settled and biological tanks played main roles in removing DMP, DEP, DnBP, DCHP, and DEHP, and the removal rate is 44.12%–58.90%. Moreover, LMW PAEs are easy to degrade under aeration conditions. The biological tank is important in the degradations of DBP and DEHP. DNOP and BBP are mainly removed from the grit chamber with a removal rate of 57.60% and 54.75%, by sorption on the suspended particles (Wu et al., 2019). The different steps of the wastewater treatment process removed various PAEs with various efficiency. In the first step, where contaminants are removed by settling, heavier PAEs (BBP and DEHP) are removed with higher efficiency. At the next step – biological degradation the lighter phthalates (DMP and DEP) are removed with an efficiency of 94% and 84%, respectively. In the last step, which is the nitrification of ammonium, the best elimination ratios are observed for DBP and DEHP 19% and 13% respectively. This is due to the adsorption of PAEs on the particles arising from nitrification (Dargnat et al., 2009). However, most conventional wastewater treatment processes do not provide good PAEs removal efficiency and it can range from 60% to 100% (Wu et al., 2019). The studies, however, revealed that Bacillus sp. LUNF1, isolated from sewage sludge can be efficient in PAEs removal from wastewater even under a range of metal ions (Fan et al., 2023). Microbial activity can be applied during composing revealing broad efficiency (25 - 100%) of PAEs removal (H.-T. Tran, Nguyen, et al., 2022).
Pressure-driven membrane techniques in phthalate removal from water revealed high effectiveness and enables 97.6-99.9% removal of PAEs. The membranes may remove compounds independent of their concentration in the filtered water and their molar masses (Bodzek et al., 2004). PAEs removal efficiency by nanofiltration depends on the type of phthalates. HMW PAEs are removed with higher efficiency than LMW, the removal efficiency of DMP, DEP, DBP, DEHP, and DNOP is 82.3%, 86.7%, 91.5%, 95.1%, and 95.4%, respectively. Different factors such as operating pressure, pH, ionic strength, and temperature have no significant effect on the PAEs removal efficiency (X. Wei et al., 2016).
There are many methods of PAEs removal that use photochemical degradation, like, photochemical degradation of DEP or DEHP via UV/H2O2 (Julinová & Slavík, 2012). Removal of PAEs from sediments may be carried out using H2O2. The efficiency of this process is 70%-100% after 14 days and increased with increasing concentration of H2O2 and when the daily doses of H2O2 are from 20 to 200 μL. Adding nutrients provides even greater efficiency from 89% to 100%. The degradation rates of various phthalates decreased with increasing log Kow of PAEs (G. C. C. Yang et al., 2016). The combination of several wastewater treatment technologies gives better removal efficiency for PAEs than a single technology (D.-W. Gao & Wen, 2016).
PAEs may be removed by adsorption where biochar and activated carbon are used as adsorbents. For this purpose biochars from vegetable wastes give a high removal efficiency and it is a cheap and environmentally friendly adsorbent (Tuan Tran et al. 2022,b). The efficiency and mechanism of this process are strongly related to the structure of PAEs. For the removal of LMW PAEs, π-π EDA interactions and pore-filling mechanisms are considered; HMW PAEs are mainly removed by hydrophobic interactions (Xiong & Pei, 2021). The ability of biochars to sorption PAEs depends on factors such as pyrolysis temperature, type of feedstock, modifications, and organic carbon content in the matrix. For instance, biochars obtained from pepper straw at 500 °C have the greatest sorption capacity, compared to biochars obtained from peanut hull which have the greatest sorption capacity when obtained at 650 °C (Xiong & Pei, 2021).
Modification of biochars with nano-manganese dioxide (nMnO2) as an oxidizer increases their capacity (Xiong & Pei, 2021). The removal efficiency increased with increasing doses of biochar and with an increasing degree of MnO2 coverage on the biochar surface (M. Gao et al., 2018). Degradation of PAEs may be achieved by persulfate (PS) oxidation. This approach is used in the remediation of groundwater and soil. PS activation and sulfate radical (SO4*-) were applied in PAEs removal from water and soil. However, the process efficiency was affected by process parameters such as PS concentration, pH, natural organic matter, and the presence of inorganic ions (e.g. HCO3-, Cl-) (Xiong & Pei, 2021).
7 Conclusions
Phthalates, recognized as dangerous endocrine disruptors, have been extensively detected in various environmental matrices, with contaminated food emerging as a primary source of human exposure. The widespread presence of PAEs in our surroundings underscores the urgent need for effective strategies to reduce their impact. Furthermore, the presented results highlight the importance of comprehensive risk assessment and regulatory measures to minimize human exposure to phthalates. Policies aimed at limiting the use of phthalates in consumer products and promoting eco-friendly alternatives are crucial steps toward mitigating their adverse effects on both environmental and human health. It is also necessary to develop novel, sustainable and effective methods for removing phthalates under various environmental conditions and from various environmental matrices. In summary, the presence of phthalates in our environment requires concerted, interdisciplinary efforts from scientists, policymakers and industry stakeholders. By prioritizing research, innovation and proactive regulatory action, we can move towards a cleaner and healthier future, free from the threats posed by phthalate contamination.
Data Availability
The datasets generated during and/or analyzed during the current study are available only in the manuscript.
References
Bai, X., Pan, K., Shoaib, N., Sun, X., Wu, X., & Zhang, L. (2024). Status of phthalate esters pollution in facility agriculture across China: Spatial distribution, risk assessment, and remediation measures. Science of The Total Environment, 908, 168416. https://doi.org/10.1016/j.scitotenv.2023.168416
Bi, M., Liu, W., Luan, X., Li, M., Liu, M., Liu, W., & Cui, Z. (2021). Production, Use, and Fate of Phthalic Acid Esters for Polyvinyl Chloride Products in China. Environmental Science & Technology, 55(20), 13980–13989. https://doi.org/10.1021/acs.est.1c02374
Bodzek, M., Dudziak, M., & Luks-Betlej, K. (2004). Application of membrane techniques to water purification. Removal of phthalates. Desalination, 162, 121–128. https://doi.org/10.1016/S0011-9164(04)00035-9
Cao, X.-L., Zhao, W., & Dabeka, R. (2015). Di-(2-ethylhexyl) adipate and 20 phthalates in composite food samples from the 2013 Canadian Total Diet Study. Food Additives & Contaminants: Part A, 32(11), 1893–1901. https://doi.org/10.1080/19440049.2015.1079742
Chen, F., Li, X., Dong, Y., Li, J., Li, Y., Li, H., et al. (2021). Biodegradation of phthalic acid esters (PAEs) by Cupriavidus oxalaticus strain E3 isolated from sediment and characterization of monoester hydrolases. Chemosphere, 266, 129061. https://doi.org/10.1016/j.chemosphere.2020.129061
Chen, L., Yu, L., Han, B., Li, Y., Zhang, J., Tao, S., & Liu, W. (2024). Pollution characteristics and affecting factors of phthalate esters in agricultural soils in mainland China. Journal of Hazardous Materials, 466, 133625. https://doi.org/10.1016/j.jhazmat.2024.133625
Chen, M., Niu, Z., Zhang, X., & Zhang, Y. (2024). Pollution characteristics and health risk of sixty-five organics in one drinking water system: PAEs should be prioritized for control. Chemosphere, 350, 141171. https://doi.org/10.1016/j.chemosphere.2024.141171
Chen, N., Shuai, W., Hao, X., Zhang, H., Zhou, D., & Gao, J. (2017). Contamination of Phthalate Esters in Vegetable Agriculture and Human Cumulative Risk Assessment. Pedosphere, 27(3), 439–451. https://doi.org/10.1016/S1002-0160(17)60340-0
Cheng, X., Ma, L., Xu, D., Cheng, H., Yang, G., & Luo, M. (2015). Mapping of phthalate esters in suburban surface and deep soils around a metropolis-Beijing, China. Journal of Geochemical Exploration, 155, 56–61. https://doi.org/10.1016/j.gexplo.2015.04.007
Cheng, Z., Chen, J.-R., Zheng, C., Yang, Z.-B., Xu, X.-X., & Wong, M.-H. (2021). Bioaccumulation and health risk assessment of phthalate esters in cultured low trophic level fish feded with food waste-based diets. Chemosphere, 276, 130189. https://doi.org/10.1016/j.chemosphere.2021.130189
Coltro, L., Saron, E. S., Ferreira, I. A. G., Santos, B. B., & Marangoni Júnior, L. (2023). Occurrence of phthalates and 2,6-diisopropylnaphthalene in dry foods packed in cellulosic materials. Journal of Consumer Protection and Food Safety, 18(1), 27–34. https://doi.org/10.1007/s00003-022-01412-x
da Costa, J. M., Kato, L. S., Galvan, D., Lelis, C. A., Saraiva, T., & Conte-Junior, C. A. (2023). Occurrence of phthalates in different food matrices: A systematic review of the main sources of contamination and potential risks. Comprehensive Reviews in Food Science and Food Safety, 22(3), 2043–2080. https://doi.org/10.1111/1541-4337.13140
Dargnat, C., Teil, M.-J., Chevreuil, M., & Blanchard, M. (2009). Phthalate removal throughout wastewater treatment plant: Case study of Marne Aval station (France). Science of The Total Environment, 407(4), 1235–1244. https://doi.org/10.1016/j.scitotenv.2008.10.027
Domínguez-Morueco, N., González-Alonso, S., & Valcárcel, Y. (2014). Phthalate occurrence in rivers and tap water from central Spain. Science of The Total Environment, 500–501, 139–146. https://doi.org/10.1016/j.scitotenv.2014.08.098
Edwards, L., McCray, N. L., VanNoy, B. N., Yau, A., Geller, R. J., Adamkiewicz, G., & Zota, A. R. (2022). Phthalate and novel plasticizer concentrations in food items from U.S. fast food chains: a preliminary analysis. Journal of Exposure Science & Environmental Epidemiology, 32(3), 366–373. https://doi.org/10.1038/s41370-021-00392-8
Erythropel, H. C., Maric, M., Nicell, J. A., Leask, R. L., & Yargeau, V. (2014). Leaching of the plasticizer di(2-ethylhexyl)phthalate (DEHP) from plastic containers and the question of human exposure. Applied Microbiology and Biotechnology, 98(24), 9967–9981. https://doi.org/10.1007/s00253-014-6183-8
Fan, S., Li, C., Guo, J., Johansen, A., Liu, Y., Feng, Y., et al. (2023). Biodegradation of phthalic acid esters (PAEs) by Bacillus sp. LUNF1 and characterization of a novel hydrolase capable of catalyzing PAEs. Environmental Technology & Innovation, 32, 103269. https://doi.org/10.1016/j.eti.2023.103269
Fierens, T., Vanermen, G., Van Holderbeke, M., De Henauw, S., & Sioen, I. (2012). Effect of cooking at home on the levels of eight phthalates in foods. Food and Chemical Toxicology, 50(12), 4428–4435. https://doi.org/10.1016/j.fct.2012.09.004
Gani, K. M., Tyagi, V. K., & Kazmi, A. A. (2017). Occurrence of phthalates in aquatic environment and their removal during wastewater treatment processes: a review. Environmental Science and Pollution Research, 24(21), 17267–17284. https://doi.org/10.1007/s11356-017-9182-3
Gao, D., Li, Z., Wen, Z., & Ren, N. (2014). Occurrence and fate of phthalate esters in full-scale domestic wastewater treatment plants and their impact on receiving waters along the Songhua River in China. Chemosphere, 95, 24–32. https://doi.org/10.1016/j.chemosphere.2013.08.009
Gao, D.-W., & Wen, Z.-D. (2016). Phthalate esters in the environment: A critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. Science of The Total Environment, 541, 986–1001. https://doi.org/10.1016/j.scitotenv.2015.09.148
Gao, M., Zhang, Y., Gong, X., Song, Z., & Guo, Z. (2018). Removal mechanism of di-n-butyl phthalate and oxytetracycline from aqueous solutions by nano-manganese dioxide modified biochar. Environmental Science and Pollution Research, 25(8), 7796–7807. https://doi.org/10.1007/s11356-017-1089-5
Ghosh, S., & Sahu, M. (2022). Phthalate pollution and remediation strategies: A review. Journal of Hazardous Materials Advances, 6, 100065. https://doi.org/10.1016/j.hazadv.2022.100065
Giuliani, A., Zuccarini, M., Cichelli, A., Khan, H., & Reale, M. (2020). Critical Review on the Presence of Phthalates in Food and Evidence of Their Biological Impact. International Journal of Environmental Research and Public Health, 17(16), 5655. https://doi.org/10.3390/ijerph17165655
Gobbato, J., Becchi, A., Bises, C., Siena, F., Lasagni, M., Saliu, F., et al. (2024). Occurrence of phthalic acid esters (PAEs) and active pharmaceutical ingredients (APIs) in key species of anthozoans in Mediterranean Sea. Marine Pollution Bulletin, 200, 116078. https://doi.org/10.1016/j.marpolbul.2024.116078
Godwin, A. (n.d.). Uses of Phthalates and Other Plasticizers, 17.
Gong, J., Yi, X., Liang, J., Liu, Z., & Du, Z. (2024). Inhibitory effects of phthalate esters (PAEs) and phthalate monoesters towards human carboxylesterases (CESs). Toxicology and Applied Pharmacology, 482, 116785. https://doi.org/10.1016/j.taap.2023.116785
Grøn, C., Laturnus, F., Mortensen, G. K., Egsgaard, H., Samsøe-Petersen, L., Ambus, P., & Jensen, E. S. (2000). Plant Uptake of LAS and DEHP from Sludge Amended Soil. In Persistent, Bioaccumulative, and Toxic Chemicals I (Vols. 1-0, Vol. 772, pp. 99–111). American Chemical Society. https://doi.org/10.1021/bk-2001-0772.ch007
Guo, W., Zhang, Z., Zhu, R., Li, Z., Liu, C., Xiao, H., & Xiao, H. (2024). Pollution characteristics, sources, and health risks of phthalate esters in ambient air: A daily continuous monitoring study in the central Chinese city of Nanchang. Chemosphere, 353, 141564. https://doi.org/10.1016/j.chemosphere.2024.141564
He, W., Yang, H., Pu, Q., & Li, Y. (2022). Novel control strategies for the endocrine-disrupting effect of PAEs to pregnant women in traffic system. Science of The Total Environment, 851, 158269. https://doi.org/10.1016/j.scitotenv.2022.158269
Heudorf, U., Mersch-Sundermann, V., & Angerer, J. (2007). Phthalates: Toxicology and exposure. International Journal of Hygiene and Environmental Health, 210(5), 623–634. https://doi.org/10.1016/j.ijheh.2007.07.011
Holland, M. (2018). Socio-economic assessment of phthalates. OECD. https://doi.org/10.1787/a38a0e34-en
Hou, H., Min, Y., Liu, X., Wang, P., Zhou, Z., & Liu, D. (2021). Occurrence and migration of phthalates in adhesive materials to fruits and vegetables. Journal of Hazardous Materials, 418, 126277. https://doi.org/10.1016/j.jhazmat.2021.126277
Huang, M., Zeng, Y., Luo, K., Lan, B., Luo, J., Zeng, L., & Kang, Y. (2023). Inhalation bioacessibility and lung cell penetration of indoor PM2.5-bound PAEs and its implication in risk assessment. Environmental Pollution, 322, 121216. https://doi.org/10.1016/j.envpol.2023.121216
Hui, K., Tang, J., Cui, Y., Xi, B., & Tan, W. (2021). Accumulation of phthalates under high versus low nitrogen addition in a soil-plant system with sludge organic fertilizers instead of chemical fertilizers. Environmental Pollution, 291, 118193. https://doi.org/10.1016/j.envpol.2021.118193
Julinová, M., & Slavík, R. (2012). Removal of phthalates from aqueous solution by different adsorbents: A short review. Journal of Environmental Management, 94(1), 13–24. https://doi.org/10.1016/j.jenvman.2011.09.006
Keyser, P., Pujar, B. G., Eaton, R. W., & Ribbons, D. W. (1976). Biodegradation of the phthalates and their esters by bacteria. Environmental Health Perspectives, 18, 159–166. https://doi.org/10.1289/ehp.7618159
Latini, G., De Felice, C., & Verrotti, A. (2004). Plasticizers, infant nutrition and reproductive health. Reproductive Toxicology, 19(1), 27–33. https://doi.org/10.1016/j.reprotox.2004.05.011
Le, T. M., Nguyen, H. M. N., Nguyen, V. K., Nguyen, A. V., Vu, N. D., Yen, N. T. H., et al. (2021). Profiles of phthalic acid esters (PAEs) in bottled water, tap water, lake water, and wastewater samples collected from Hanoi, Vietnam. Science of The Total Environment, 788, 147831. https://doi.org/10.1016/j.scitotenv.2021.147831
Li, C., Chen, J., Wang, J., Han, P., Luan, Y., Ma, X., & Lu, A. (2016). Phthalate esters in soil, plastic film, and vegetable from greenhouse vegetable production bases in Beijing, China: Concentrations, sources, and risk assessment. Science of The Total Environment, 568, 1037–1043. https://doi.org/10.1016/j.scitotenv.2016.06.077
Li, J., Han, S., Xu, R., Zhang, X., Liang, J., Wang, M., & Han, B. (2024). Insight into the effect of geographic location and intercropping on contamination characteristics and exposure risk of phthalate esters (PAEs) in tea plantation soils. Journal of Integrative Agriculture, S209531192400090X. https://doi.org/10.1016/j.jia.2024.03.018
Li, K., Ma, D., Wu, J., Chai, C., & Shi, Y. (2016). Distribution of phthalate esters in agricultural soil with plastic film mulching in Shandong Peninsula, East China. Chemosphere, 164, 314–321. https://doi.org/10.1016/j.chemosphere.2016.08.068
Li, X., Wang, Q., Jiang, N., Lv, H., Liang, C., Yang, H., et al. (2023). Occurrence, source, ecological risk, and mitigation of phthalates (PAEs) in agricultural soils and the environment: A review. Environmental Research, 220, 115196. https://doi.org/10.1016/j.envres.2022.115196
Liang, D.-W., Zhang, T., Fang, H. H. P., & He, J. (2008). Phthalates biodegradation in the environment. Applied Microbiology and Biotechnology, 80(2), 183–198. https://doi.org/10.1007/s00253-008-1548-5
Liu, B., Lv, L., Ding, L., Gao, L., Li, J., Ma, X., & Yu, Y. (2024). Comparison of phthalate esters (PAEs) in freshwater and marine food webs: Occurrence, bioaccumulation, and trophodynamics. Journal of Hazardous Materials, 466, 133534. https://doi.org/10.1016/j.jhazmat.2024.133534
Liu, H., Liang, H., Liang, Y., Zhang, D., Wang, C., Cai, H., Shvartsev, S., & L. (2010). Distribution of phthalate esters in alluvial sediment: A case study at JiangHan Plain, Central China. Chemosphere, 78(4), 382–388. https://doi.org/10.1016/j.chemosphere.2009.11.009
Müller, J., & Kördel, W. (1993). Occurrence and fate of phthalates in soil and plants. Science of The Total Environment, 134, 431–437. https://doi.org/10.1016/S0048-9697(05)80044-0
Naveen, K. V., Saravanakumar, K., Zhang, X., Sathiyaseelan, A., & Wang, M.-H. (2022). Impact of environmental phthalate on human health and their bioremediation strategies using fungal cell factory- A review. Environmental Research, 214, 113781. https://doi.org/10.1016/j.envres.2022.113781
Ou, Y., Zhou, J. L., Jia, Y., Liang, M., Hu, H., & Ren, L. (2023). Complete genome of Mycolicibacterium phocaicum RL-HY01, a PAEs-degrading marine bacterial strain isolated from Zhanjiang Bay, China. Marine Genomics, 69, 101019. https://doi.org/10.1016/j.margen.2023.101019
Poopal, R.-K., Zhang, J., Zhao, R., Ramesh, M., & Ren, Z. (2020). Biochemical and behavior effects induced by diheptyl phthalate (DHpP) and Diisodecyl phthalate (DIDP) exposed to zebrafish. Chemosphere, 252, 126498. https://doi.org/10.1016/j.chemosphere.2020.126498
Przybylińska, P. A., & Wyszkowski, M. (2016). Environmental contamination with phthalates and its impact on living organisms. Ecological Chemistry and Engineering S, 23(2), 347–356. https://doi.org/10.1515/eces-2016-0024
Puri, M., Gandhi, K., & Kumar, M. S. (2023). The occurrence, fate, toxicity, and biodegradation of phthalate esters: An overview. Water Environment Research, 95(1), e10832. https://doi.org/10.1002/wer.10832
Ren, L., Weng, L., Chen, D., Hu, H., Jia, Y., & Zhou, J. L. (2023). Bioremediation of PAEs-contaminated saline soil: The application of a marine bacterial strain isolated from mangrove sediment. Marine Pollution Bulletin, 192, 115071. https://doi.org/10.1016/j.marpolbul.2023.115071
Rudel, R. A., & Perovich, L. J. (2009). Endocrine disrupting chemicals in indoor and outdoor air. Atmospheric Environment, 43(1), 170–181. https://doi.org/10.1016/j.atmosenv.2008.09.025
Sablayrolles, C., Montréjaud-Vignoles, M., Benanou, D., Patria, L., & Treilhou, M. (2005). Development and validation of methods for the trace determination of phthalates in sludge and vegetables. Journal of Chromatography A, 1072(2), 233–242. https://doi.org/10.1016/j.chroma.2005.02.074
Saeidnia, S., & Abdollahi, M. (2013). Are medicinal plants polluted with phthalates? DARU Journal of Pharmaceutical Sciences, 21(1), 43. https://doi.org/10.1186/2008-2231-21-43
Sathyanarayana, S. (2008). Phthalates and Children’s Health. Current Problems in Pediatric and Adolescent Health Care, 38(2), 34–49. https://doi.org/10.1016/j.cppeds.2007.11.001
Sharma, R., & Kaur, R. (2020). Physiological and metabolic alterations induced by phthalates in plants: possible mechanisms of their uptake and degradation. Environmental Sustainability, 3(4), 391–404. https://doi.org/10.1007/s42398-020-00141-x
Shukur, S. A., Hassan, F. M., & Fakhry, S. S. (2024). Unveiling the Nexus the link between water quality index and phthalic acid ester concentrations in Tigris River. Emerging Contaminants, 10(1), 100279. https://doi.org/10.1016/j.emcon.2023.100279
Staples, C. A. (2003). Series Anthropogenic Compounds: Phtalate Esters (Vol. 3Q). Springer Berlin Heidelberg. https://doi.org/10.1007/b11472
Sun, Q., Zhang, X., Liu, C., Nier, A., Ying, S., Zhang, J., et al. (2023). The content of PAEs in field soils caused by the residual film has a periodical peak. Science of The Total Environment, 864, 161078. https://doi.org/10.1016/j.scitotenv.2022.161078
Sun, S., Zhang, B., Hu, J., Gu, W., Wang, Z., Fan, D., et al. (2025). Accumulation characteristics and fate modeling of phthalic acid esters in surface water from the Three Gorges Reservoir area, China. Journal of Environmental Sciences, 149, 46–56. https://doi.org/10.1016/j.jes.2024.01.027
Tran, B. C., Teil, M.-J., Blanchard, M., Alliot, F., & Chevreuil, M. (2015). Fate of phthalates and BPA in agricultural and non-agricultural soils of the Paris area (France). Environmental Science and Pollution Research, 22(14), 11118–11126. https://doi.org/10.1007/s11356-015-4178-3
Tran, H. T., Lin, C., Bui, X.-T., Ky Nguyen, M., Cao, N. D. T., Mukhtar, H., et al. (2022). Phthalates in the environment: characteristics, fate and transport, and advanced wastewater treatment technologies. Bioresource Technology, 344, 126249. https://doi.org/10.1016/j.biortech.2021.126249
Tran, H.-T., Nguyen, M.-K., Hoang, H.-G., Hutchison, J. M., & Vu, C. T. (2022). Composting and green technologies for remediation of phthalate (PAE)-contaminated soil: Current status and future perspectives. Chemosphere, 307, 135989. https://doi.org/10.1016/j.chemosphere.2022.135989
Vasseghian, Y., Alimohamadi, M., Dragoi, E.-N., & Sonne, C. (2023). A global meta-analysis of phthalate esters in drinking water sources and associated health risks. Science of The Total Environment, 903, 166846. https://doi.org/10.1016/j.scitotenv.2023.166846
Ventrice, P., Ventrice, D., Russo, E., & De Sarro, G. (2013). Phthalates: European regulation, chemistry, pharmacokinetic and related toxicity. Environmental Toxicology and Pharmacology, 36(1), 88–96. https://doi.org/10.1016/j.etap.2013.03.014
Wang, H., Li, C., Yan, G., Zhang, Y., Wang, H., Dong, W., et al. (2023). Seasonal distribution characteristics and ecological risk assessment of phthalate esters in surface sediment of Songhua River basin. Environmental Pollution, 337, 122567. https://doi.org/10.1016/j.envpol.2023.122567
Wang, J., Bo, L., Li, L., Wang, D., Chen, G., Christie, P., & Teng, Y. (2014). Occurrence of phthalate esters in river sediments in areas with different land use patterns. Science of The Total Environment, 500–501, 113–119. https://doi.org/10.1016/j.scitotenv.2014.08.092
Wang, J., Chen, G., Christie, P., Zhang, M., Luo, Y., & Teng, Y. (2015). Occurrence and risk assessment of phthalate esters (PAEs) in vegetables and soils of suburban plastic film greenhouses. Science of The Total Environment, 523, 129–137. https://doi.org/10.1016/j.scitotenv.2015.02.101
Wang, J., Luo, Y., Teng, Y., Ma, W., Christie, P., & Li, Z. (2013). Soil contamination by phthalate esters in Chinese intensive vegetable production systems with different modes of use of plastic film. Environmental Pollution, 180, 265–273. https://doi.org/10.1016/j.envpol.2013.05.036
Wang, J., Zhang, M.-Y., Chen, T., Zhu, Y., Teng, Y., Luo, Y.-M., & Christie, P. (2015). Isolation and Identification of a Di-(2-Ethylhexyl) Phthalate-Degrading Bacterium and Its Role in the Bioremediation of a Contaminated Soil. Pedosphere, 25(2), 202–211. https://doi.org/10.1016/S1002-0160(15)60005-4
Wang, L., Liu, M., Tao, W., Zhang, W., Wang, L., Shi, X., et al. (2018). Pollution characteristics and health risk assessment of phthalate esters in urban soil in the typical semi-arid city of Xi’an, Northwest China. Chemosphere, 191, 467–476. https://doi.org/10.1016/j.chemosphere.2017.10.066
Wang, L., Liu, Y., Zhang, Y., Chen, S., Zhang, N., Wang, Z., & Liu, H. (2023). Estimation and potential ecological risk assessment of multiphase PAEs in mangrove wetlands in Dongzhai Harbor, Hainan. Science of The Total Environment, 870, 161835. https://doi.org/10.1016/j.scitotenv.2023.161835
Wang, S. Y., Wang, M. Q., Yang, E. Q., Chen, X. M., & Pan, F. G. (2022). Review on Occurrence, Sources of Contamination, and Mitigation Strategies of Phthalates in Vegetable Oils. European Journal of Lipid Science and Technology, 124(5), 2100086. https://doi.org/10.1002/ejlt.202100086
Wang, X., Zhang, Y., Huang, B., Chen, Z., Zhong, M., Wang, W., et al. (2021). Atmospheric phthalate pollution in plastic agricultural greenhouses in Shaanxi Province. China. Environmental Pollution, 269, 116096. https://doi.org/10.1016/j.envpol.2020.116096
Wang, Z., Ma, J., Wang, T., Qin, C., Hu, X., Mosa, A., & Ling, W. (2023). Environmental health risks induced by interaction between phthalic acid esters (PAEs) and biological macromolecules: A review. Chemosphere, 328, 138578. https://doi.org/10.1016/j.chemosphere.2023.138578
Wei, L., Li, Z., Sun, J., & Zhu, L. (2020). Pollution characteristics and health risk assessment of phthalate esters in agricultural soil and vegetables in the Yangtze River Delta of China. Science of The Total Environment, 726, 137978. https://doi.org/10.1016/j.scitotenv.2020.137978
Wei, X., Shi, Y., Fei, Y., Chen, J., Lv, B., Chen, Y., et al. (2016). Removal of trace phthalate esters from water by thin-film composite nanofiltration hollow fiber membranes. Chemical Engineering Journal, 292, 382–388. https://doi.org/10.1016/j.cej.2016.02.037
Wofford, H. W., Wilsey, C. D., Neff, G. S., Giam, C. S., & Neff, J. M. (1981). Bioaccumulation and metabolism of phthalate esters by oysters, brown shrimp, and sheepshead minnows. Ecotoxicology and Environmental Safety, 5(2), 202–210. https://doi.org/10.1016/0147-6513(81)90035-X
Wu, J., Ma, T., Zhou, Z., Yu, N., He, Z., Li, B., et al. (2019). Occurrence and fate of phthalate esters in wastewater treatment plants in Qingdao, China. Human and Ecological Risk Assessment: An International Journal, 25(6), 1547–1563. https://doi.org/10.1080/10807039.2018.1471341
Xiong, Y.-H., & Pei, D.-S. (2021). A review on efficient removal of phthalic acid esters via biochars and transition metals-activated persulfate systems. Chemosphere, 277, 130256. https://doi.org/10.1016/j.chemosphere.2021.130256
Yang, G. C. C., Huang, S.-C., Jen, Y.-S., & Tsai, P.-S. (2016). Remediation of phthalates in river sediment by integrated enhanced bioremediation and electrokinetic process. Chemosphere, 150, 576–585. https://doi.org/10.1016/j.chemosphere.2015.12.044
Yang, Y., Zhao, Z., Chang, Y., Wang, H., Wang, H., Dong, W., & Yan, G. (2023). PAHs and PAEs in the surface sediments from Nenjiang River and the Second Songhua River, China: Distribution, composition and risk assessment. Process Safety and Environmental Protection, 178, 765–775. https://doi.org/10.1016/j.psep.2023.08.037
Zeng, L.-J., Huang, Y.-H., Chen, X.-T., Chen, X.-H., Mo, C.-H., Feng, Y.-X., et al. (2020). Prevalent phthalates in air-soil-vegetable systems of plastic greenhouses in a subtropical city and health risk assessments. Science of The Total Environment, 743, 140755. https://doi.org/10.1016/j.scitotenv.2020.140755
Zhang, Q.-Q., Ma, Z.-R., Cai, Y.-Y., Li, H.-R., & Ying, G.-G. (2021). Agricultural Plastic Pollution in China: Generation of Plastic Debris and Emission of Phthalic Acid Esters from Agricultural Films. Environmental Science & Technology, 55(18), 12459–12470. https://doi.org/10.1021/acs.est.1c04369
Zhang, T., Ma, B., & Wang, L. (2023). Phthalic acid esters in grains, vegetables, and fruits: concentration, distribution, composition, bio-accessibility, and dietary exposure. Environmental Science and Pollution Research, 30(2), 2787–2799. https://doi.org/10.1007/s11356-022-22415-z
Zhang, Y., Jiao, Y., Li, Z., Tao, Y., & Yang, Y. (2021). Hazards of phthalates (PAEs) exposure: A review of aquatic animal toxicology studies. Science of The Total Environment, 771, 145418. https://doi.org/10.1016/j.scitotenv.2021.145418
Zhang, Y., Yang, Y., Tao, Y., Guo, X., Cui, Y., & Li, Z. (2023). Phthalates (PAEs) and reproductive toxicity: Hypothalamic-pituitary-gonadal (HPG) axis aspects. Journal of Hazardous Materials, 459, 132182. https://doi.org/10.1016/j.jhazmat.2023.132182
Zhao, F., Ma, Z., Ping, H., He, Z., Li, B., Gao, Y., & Li, C. (2022). Tissue distribution of phthalates in celery under different cultivation patterns and associated dietary exposure. Environmental Pollution, 292, 118391. https://doi.org/10.1016/j.envpol.2021.118391
Zhao, H.-M., Du, H., Xiang, L., Chen, Y.-L., Lu, L.-A., Li, Y.-W., et al. (2015). Variations in phthalate ester (PAE) accumulation and their formation mechanism in Chinese flowering cabbage (Brassica parachinensis L.) cultivars grown on PAE-contaminated soils. Environmental Pollution, 206, 95–103. https://doi.org/10.1016/j.envpol.2015.06.008
Zhao, J., Ji, Y., Zhu, Z., Zhang, W., Zhang, L., & Zhao, J. (2018). PAEs occurrence and sources in road dust and soil in/around parks in May in Tianjin, China. Ecotoxicology and Environmental Safety, 147, 238–244. https://doi.org/10.1016/j.ecoenv.2017.08.014
Zheng, X., Zhang, B.-T., & Teng, Y. (2014). Distribution of phthalate acid esters in lakes of Beijing and its relationship with anthropogenic activities. Science of The Total Environment, 476–477, 107–113. https://doi.org/10.1016/j.scitotenv.2013.12.111
Zhou, B., Zhao, L., Sun, Y., Li, X., Weng, L., & Li, Y. (2021). Contamination and human health risks of phthalate esters in vegetable and crop soils from the Huang-Huai-Hai region of China. Science of The Total Environment, 778, 146281. https://doi.org/10.1016/j.scitotenv.2021.146281
Acknowledgment
This study was supported by grant No. 2021/40/Q/NZ8/00006 from the National Science Centre, Poland.
Author information
Authors and Affiliations
Contributions
A. Sokołowski: Visualization, Writing – original draft; M. Kończak – Visualization, Reviewing
P. Oleszczuk: Reviewing, Supervision; Y. Gao: Reviewing, Supervision; B. Czech: Investigation, Methodology, Visualization, Writing- Reviewing and Editing Conceptualization, Funding acquisition; Supervision; All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sokołowski, A., Kończak, M., Oleszczuk, P. et al. Environmental and Food Contamination by Phthalic Acid Esters (PAEs): Overview. Water Air Soil Pollut 235, 313 (2024). https://doi.org/10.1007/s11270-024-07121-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07121-5