Abstract
This exploration focuses on the removal of chromium from actual tannery wastewater, collected from the HARBY TANNERY factory in Rubiki (Badr City), using an economical sorbent made from activated carbon derived from rice straw (CRS). The CRS sorbent is activated using H3PO4. The experiment aims to assess the impact of various parameters, including chromium initial concentration, sorbent dosage, treatment time, agitation velocity (rpm), sorbent particle size, and solution pH, on chromium removal from tannery wastewater. Structural, morphological, and electronic distinctive of raw and treated CRS, as well as carbonized CRS, were analyzed using FTIR, SEM, and TEM techniques. XRF analysis was conducted to investigate the chemical elemental composition of carbonized CRS before and after sorption. Zeta potential measurement was performed to assess the electrical charges of particles present in a suspension. The adsorption data was tested for both Langmuir and Freundlich adsorption isotherms, and most of the factors suggested that it follows the Langmuir adsorption isotherm with an R2 value of 99.67%. Additionally, adsorption kinetics were performed to identify the reaction order, which exhibited that sorption pursued pseudo-second-order kinetics with a rate constant (k) of 0.0658 g/mg g/min, a high correlation factor (R2) of 99.76%, and an estimated equilibrium chromium ion adsorption capacity (qe) of 1.597 mg/g, which closely matched the experimental data (1.4835 mg/g). The improved surface morphology and increased surface area of CRS resulted in approximately 98.9% chromium removal. Mechanism studies confirmed that intraparticle diffusion is not the sole rate-controlling step, and Boyd’s model demonstrated that film diffusion limited the rate of chromium adsorption. The desorption of chromium from the carbonized rice straw surface could be achieved by up to 96.4% of the sorbed amount by raising the solution pH to 10, indicating the potential reusability of carbonized rice straw for additional adsorption cycles. Finally, a statistical regression analysis and least square multivariate analysis were used to establish a correlation for predicting efficiency, yielding an R2 value of 97.54%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The discharge of industrial wastewater is a serious socio-environmental issue that leads to water contamination due to the presence of certain heavy metal ions. These metal ions need to be removed urgently owing to their toxicity, non-biodegradability, and persistent nature. Chromium, one of the principal contaminants in the environment, is commonly found in wastewater from various industrial units, and it can exist in two forms: Cr (III) and Cr (VI). While Cr(III) is not considered toxic, Cr(VI) is a highly toxic, carcinogenic, and mutagenic substance that can cause cancer of the lung, stomach, liver, and renal dysfunction (Kerur et al., 2022). The tanning process is one of the leading causes of chromium pollution globally. Most tanneries use chrome for their tanning process owing to its quick processing time, low cost, ability to produce light leather color, and increased leather stabilization (Namasivayam & Kadirvelu, 1994). During the tanning process, approximately 60 to 80% of the chromium used is utilized, while the remaining amount is typically released into the sewer water, causing harmful effects on the environment. The maximum allowable level of trivalent chromium in wastewater is 5 mg/L, while hexavalent chromium has a limit of 0.05 mg/L (Acar & Malkoc, 2004). In addition to these restrictions, it is essential for industries to treat their discharges by reducing chromium to acceptable concentrations in order to prohibit the negative impact of Cr(VI) on public health and the environment. Several physical and chemical treatment systems have been studied for their potential to lower the Cr concentration in tannery effluents to acceptable levels, given the properties of this effluent (Aboulhassan et al., 2008)(Kara & Demirbel, 2012). Physical and chemical techniques for treating tannery effluents include adsorption, chemical precipitation, coagulation/flocculation, ultrafiltration, reverse osmosis, membrane filtration, and electrodialysis, among others. Among these methods, adsorption is the most effective and cost-efficient technology owing to its relatively low cost, simplicity, and easy use in treatment systems, as well as its high disposal capacity and low sludge production. However, the major drawback of the sorption technique is the high cost of sorbents, which increases the cost of the treatment process. So, it is essential to improve sorbents with high competence, excellent recycling ability, and inexpensive (Machado & Mulky, 2023)(Ewis et al., 2022). In the sorption process, chromium ions are eliminated from the solution by direct precipitation onto the surface of an especially selected sorbent material (Rajczykowski & Loska, 2018). The process of sorption is governed by surface affinity, and important parameters for adsorption include chemical reactivity, pH, surface area available for sorption per unit volume, and reduction in surface tension (Babel & Kurniawan, 2003).
In recent years, agricultural by-products have received considerable attention as sorbents for demineralization from water. These by-products include nut wastes, waste wool, modified cotton, tree bark, sawdust, coconut coir and shell, rice husk, wheat straw, and sugar cane bagasse. Agricultural wastes contain hemicellulose, lignin, lipids, proteins, simple sugars, water, hydrocarbons, and starch, as well as functional groups that can aid in the sorption process. Agricultural wastes can be used in their natural state, although pre-treatment may be necessary to enhance their sorption capacity. The use of agricultural waste such as rice straw has been identified as a promising alternative, especially in countries where agriculture is a major economic activity and rice straw is readily available (Diyanati et al., 2013). It is inexpensive and exhibits strong sorption abilities while properly processed (Chuah et al., 2005)(Bansal et al., 2009).
The major purpose of this study is to appreciate the effectiveness of carbonized rice straw waste as a sorbent for eliminating chromium from genuine tannery wastewater obtained from the leather tanning industry at the HARBY TANNERY factory in Rubiki (Badr City). Rice straw is employed as an activated carbon to eliminate chromium due to its enlarged surface area, fine pore structures, high sorption capacity, and high surface reactivity. The study examines the impact of various parameters, including the initial concentration of chromium, the amount of sorbent, treatment time, agitation velocity (rpm), sorbent particle size, and solution pH, on the removal of chromium from tannery wastewater. Additionally, the study investigates the reusability of carbonized rice straw for four adsorption-desorption cycles.
2 Test Appliance
The processing equipment employed in this study consists of a 500-mL cylindrical glass vessel with an inner diameter of 10.5 cm. A paddle impeller with an outer diameter of 4 cm and made of 316 S.S. was installed in the center of the container, 4 cm above the base. Epoxy resin was applied to the shaft and impeller to prevent them from contacting the treated solution and interfering with the adsorption process. A thermometer was placed inside the vessel to monitor and control the temperature of the treated solution. The vessel and its contents were immersed in a rectangular water bath with manual temperature adjustment, which allowed for adjusting the temperature of the treated solution. A vertical water condenser was attached to the top of the container to condense any water vapor released and maintain the water content of the treated solution.
3 Reagents
The reagents exercised in this experimental work were of analytical grade and were acquired from El-Gomhouria Company for Trading Chemicals and Medical Appliances in Egypt. The materials utilized in this study include phosphoric acid (H3PO4, 70% V/V), hydrochloric acid (HCl), and sodium hydroxide (NaOH).
Agricultural waste material, such as rice straw, was utilized as the sorbent in this study to evaluate the adsorption competence as a function of treatment time, initial ion concentration, sorbent quantity, sorbent particle size, solution pH, and mixing speed (rpm). Rice straw was compiled from an agricultural field in Port Said, Egypt, and its chemical composition, including 35% ash content, 55.5% cellulose, 6.48% lignin, and 6% hemicellulose, was determined following the TAPPI T257 om-85, TAPPI T222 om-88, and TAPPI om-85 standard methods. Additionally, the moisture content of the rice straw was found to be 7.34% (Amanuel, 2020).
4 Collection of Tannery Wastewater
Tannery wastewater was gathered from the leather tanning industry at the HARBY TANNERY factory in Rubiki (Badr City) and filtered through a Whatman No. 45 filter paper. The initial characteristics of tannery industry sewer water are presented in Table 1.
5 Experimental Procedure
5.1 A Sorbent Preparation
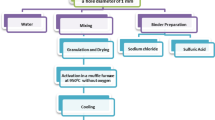
The rice straw sample was initially washed 3 to 4 times with distilled water to eliminate dirt particles and color materials. Next, the washed rice straw was dehydrated in an electric oven at 105 °C for 24 h to remove any residual moisture. The dried rice straw was then divided into small pieces.
To enhance the proportion of active surfaces and remove soluble components such as resins, tannins, coloring agents, and reducing sugars, a (70% V/V) H3PO4 solution in a W/V ratio of 2:1 was utilized in an adjustment process. In this treatment, the dried rice straw was immersed in the H3PO4 solution for 3 h at 100–130 °C and stirred on an electrical stirrer at 240 rpm.
The next step was an activation process, in which the rice straw was placed in pre-weighed glass crucibles and carbonized in an oven away from the air at a temperature of 450 ± 20 °C for 2 h. After treatment, the resulting activated carbons were allowed to cool to room temperature before use. They were then separated, crushed, and ground to a size of 200–500 μm using a cracking device and sieved using a mechanical screening apparatus. The samples were rinsed with ionized water until neutral (pH: 6–7) and then dehydrated at 105 °C in an oven until a constant weight was achieved. The resulting carbonized rice straw was used as an activated carbon for characterization experiments and tested for chromium adsorption capacity, as described in the study (Farahmand, 2016).
The moisture content of the carbonized rice straw was specified by measuring the loss of moisture after drying in an oven at 110 °C for 1 h. To do this, 1 g of carbonized rice straw powder was placed in a crucible that had previously been weighed (12.47 gm). The crucible was then placed in an electric hot air oven at approximately 110 °C. After drying, the crucible was removed from the oven and allowed to cool in a desiccator before being weighed again. The moisture content of the sample was estimated using the following equation:
where: W1 is the weight of the empty crucible, W2 is the weight of carbonic rice straw, and W3 is the weight of residue after drying at 110 °C.
The bulk density of the carbonized rice straw powder was specified by packing it into a measuring cylinder (50 mL) and measuring the amount taken up by the cylinder. The bulk density was calculated using the formula:
where: M is the amount (in grams) of carbonized rice straw placed in the cylinder, and V is the volume of the cylinder occupied by the carbonized rice straw powder.
Furthermore, the functional groups, surface morphology, and electronic structures of the sorbent were analyzed using Fourier transform infrared (FTIR) spectroscopy, scanning electron microscopy (SEM), and transmission electron microscope (TEM) (F200i TEM, catalogue No. TALOSF 200I, a 20-200 kV), respectively. The elemental composition of carbonic rice straw before and after sorption was also analyzed by X-ray fluorescence (XRF) (ARL™ OPTIM'X WDXRF Spectrometer, catalogue No. IQLAAHGABMFAASMACH) analysis. Zeta potential analysis was conducted to evaluate the electrical charges of particles within a suspension. The SZ-100 nanoparticle size and Zeta potential analyzer were utilized to determine the zeta potential of the sorbent.
5.2 Batch Adsorption Studies
In this study, the tannery sewer water was stirred in a batch process using a series of beakers with stirrers. The batch technique was chosen for its simplicity and ease of use. Adsorbate solutions with various concentrations were prepared by diluting the tannery sewer water sample containing 3300 mg/L of chromium ions. A fixed volume of the sample effluent (250 mL) was used to evaluate the impact of different variables on the removal of chromium, including the initial concentration, quantity of sorbent, treatment time, agitation velocity, particle size, and pH. As indicated by Sharma & Ayub (2019), the maximum sorption capacity was observed between 25 and 30 °C, as desorption began to occur at higher temperatures. Therefore, all experimental runs were conducted at room temperature to evaluate the adsorption capacities of rice straw for removing chromium from tannery wastewater. The pH of the adsorptive solution was adjusted using NaOH/HCl solution, and the pH of the waste solution was measured using a digitally calibrated pH-meter (model 3505 made by JENWAY Instruments, with a resolution of 0.01 and accuracy of ± 0.02).
After the specified time elapsed, the suspension was filtered, and the remaining metal ion concentration in the aqueous phase was determined from the calibration curve prepared by measuring the absorbance at the maximum wavelength of 540 nm using a UV spectrometer. Figure 1 depicts a schematic diagram of the sorption process of chromium from authentic tannery effluents onto carbonized rice straw. The percentage of chromium removal was calculated by means of the following equation:
where, C0 and Ce are the initial and equilibrium chromium concentrations in solution, individually (mg/L). The adsorption capacity qe (mg/g) was calculated by means of the bellow equation:
where: V and W are the volume of solution (L) and the weight of a sorbent (g), individually.
6 Results and Discussion
The graphs displayed below demonstrate how the results are exhibited. The physicochemical properties, such as moisture content and bulk density, of carbonized rice straw activated with H3PO4 were specified. The structural, morphological, and electronic distinctive of raw rice straw, H3PO4-treated rice straw, and carbonized rice straw were analyzed using FTIR, SEM, EDX, XRF, zeta potential, and TEM techniques. In a batch adsorption system, various factors were optimized.
6.1 A Sorbent Characterization
The high surface area, porous character and natural chemical surface of carbonic rice straw activated by H3PO4, make it have an enhanced ability to get rid of heavy metals (Temesgen & Gebrie, 2022). A rough analysis of carbonic rice straw powder with a diameter less than 500 μm was executed to comprehend the physicochemical characteristics, including moisture content (7.2%) and bulk density (0.704 g/mL).
Carbonized rice straw treated with H3PO4 can achieve a maximum chromium removal efficiency of 99.5% due to its favorable cloth characteristic values. Singh and Singh (2012) (Singh & Singh, 2012) acquired utmost chromium removal (94%) using activated rice husk treated with H3PO4 (40%), which had a moisture content of (13.82%) and a bulk density of (0.68 g/mL). Both activated rice husk and carbonized rice straw share similarities in terms of bulk density and activating agent. However, the lower moisture content of carbonized rice straw, compared to activated rice husk, may have contributed to its superior chromium removal efficiency. This could be owing to the pores in the interspaces of activated rice husk being filled with water, which interfered with the evolution of the pore structure and prevented the diffusion of chromium ions onto the adsorbent sites (Devi et al., 2012). A lower bulk density value denotes a highly porous structure with a larger void space. The American Water Works Association has specified a lower limit of 0.25 g/mL for the practical utilization of activated sorbents. Hence, the bulk density of the carbonized rice straw powder used in this experiment fulfills this criterion.
6.2 Scanning Electron Microscope Image (SEM)
Scanning electron microscope image (SEM), a layer of raw straw, treated rice straw with H3PO4, carbonic rice straw before the sorption, and carbonic rice straw after the sorption, is revealed in Fig. 2. The outcomes verify that the carbonic rice straw is a suitable choice for chromium elimination from tannery sewer water owing to its high carbon content, porosity, and surface area as elucidated in Fig. 2C. SEM image reveals that the pores serve as channels to the microporous network. As shown in the figure, carbonic rice straw possesses a rough texture with a heterogeneous surface and a range of randomly distributed pore sizes. The surface topography of carbonic rice straw before and after the sorption was noticed to be dissimilar in SEM images. Figure 2C depicts how chromium adsorption has coated active sites of the sorbent. After the sorption of chromium, a film forms on the sorbent surface, and some chromium has also engaged the pores (Aragaw et al., 2022), as depicted in the SEM image. After the sorption of chromium, the sorbent surface led to a metallic particle cumulation, which was identified as chromium through the EDx spectra as exposed in Fig. 2D. The morphology of adsorbed chromium on carbonic rice straw surface was corroborated by a uniform dispersal of chromium on the surface. The surface morphology of the formed chromium is irregularly shaped.
6.3 Transmission Electron Microscope (TEM)
TEM analysis was conducted to examine the structure of the sorbent utilized (Mohamed et al., 2017). Treated rice straw with H3PO4, Carbonic rice straw with H3PO4 before and after a sorption were characterized by a transmission electron microscope (TEM) to observe the internal microporous network as elucidated in Fig. 3. Figure 3a depicts that the microstructure of rice straw underwent some modification in surface structure after pre-treatment. The surface of untreated rice straw was regular and compact (Jisha et al., 2022)(Ahmed et al., 2023)(Zhang et al., 2022), whereas after pre-treatment with H3PO4, it became more porous with a looser, more agglomerated structure, and developed hollows, cracks, and tiny particulates, resulting in smaller particle size distribution. Although the pre-treated rice straw surface became flat, rough, and fluffy, the pre-treatment did not significantly damage the raw material (Huang et al., 2022)(Li et al., 2022)(Zhang et al., 2021). The carbonization of the pre-treated rice straw led to an improved pore structure with irregular cavities and holes, as shown in Fig. 3b. This porous structure can provide channels for the adsorption of electrolyte ions, creating an effective electric double layer and improving the accumulation of charge. Additionally, this structure can facilitate the rapid transfer and diffusion of ions, as demonstrated in Fig. 3c. During the carbonization process, chromium quickly migrated and was adsorbed into the inner pores of the sorbent due to the well-developed hole structure (Li et al., 2022)(Jorn-am et al., 2021).
XRF analysis was conducted to determine the elemental composition of carbonized rice straw before and after the sorption of chromium. This approach yielded reliable and accurate data regarding the adsorbed elements. As elucidated in Table 2, the results revealed the presence of trace metals like Cl, Ca, Na, Mn, Mg, K, S, and P with their respective percentage compositions. These findings confirmed the effectiveness of the adsorption process.
6.4 IR Spectra—Fourier Transforms Infrared (FTIR)
Figure 4 exhibits the functional groups, their formed bonds, and their range for raw straw, treated rice straw and carbonic rice straw analyzed by means of IR spectra—Fourier transforms infrared.
The IR spectra of raw rice straw (R1) depict the board band characteristic of the O–H extended from holocellulose (cellulose and hemicellulose) at 3300 cm−1 and C–O–C absorption band at 1035 cm−1 as exhibited in Table 3. The adsorption bands of 2918 cm−1 and 2849 cm−1 are pointed to the C–H extension. The characteristic peak at 795 cm−1 is the glycosidic β (1–4) linkage of cellulose. The characteristic peak of C=O of the acetyl group at 1736 cm−1 is owing to hemicellulose/lignin. The bond ascribed to 1646 cm−1 is that of the aromatic skeleton stretching. Lastly, the band at 1370 cm−1 is owing to the phenolic compound, phenol, syringol, and guaiacol (Bhattacharyya et al., 2020)(Srivastava et al., 2023)(Pang et al., 2022) as exhibited in Table 3. The high-intensity percentage of functional groups pointed out that the rice straw has a high content of cellulose, followed by hemicellulose, and lignin (Bhattacharyya et al., 2020).
Treating rice straw (R2) with 70% H3P04 at 100 −130 °C lowers the intensity of the O–H chemical bond as a result of heating during the treatment processes (dehydration and depolymerization processes) (Taha et al., 2014)(Neme et al., 2022)(Abaide et al., 2019)(Goswami et al., 2022)(Hsu et al., 2010)(Huang et al., 2022) and raises the intensity of the C–O–C, C=O, and glycosidic bond as exposed in Fig. 4 and Table 3. The small peak at 663 cm−1 is caused by bending vibrations of O–P–O (Jastrzębski et al., 2011).
Meanwhile, IR spectra of carbonized rice straw (R3) exhibit the loss of intensity of C–H aliphatic, C–O–C, C=O, and glycosidic bond at 2849, 1032, 1736, and 795 cm−1, respectively. As a result of the increase of aromatization at 450 °C during the carbonization process, the two main characteristic bands were obtained for carbonized rice straw, the first peak was at1557 cm−1 of C=C and the second peak was at 2119 cm−1 of aromatic ring. There is a small peak at 3743 cm−1 of O–H at low intensity due to heating, carbonization, and activation processes (Rahman & Chin, 2019)(Kumar et al., 2021)(Motlagh et al., 2021)(Griffin et al., 2022). The silicate composition was verified by small peaks at 566 cm−1 (Rahman & Chin, 2019)(Sakhiya et al., 2021). The prepared carbonized rice straw exhibited a good surface since it is composed of numerous functional groups accountable for the sorption characteristics for example amines, alcohols, and so on.
6.5 Zeta Potential (ZP)
Zeta potential (ZP) represents the surface potential associated with surface charge. The ZP values of raw rice straw (R1), rice straw treated with H3PO4 (R2), and carbonized rice straw (R3) were determined by placing 2 g of each sample in separate conical flasks and adding 100 mL of deionized water. As depicted in Fig. 5a, the measured zeta potentials of R1, R2, and R3 were −9 mV, −12.4 mV, and −50.8 mV, respectively, indicating their negatively charged nature. Raw rice straw (R1), rice straw treated with H3PO4 (R2), and carbonized rice straw (R3) exhibit a negative zeta potential, indicating their potential for effectively adsorbing heavy metals (Lukman, 2022). The treatment processes involving phosphoric acid and subsequent carbonization of rice straw increased the negative charge on the straw molecules, leading to enhanced attraction with positive chromium ions (Cr+2) produced in the solution. The increase in zeta potential from −9 to −50.8 mV in carbonized rice straw (CRS) resulted in improved suspension stability. Furthermore, the enhancements in surface area and porosity of CRS indicated an increased capacity for the adsorption of wastewater pollutants (Hossain et al., 2020. Figure 5b demonstrates similar trends in adsorption efficiency profiles with respect to zeta potential values.
7 Influence of Operating Factors
The chromium disarmament from sewer water by means of an adsorption technique applying carbonic rice straw was inspected based on numerous factors. The sorption technique was enhanced in a batch trial to find the best conditions namely an initial chromium concentration (30–330 mg/L), sorbent quantity (1–7 g), solution pH (3–10), sorbent particle size (200–500 μm), treatment time (20–100 min), and agitation speed (100–500 rpm).
7.1 Influence of pH on Chromium Disarmament Efficacy
The sorption of chromium ions onto CRS is shown in Fig. 6 to be affected by pH (C0 = 30 mg/L, 5 g of adsorbent, 200 μm particle size, 80min, and 200 rpm). The greatest removal was attained at pH = 3.0. The chromium ions elimination was 98.9% at pH = 3, but this rate reduced to 81.44% at pH = 7 and reduced to 50.23% at pH = 10. The figure clearly shows that the ideal solution pH is 3. The increased adsorption at a small pH can be clarified by means of the hypothesis that the high concentration of hydrogen ions in solution at a small pH can equalize the negatively charged surface of CRS or change the neutral groups into positively charged groups. In light of this, the elevating grade of chromium sorption is a consequence of the strong electrostatic attraction between the sorbent material and adsorbate (Kabir et al., 2019)(Adhikari et al., 2017). The sorption influence is high at low pH since prevalent chromium types happen mainly in monovalent HCrO4− formula, which is subsequently progressively converted to divalent CrO42− and Cr2O72− as pH rises. The free energy of HCrO4− sorption is smaller than that of CrO42− and Cr2O72−, and accordingly, HCrO4− is more readily sorbed than CrO42− and Cr2O72− at identical concentration. With rising pH, the surface of composite materials turns out to be increasingly deprotonated so as to the positive surface charges quantity is greatly reduced, resulting in a drop in the sorption capability of Chromium. Consequently, the chromium sorption amounts at low pH are greater than that at high pH (Chen et al., 2013)(Zhu et al., 2016).
7.2 Influence of Treatment Time on Chromium Disarmament Efficacy
Figure 7 represents the contact time effect on Cr (VI) sorption (pH = 3, 5 g of adsorbent dose, 200 μm particle size, C0 = 30 mg/L, and 200 rpm). As can be shown, the chromium elimination by CRS raised beside rising contact times, reaching a peak at 80 min. The fast rise in chromium elimination with the rise in the processing time is a result of the great number of unoccupied sites available for sorption till equilibrium is reached (Santhosh & Dhandapani, 2013). After the equilibrium time, no more chromium elimination was noticed, pointing saturation of adsorption sites (Shirzad et al., 2011). This was owing to dissonance between the solute molecules and the bulk phase (Qaiser et al., 2009).
7.3 Influence of Sorbent Amount on Chromium Disarmament Efficacy
In Fig. 8, the impact of a sorbent amount on the adsorption of chromium is indicated for a fixed initial chromium concentration of 30 mg/L in the solution, a pH value of 3, 200 rpm, 200 μm particle size, and 80-min contact time. The chromium elimination percentage rises from 58.84 to 98.9% when the sorbent amount is raised from 1 to 5 g, as a consequence of the raised surface area of sorbent (Abd El Gawad & Zahran, 2023) and the availability of further binding sites for sorption (Kabir et al., 2019)(Devi & Manonmani, 2015). After 5 g of sorbent, the removal percentage was reduced by reason of the unaltered chromium ion initial concentration and the significant sorption capability of the minimum sorbent amount or fewer surface area per unit weight obtainability (Ajmani et al., 2019).
7.4 Influence of Initial Chromium Concentration on Chromium Disarmament Efficacy
The impact of chromium initial concentration on the elimination rate was determined at various initial concentrations (30 to 330 mg/L) via saving contact time, adsorbent dose, particle size, pH and stirring speed at the values 80 min, 5 g, 200 μm particle size, 3, and 200 rpm, respectively. The final result indicates that a rise in an initial chromium concentration from 30 to 330 mg/L reduces the removal percentage from 98.9 to 88.76% as is also displayed in Fig. 9. In fact, the reason for this is that all sorbents contain a finite numeral of active sites, and they become saturated at a certain concentration of ions which results in a lower chromium elimination percentage (Banat et al., 2000)(Tsai & Chen, 2010)(Birhanu et al., 2020)(Yari et al., 2016).
7.5 Influence of Shaking Speed on Chromium Disarmament Efficacy
At the optimal pH (3), treatment time (80 min), C0 (30 ppm), a sorbent quantity (5 g), and 200 μm particle a sorbent size, the influence of the sorbent/adsorbate system on the chromium removal from hydrous solution was examined. Low, medium, and high agitation rates were used to determine the effect of the sorbent/adsorbate system (100, 200, 300, 500 rpm). As soon as the shaking velocity was raised from 100 to 200 rpm, the chromium removal percentage rapidly raised from 63.42 to 98.9% as shown in Fig. 10, demonstrating that the operation was controlled by mass transfer and that the precipitated chromium has a dendritic structure that permits mass transfer between discrete dendritic parts such that the efficient surface area of mass transfer through precipitation rises (El-Gawad et al., 2017). When the shaking speed is raised beyond 200 rpm, the efficiency for the removal of chromium is reduced to 97.8% at a shaking speed of 300 rpm. The fast elimination efficiency at faster velocities can be referred to as providing perfect contact between the sorbent and adsorbate ions (Barot & Bagla, 2012). Moreover, as the shaking speed rises, the chromium ions diffusion rate from the bulk liquid to the liquid boundary layer surrounding the sorbent particles rises as a result of the rise in turbulence and the decrease in the liquid boundary layer thickness (Abd El Gawad & Zahran, 2023) (Vunain et al., 2021)(McKay, 1982). Wherefore, the sorption of chromium on carbonic rice straw relies on the speed of shaking.
7.6 Influence of Sorbent Particle Size on Chromium Disarmament Efficacy
The influence of sorbent particle size on the elimination rate of chromium was determined at various particle sizes (200 to 500 μm) via initial chromium ions concentration, treatment time, adsorbent dose, pH, and shaking speed at the values 30 mg/L, 80 min, 3, 5 g, and 200 rpm, respectively. The final result clarified that higher particle size causes lower chromium removal percentage, as represented in Fig. 11. This is a consequence of the decrease in a sorbent external surface area with an increase in particle size. On the contrary, when the particle size decreases, the sorbent external surface area increases at a constant amount of the adsorbent, thus providing more active sites for adsorption (El-Gawad et al., 2016)(Srivastava et al., 2006)(Kumar et al., 2017).
8 Isothermal Modeling
Isotherms are utilized to elucidate the adsorption process, with the Langmuir and Freundlich isotherms being the most commonly used. The Langmuir isotherm elucidates adsorption at uniform sites, where a monolayer is formed, resulting in a linear equation given by the equation:
where: qe (mg/g) is the equilibrium concentration of chromium, and Ce (mg/L) is the equilibrium concentration in the liquid phase. The plot of Ce/qe vs Ce is linear which means that the adsorption data fitted reasonably to the Langmuir isotherm as elucidated in Fig. 12a. The constants were assessed from the slope (1/qm) and intercept (1/KL) and listed in Table 4. The Langmuir equation in terms of dimensionless factor, RL is given by:
The value of RL, a positive number (0 < RL < 1), denotes the feasibility of the sorption process.
The Freundlich isotherm model assumes that adsorption occurs on heterogeneous surfaces that possess different adsorption energies. The linear form of the Freundlich equation is given by:
where: 1/n is the heterogeneity factor related to intensity, and Kf is the Freundlich constant. The slope and intercept of log qe against log Ce give 1/n and Kf values as elucidated in Fig. 12b.
The comparison of correlation values (R2) between the Langmuir and Freundlich adsorption isotherms suggests that the Langmuir isotherm is superior to the Freundlich isotherm.
9 The Sorption Kinetic Models of Chromium on Carbonic Rice Straw
Accordingly in the results and discussion division, the operation is influenced by diverse operative parameters. So, this division was performed to define the order of reaction. The research utilizes pseudo-first-order, pseudo-second-order, and Elovich kinetic models and particle diffusion and film diffusion models to describe the sorption mechanism of chromium ions by means of carbonic rice straw and its potential rate-controlling step involving mass transfer and chemical reaction. The pseudo-first-order kinetic model is specified by:
where qe and qt represent the quantity of chromium ions adsorbed (mg/g) at equilibrium and at time t, individually, K1 (min−1) is the rate constant.
The plot of ln (qe−qt) vs time at ideal processing factors (3 pH, 80-min sorption time, 30 mg/L initial chromium concentration, 200 rpm, 200 μm a sorbent particle size, and a sorbent quantity of 5 g at ambient temperature) is represented in Fig. 13a to define K1, qe calculated, and the (R2) correlation coefficient. Consistent with the plotted data, the chromium adsorption kinetic on carbonic rice straw is not exactly preferred by the pseudo-first-order model. This is a consequence that the ln (qe−qt) plot vs time does not present a linear relation. A similar inspection was stated by Vunain et al. (2021) in the chromium disarmament from aqueous solution. The correlation factor R2, qe calculated, and the rate constant (k) are 58.04%, 1.188 mg/g, and 0.043/min, individually as elucidated in Table 5. The pseudo-second-order linear plot is indicated by:
As indicated by the data plotted in Fig. 13b, the adsorption obeys a pseudo-second-order kinetic model with the rate constant (k) of 0.0658 g/mg/min, qe calculated of 1.597 mg/g and correlation factor (R2) of 99.76%. This is owing to the acquired correlation factors being superior compared to the pseudo-first-order correlation factors indicating that the rate control process may be chemisorption embracing valency forces through the sharing or exchange of electrons between the sorbent and the sorbate. Also, the estimated qe from a pseudo-second-order kinetic model was approximately identical to the qe expected (1.4835 mg/g). The prior authors stated an identical conclusion on the sorption of Cr ions from aqueous solution by various sorbents (Singh et al., 2014)(Khandanlou et al., 2015).
The Elovich equation is commonly utilized to examine the kinetics of gas chemisorption on solid surfaces (Zhu et al., 2014). However, recent findings (Zhu et al., 2014)(Wang et al., 2011) have exhibited that this kinetic equation can also be applied to inspect the sorption of liquids on an adsorbent, and can be expressed linearly as:
The Elovich parameters, 𝑎 (mg/g) and , can be acquired from the intercept and slope of the straight line. The Elovich model was applied to inspect the adsorption of chromium ions on carbonic rice straw, and the outcomes are exhibited in Fig. 13c. However, as depicted in the figure, the linear fit curve did not exhibit a good fit with the Elovich model (𝑅2 = 84.77%), indicating that the model cannot be used to describe the adsorption of chromium ions on carbonic rice straw. This finding suggests that chemical adsorption between the active sites on the sorbent surface and chromium ions did not occur, and instead, the adsorption behavior of chromium ions was primarily influenced by the electrostatic interaction between the ionic groups and the ions.
10 The Sorption Mechanism Analysis
In order to comprehend the behavior of the sorption of chromium, it is essential to identify the rate-limiting step in the sorption process. In solid-liquid adsorption processes, solute transfer is typically described by external mass transfer, intraparticle diffusion, or a combination of both. The four steps involved in the adsorption mechanism are listed below:
-
(1)
Transfer of metal ions from the bulk liquid to the liquid film or boundary layer surrounding the adsorbent.
-
(2)
Transfer of solute ions from the boundary film to the external surface of the sorbent (film diffusion).
-
(3)
Transfer of ions from the surface to the intra-particular active sites (particle diffusion).
-
(4)
The sorption of ions by the active sites of a sorbent.
Since the 1st step does not include sorbents and the 4th step is a very fast process, they are not subject to the rate control steps. For that reason, the rate control steps mainly rely on film diffusion or particle diffusion. Figure 14 depicts the sorption mechanism of chromium onto the carbonic rice straw.
In order to prophesy the rate control step, an intraparticle diffusion model is widely utilized (Weber Jr & Morris, 1963)(Wang et al., 2010). When mass transfer is the control step, it is substantial to define the diffusion mechanism. In accordance with the intraparticle diffusion model, the initial rate of diffusion is specified by the following relation (Eltaweil et al., 2020):
where: qt (mg/g) is the quantity of chromium ions adsorbed (mg/g) at time t, kd is the rate constant of intraparticle diffusion, and C is the intercept. The value of kd (mg/g min1/2) was defined from the slope of the respective plot of qt vs t1/2 at ideal factors and recorded in Table 5. Obviously, the estimated values of rate constant (kd) and regression coefficient (R2) were 0.2223 mg/g min1/2 and 99.81%, respectively, as shown in Table 5. The plot of qt vs t1/2 exhibited multilinear characterizations of two steps that appeared in the sorption process as elucidated in Fig. 15a (Wu et al., 2005). The first step part is owing to the immediate adsorption on the outer surface, and the second step is the gradual adsorption stage, wherever intraparticle diffusion is rate control (Cheung et al., 2007). The larger slope (0.2223 mg/g min1/2) of the first step part signifies that the rate of chromium adsorption is initially higher owing to the immediate availability of the bigger surface area and active sites. The lower slope (0.0071 mg/g min1/2) of the second step part is owing to a lower concentration gradient causing the diffusion of chromium ions into the sorbent pores. The two clear steps of the process signify that intraparticle diffusion is not only the rate control step for the sorption of chromium but other mechanisms are involved in chromium adsorption (Ho & Ofomaja, 2005).
By reason of the dual nature of intraparticle diffusion (both film and pore diffusion), and so as to specify the veritable rate control step inclusive in the sorption of chromium, kinetic data were examined by using the Boyd model. Boyd’s kinetic equation is exemplified as (Zhu et al., 2014):
where: F is the fraction of solute adsorbed at any time t, Bt is the mathematical function of F, D is the diffusion coefficient (m2/g), and r is the sorbent radius (m). The plot of [−0.4977 − ln (1 − F)] vs t was utilized to inspect the experiential data linearity as exhibited in Fig. 15b. The linearity of this plot is utilized to differentiate between exterior transfer and intraparticle transfer controlled rates of chromium adsorption (Wang et al., 2006). Crossing the straight line through the original point indicates that the sorption processes are subjected to particle diffusion mechanisms; otherwise, they are subject to film diffusion or exterior mass transport (Zhu et al., 2014). The Boyd plot of the chromium adsorption data is linear but does not pass through the original point. This reveals that the film diffusion is the rate-limiting step for the chromium adsorption. The estimated values of Boyd model parameters are B (0.0502), D (5.091 *10−11), and R2 (93.16%) as exhibited in Fig. 15b. Other authors acquired identical outcomes to these outcomes in several investigations (Wang et al., 2006).
11 Desorption Process of Chromium and Reusability of Carbonic Rice Straw
The desorption technique is substantial because it can significantly minimize the total cost. The desorption of chromium from carbonic rice straw surface could be performed by raising the pH values from 3 to 10 as the sorption of chromium was adequate at low pH. The adsorption was implemented in 250 mL of 30 mg/L initial chromium concentration at a pH of 3 with 5 g of carbonic rice straw and 200 μm a sorbent particle size at 25 °C and 200 rpm for 80 min. At these conditions, the elimination percentage of chromium was 98.9%. After filtration, the carbonic rice straw was dispersed in 50 mL of HCl solution and agitated at 25 °C for 80 min. Then, the desorbed adsorbate into the solution was discrete by filtration and analyzed for the corresponding metal ion concentration. Thus, the adsorbate desorption percent was estimated by means of the subsequent equation (Berhe et al., 2015)(Aragaw et al., 2022):
where, the desorbed is the concentration of the metal ion in the desorbing solution after the desorption process, and adsorbed is the product of Co–Ce.
The results clarified that the chromium desorption increases with raising pH values to 10 as indicated in Fig. 16. This means that the desorption process relies on the acid–base nature of chromium. This is a consequence of the increased negative charge density of chromium at a pH greater than 7, which leads to a higher electrostatic repulsion with the carbonic rice straw surface. In addition, it could be observed that the maximum chromium desorption percentage was close to 96.4% at pH 10. After that, the carbonic rice straw was reused by iterating the successive adsorption–desorption cycles four times by using the same carbonic rice straw to determine the efficacy of recycled carbonic rice straw in the sorption of chromium. The consumed carbonic rice straw was washed thoroughly with distilled water more than once to remove chromium residue from the carbonic rice straw surface for each reusability cycle. Figure 17 elucidates the competence of reused carbonic rice straw for chromium adsorption. It is evident that there was no significant variation in the elimination% of chromium, but it reduced slightly with each cycle of carbonic rice straw reuse.
12 Statistical Analysis
The above results indicated that the elimination efficiency of chromium was affected non-linearly, by the numerous processing factors such as chromium initial concentration, solution pH, shaking speed (rpm), sorbent amount, sorbent particle size, and treatment time. The statistical and least square multivariate regression technique was utilized so as to obtain the mathematical correlation that can obvious the influence of the processing factors on the elimination chromium efficiency. The model takes the following form:
Where A is the chromium initial concentration (mg/L), B is shaking speed (rpm), C is the pH of the solution, D sorbent amount, E is the sorbent particle size, F is the treatment time, and ε is the error which is the variation between the detected experimental values and the correlation expected values (equal zero).
Data for ANOVA are depicted in Table 6, while the values, p-values, standard error, and t-test for all coefficients are specified in Table 7. All the p-values less than 0.001 denote that the correlation term is significant. The normal probability of standardized residuals with average correlation errors of zero is exhibited in Fig. 18a, while Fig. 18b clarifies the comparison between the experimentally detected elimination percent and expected values. Figure 19 illustrates 3D surface plots that depict the interactive effects of two variables on the removal efficiency. The first 3D surface plot (Fig. 19a) demonstrates the impact of stirring speed and sorbent amount on the removal efficiency. The second 3D surface plot (Fig. 19b) showcases the influence of sorbent particle size and sorbent amount on the removal efficiency. Lastly, the third 3D surface plot (Fig. 19c) reveals the correlation between stirring speed, solution pH, and the removal efficiency.
13 Comparison of Carbonic Rice Straw and Other Sorbents
Comparison outcomes between the maximum chromium elimination percentage onto carbonic rice straw and other sorbents are elucidated in Table 8.
14 Conclusion
The current study deals with the chromium elimination from tanneries industry wastewater by using carbonic rice straw as a sorbent. Adsorption experiments were completed by adjusting pH, stirring speed, sorbent quantity, sorbent particle size, treatment time, and chromium initial concentration. The research outcomes elucidated that:
-
(i)
The chromium elimination efficiency by means of carbonic rice straw rises by raising contact time and adsorbent dosage.
-
(ii)
The ultimate elimination percentage of chromium (98.9%) was obtained at ideal operators of pH 3, 80-min sorption time, initial concentration of chromium of 30 mg/L, 200 rpm, 200 μm a sorbent particle size, and a sorbent quantity of 5 g at ambient temperature.
-
(iii)
The carbonic rice straw enhanced from rice straw activated by phosphoric acid (H3PO4) can be efficiently applied as a premium alternative for the chromium elimination from actual tannery sewer water.
-
(iv)
When analyzing experimental adsorption data, it is common to test multiple adsorption isotherm models to identify the best fit for the data. In this case, both the Langmuir and Freundlich adsorption isotherms were tested, and the Langmuir isotherm was found to provide a better fit to the experimental data. The high value of R2 (99.67%) suggests a strong correlation between the Langmuir model’s predicted values and the experimental data.
-
(v)
The chromium adsorption followed the pseudo-second-order kinetics for a rate constant (k) of 0.0658 g/mg/min, 1.597 mg/g of the calculated qe and 99.76% correlation factor (R2).
-
(vi)
The Elovich model confirmed that chemisorption is not a suitable method to depict the adsorption behavior of chromium ions on carbonized rice straw.
-
(vii)
Mechanism studies confirmed that intraparticle diffusion is not only rate control step, and Boyd’s model exhibited that the film diffusion is the rate-limiting step for the chromium adsorption.
-
(viii)
The maximum percentage of chromium desorption from the carbonic rice straw surface was about 96.4% at pH 10, and this consequence is significant as carbonic rice straw can be reused for four cycle adsorption-desorption process. It is evident that there was no significant variation in the elimination% of chromium, but it reduced slightly with each cycle of carbonic rice straw reuse.
-
(ix)
Ultimately, the acquired correlation in terms of momentous factors only has the following form:
Elimination chromium% = −13320.45 − 0.02 A − 0.37 B − 18.54 C − 23.31 D − 0.38 E − 0.494 F + 594.74 C0.1 + 105.93 D0.5 + 3738.99 F0.01 + 8012.12 E0.01 + 181.4 B0.2
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Abaide, E. R., Dotto, G. L., Tres, M. V., Zabot, G. L., & Mazutti, M. A. (2019). Adsorption of 2–nitrophenol using rice straw and rice husks hydrolyzed by subcritical water. Bioresource Technology, 284, 25–35.
Abd El Gawad, H. A., & Zahran, H. (2023). Silver withdrawal from X-Ray waste via leaching and sorption techniques: Appraisal ideal treatment factors. Egyptian Journal of Chemistry, 66(5), 509–522.
Aboulhassan, M. A., Souabi, S., & Yaacoubi, A. (2008). Pollution reduction and biodegradability index improvement of tannery effluents. International Journal of Environmental Science and Technology, 5, 11–16.
Acar, F. N., & Malkoc, E. (2004). The removal of chromium (VI) from aqueous solutions by Fagus orientalis L. Bioresource Technology, 94(1), 13–15.
Adhikari, D. L., Aryal, R. L., Bhattarai, S., Gautam, S. K., & Poudel, B. R. (2017). Removal of chromium (VI) from aqueous solution using chemically-modified sweet lime (Citrus limetta) Peels as Adsorbent. Journal of Nepal Chemical Society, 36, 82–95.
Ahmed, W., et al. (2023). Adsorption of Pb (II) from wastewater using a red mud modified rice-straw biochar: Influencing factors and reusability. Environmental Pollution, 326, 121405.
Ajmani, A., Shahnaz, T., Narayanan, S., & Narayanasamy, S. (2019). Equilibrium, kinetics and thermodynamics of hexavalent chromium biosorption on pristine and zinc chloride activated Senna siamea seed pods. Chemistry and Ecology, 35(4), 379–396.
Amanuel, L. (2020). Palm leaf sheath fiber extraction and surface modification. Journal of Engineered Fibers and Fabrics, 15, 1558925020950724.
Aragaw, T., Leta, S., Alayu, E., & Mekonnen, A. (2022). Chromium removal from electroplating wastewater using activated coffee husk carbon. Adsorption Science and Technology, 2022, 7646593.
Babel, S., & Kurniawan, T. A. (2003). Low-cost adsorbents for heavy metals uptake from contaminated water: A review. Journal of Hazardous Materials, 97(1–3), 219–243.
Banat, F. A., Al-Bashir, B., Al-Asheh, S., & Hayajneh, O. (2000). Adsorption of phenol by bentonite. Environmental Pollution, 107(3), 391–398.
Bansal, M., Garg, U., Singh, D., & Garg, V. K. (2009). Removal of Cr (VI) from aqueous solutions using pre-consumer processing agricultural waste: A case study of rice husk. Journal of Hazardous Materials, 162(1), 312–320.
Barot, N. S., & Bagla, H. K. (2012). Eco-friendly waste water treatment by cow dung powder (adsorption studies of Cr (III), Cr (VI) and Cd (II) using tracer technique). Desalination and Water Treatment, 38(1–3), 104–113.
Berhe, S., Ayele, D., Tadesse, A., & Mulu, A. (2015). Adsorption efficiency of coffee husk for removal of lead (II) from industrial effluents: Equilibrium and kinetic study. International Journal of Scientific and Research Publications, 5(9), 1–8.
Bhattacharyya, P., et al. (2020). Characterization of rice straw from major cultivars for best alternative industrial uses to cutoff the menace of straw burning. Industrial Crops and Products, 143, 111919.
Birhanu, Y., Leta, S., & Adam, G. (2020). Removal of chromium from synthetic wastewater by adsorption onto Ethiopian low-cost Odaracha adsorbent. Applied Water Science, 10(11), 1–11.
Chen, J.-H., Hsu, K.-C., & Chang, Y.-M. (2013). Surface modification of hydrophobic resin with tricaprylmethylammonium chloride for the removal of trace hexavalent chromium. Industrial and Engineering Chemistry Research, 52(33), 11685–11694.
Cheung, W. H., Szeto, Y. S., & McKay, G. (2007). Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresource Technology, 98(15), 2897–2904.
Chuah, T. G., Jumasiah, A., Azni, I., Katayon, S., & Choong, S. Y. T. (2005). Rice husk as a potentially low-cost biosorbent for heavy metal and dye removal: An overview. Desalination, 175(3), 305–316.
Devi, B. V., Jahagirdar, A., & Ahmed, M. Z. (2012). Adsorption of chromium on activated carbon prepared from coconut shell. International Journal of Engineering Research and Applications, 2(5), 364–370.
Devi, M. M., & Manonmani, S. (2015). Removal of hexavalent chromium ions from aqueous solution by adsorption using activated carbon prepared from Cucumis melo peel activated carbon. Oriental Journal of Chemistry, 31(1), 531–539.
Diyanati, R. A., Balarak, D., & Ghasemi, M. (2013). Survey of efficiency agricultural weast in removal of acid orang 7 (AO7) dyes from aqueous solution: Kinetic and equilibrium study. Iranian Journal of Health Sciences, 2(1), 35–40.
El-Gawad, H. A., Kattab, I. A., Mady, A. N., Moselhy, H., & Ibrahim, O. A. (2016). Cementation of copper with zinc metal from monometallic sulphate solution using stirred reactor. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 7(6), 1015–1029.
El-Gawad, H. A., Kattab, I. A., Mahdy, A. N., Moselhy, H., & Ibrahim, O. A. (2017). Cementation of lead from mono-metallic nitrate solution using a modest-agitated reactor. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 8(1), 1366–1380.
Eltaweil, A. S., Abd El-Monaem, E. M., Omer, A. M., Khalifa, R. E., Abd El-Latif, M. M., & El-Subruiti, G. M. (2020). Efficient removal of toxic methylene blue (MB) dye from aqueous solution using a metal-organic framework (MOF) MIL-101 (Fe): Isotherms, kinetics, and thermodynamic studies. Desalination and Water Treatment, 189, 395–407.
Ewis, D., Mahmud, N., Benamor, A., Ba-Abbad, M. M., Nasser, M., & El-Naas, M. (2022). Enhanced removal of diesel oil using new magnetic bentonite-based adsorbents combined with different carbon sources. Water, Air, & Soil Pollution, 233(6), 195.
Farahmand, E. (2016). Adsorption of cerium (IV) from aqueous solutions using activated carbon developed from rice straw. Open Journal of Geology, 6(3), 189–200.
Goswami, J., et al. (2022). Phosphoric acid assisted synthesis of fluorescent carbon dots from waste biomass for detection of Cr (VI) in aqueous media. Materials Chemistry and Physics, 286, 126133.
Griffin, G. J., Ward, L. P., Madapusi, S., Shah, K. V., & Parthasarathy, R. (2022). A study of chemical pre-treatment and pyrolysis operating conditions to enhance biochar production from rice straw. Journal of Analytical and Applied Pyrolysis, 163, 105455.
Ho, Y.-S., & Ofomaja, A. E. (2005). Kinetics and thermodynamics of lead ion sorption on palm kernel fibre from aqueous solution. Process Biochemistry, 40(11), 3455–3461.
Hossain, N., Nizamuddin, S., Griffin, G., Selvakannan, P., Mubarak, N. M., & Mahlia, T. M. I. (2020). Synthesis and characterization of rice husk biochar via hydrothermal carbonization for wastewater treatment and biofuel production. Scientific Reports, 10(1), 18851.
Hsu, T.-C., Guo, G.-L., Chen, W.-H., & Hwang, W.-S. (2010). Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresource Technology, 101(13), 4907–4913.
Huang, X., Sheng, X., Guo, Y., Sun, Y., Fatehi, P., & Shi, H. (2022). Rice straw derived adsorbent for fast and efficient phosphate elimination from aqueous solution. Industrial Crops and Products, 184, 115105.
Jastrzębski, W., Sitarz, M., Rokita, M., & Bułat, K. (2011). Infrared spectroscopy of different phosphates structures. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 79(4), 722–727.
Jisha, K. J., Rajamani, S., Singh, D., Sharma, G., & Gardas, R. L. (2022). A comparative study of ionothermal treatment of rice straw using triflate and acetate-based ionic liquids. Journal of Ionic Liquids, 2(2), 100037.
Jorn-am, T., Praneerad, J., Attajak, R., Sirisit, N., Manyam, J., & Paoprasert, P. (2021). Quasi-solid, bio-renewable supercapacitor with high specific capacitance and energy density based on rice electrolytes and rice straw-derived carbon dots as novel electrolyte additives. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 628, 127239.
Kabir, M. M., Ferdousi, S., Rahman, M. M., & Uddin, M. K. (2019). Chromium (VI) removal efficacy from aqueous solution by modified tea wastes-polyvinyl alcohol (TW-PVA) composite adsorbent. Desalination and Water Treatment, 174, 311–323.
Kara, A., & Demirbel, E. (2012). Kinetic, isotherm and thermodynamic analysis on adsorption of Cr (VI) ions from aqueous solutions by synthesis and characterization of magnetic-poly (divinylbenzene-vinylimidazole) microbeads. Water, Air, & Soil Pollution, 223, 2387–2403.
S. S. Kerur, M. S. Hanagadakar, S. S. Nandi, R. Sholapurmath, and S. N. Hosamane, “Optimization, statistical and adsorption analysis of Cr (VI) using corn industry sludge: Kinetic and isotherm studies,” 2022.
Khandanlou, R., Ahmad, M. B., Fard Masoumi, H. R., Shameli, K., Basri, M., & Kalantari, K. (2015). Rapid adsorption of copper (II) and lead (II) by rice straw/Fe3O4 nanocomposite: Optimization, equilibrium isotherms, and adsorption kinetics study. PLoS One, 10(3), e0120264.
Kumar, A., Chauhan, A. S., Bains, R., & Das, P. (2021). Rice straw (Oryza sativa L.) biomass conversion to furfural, 5-hydroxymethylfurfural, lignin and bio-char: A comprehensive solution. Journal of Industrial and Engineering Chemistry, 104, 286–294.
Kumar, R., Arya, D. K., Singh, N., & Vats, H. K. (2017). Removal of Cr (VI) using low cost activated carbon developed by agricultural waste. IOSR Journal of Applied Chemistry, 10(1), 76–79.
Li, H., et al. (2022). Removal of elemental mercury from flue gas over a low-cost and magnetic sorbent derived from FeSO4-flocculated sludge and rice straw. Journal of the Energy Institute, 105, 406–414.
Lukman, H. S. (2022). Adsorption potentials of rice husk for treatment of tannery effluent in Zaria, Nigeria. International Journal of Information, Engineering & Technology, 11, 99.
Machado, A. A., & Mulky, L. (2023). A comparative study of treatment methods of raw sugarcane bagasse for adsorption of oil and diesel. Water, Air, & Soil Pollution, 234(4), 213.
McKay, G. (1982). Adsorption of dyestuffs from aqueous solutions with activated carbon I: Equilibrium and batch contact-time studies. Journal of Chemical Technology and Biotechnology, 32(7-12), 759–772.
Mohamed, G., El-Shafey, O., & Fathy, N. A. (2017). Preparation of carbonaceous hydrochar adsorbents from cellulose and lignin derived from rice straw. Egyptian Journal of Chemistry, 60(5), 793–804.
Motlagh, E. K., Asasian-Kolur, N., Sharifian, S., & Pirbazari, A. E. (2021). Sustainable rice straw conversion into activated carbon and nano-silica using carbonization-extraction process. Biomass and Bioenergy, 144, 105917.
Namasivayam, C., & Kadirvelu, K. (1994). Coirpith, an agricultural waste by-product, for the treatment of dyeing wastewater. Bioresource Technology, 48(1), 79–81.
Neme, I., Gonfa, G., & Masi, C. (2022). Activated carbon from biomass precursors using phosphoric acid: A review. Heliyon, e11940.
Pang, B., et al. (2022). Performance and environmental implication assessments of green bio-composite from rice straw and bamboo. Journal of Cleaner Production, 375, 134037.
Qaiser, S., Saleemi, A. R., & Umar, M. (2009). Biosorption of lead from aqueous solution by Ficus religiosa leaves: Batch and column study. Journal of Hazardous Materials, 166(2–3), 998–1005.
Rahman, H. A., & Chin, S. X. (2019). Physical and chemical properties of the rice straw activated carbon produced from carbonization and KOH activation processes. Sains Malays, 48(2), 385–391.
Rajczykowski, K., & Loska, K. (2018). Stimulation of heavy metal adsorption process by using a strong magnetic field. Water, Air, & Soil Pollution, 229, 1–7.
Sakhiya, A. K., Anand, A., Vijay, V. K., & Kaushal, P. (2021). Thermal decomposition of rice straw from rice basin of India to improve energy-pollution nexus: Kinetic modeling and thermodynamic analysis. Energy Nexus, 4, 100026.
Santhosh, P., & Dhandapani, C. (2013). Adsorption studies on the removal of chromium (VI) from wastewater using activated carbon derived from water hyacinth. Nature, Environment and Pollution Technology, 12(4), 563.
Sharma, P. K., & Ayub, S. (2019). The cost analysis and economic feasibility of agro wastes to adsorb chromium (VI) from wastewater. International Journal of Civil Engineering and Technology, 10(2), 2387–2402.
Shirzad, S. M., Samarghandi, M. R., Azizian, S., Kim, W. G., & Lee, S. M. (2011). The removal of hexavalent chromium from aqueous solutions using modified holly sawdust: Equilibrium and kinetics studies. Environmental Engineering Research, 16(2), 55–60.
Singh, D., Gautam, R. K., Kumar, R., Shukla, B. K., Shankar, V., & Krishna, V. (2014). Citric acid coated magnetic nanoparticles: Synthesis, characterization and application in removal of Cd (II) ions from aqueous solution. Journal of Water Process Engineering, 4, 233–241.
S. R. Singh and A. P. Singh, “Treatment of water containg chromium (VI) using rice husk carbon as a newlow cost adsorbent,” 2012.
Srivastava, P. K., Singh, A., Kumari, S., Arora, S., Choubey, A. K., & Sinha, A. S. K. (2023). Production and characterization of sustainable vermimanure derived from poultry litter and rice straw using tiger worm Eisenia fetida. Bioresource Technology, 369, 128377.
Srivastava, V. C., Mall, I. D., & Mishra, I. M. (2006). Equilibrium modelling of single and binary adsorption of cadmium and nickel onto bagasse fly ash. Chemical Engineering Journal, 117(1), 79–91.
Taha, S. M., Amer, M. E., Elmarsafy, A. E., & Elkady, M. Y. (2014). Adsorption of 15 different pesticides on untreated and phosphoric acid treated biochar and charcoal from water. Journal of Environmental Chemical Engineering, 2(4), 2013–2025.
G. M. Temesgen and G. S. Gebrie, “Assessment of cactus biosorption potential of Cr (VI) ions removal from synthetic and tannery wastewater,” 2022.
Tsai, W.-T., & Chen, H.-R. (2010). Removal of malachite green from aqueous solution using low-cost chlorella-based biomass. Journal of Hazardous Materials, 175(1–3), 844–849.
Vunain, E., Njewa, J. B., Biswick, T. T., & Ipadeola, A. K. (2021). Adsorption of chromium ions from tannery effluents onto activated carbon prepared from rice husk and potato peel by H3PO4 activation. Applied Water Science, 11(9), 150.
Wang, L., Zhang, J., Zhao, R., Li, Y., Li, C., & Zhang, C. (2010). Adsorption of Pb (II) on activated carbon prepared from Polygonum orientale Linn.: Kinetics, isotherms, pH, and ionic strength studies. Bioresource Technology, 101(15), 5808–5814.
Wang, X., Qin, Y., & Li, Z. (2006). Biosorption of zinc from aqueous solutions by rice bran: Kinetics and equilibrium studies. Separation Science and Technology, 41(4), 747–756.
Wang, X. S., Lu, Z. P., Miao, H. H., He, W., & Shen, H. L. (2011). Kinetics of Pb (II) adsorption on black carbon derived from wheat residue. Chemical Engineering Journal, 166(3), 986–993.
Weber, W. J., Jr., & Morris, J. C. (1963). Kinetics of adsorption on carbon from solution. Journal of the Sanitary Engineering Division, 89(2), 31–59.
Wu, F.-C., Tseng, R.-L., & Juang, R.-S. (2005). Comparisons of porous and adsorption properties of carbons activated by steam and KOH. Journal of Colloid and Interface Science, 283(1), 49–56.
Yari, A. R., Kord Mostafapour, F., Mahdavi, Y., & Joghataei, A. (2016). Agricultural waste as adsorbent for removal of chromium (VI) from aqueous solution. Archives of Hygiene Sciences, 5(4), 310–318.
Zhang, C., Yang, W., Chen, W.-H., Ho, S.-H., Pétrissans, A., & Pétrissans, M. (2022). Effect of torrefaction on the structure and reactivity of rice straw as well as life cycle assessment of torrefaction process. Energy, 240, 122470.
Zhang, J., et al. (2021). N-doped hierarchically porous carbon derived from grape marcs for high-performance supercapacitors. Journal of Alloys and Compounds, 854, 157207.
Zhu, K., Gao, Y., Tan, X., & Chen, C. (2016). Polyaniline-modified Mg/Al layered double hydroxide composites and their application in efficient removal of Cr (VI). ACS Sustainable Chemistry & Engineering, 4(8), 4361–4369.
Zhu, W., Liu, J., & Li, M. (2014). Fundamental studies of novel zwitterionic hybrid membranes: Kinetic model and mechanism insights into strontium removal. Scientific World Journal, 2014, 485820.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare there is no conflicting of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Gawad, H.A., Kadry, G., Zahran, H.A. et al. Chromium Disarmament from Veritable Tanneries Sewer Water Utilizing Carbonic Rice Straw as a Sorbent: Optimization and Carbonic Rice Straw Characteristics. Water Air Soil Pollut 234, 659 (2023). https://doi.org/10.1007/s11270-023-06644-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06644-7