Abstract
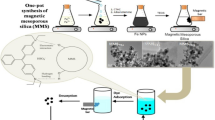
Dyes are hazardous to public health and the environment. Herein, we synthesized Ti-doped porous SiO2 microspheres utilizing a one-step sol-gel method as an effective adsorbent for the removal of cationic dyes from wastewater. The study of the varying individual parameters such as titanium-silica ratio, pH, adsorbent dose, and dye concentration indicated that the adsorption of methylene blue (MB) by Si3TiO10 microspheres exhibited excellent performance, including a wide range of applications (pH = 4–11), fast adsorption rate (15 min removed 90.8% of MB), high adsorption capacity (215.29 mg/g), excellent selectivity (adsorption capacity toward cationic dyes (98%) and < 5% toward anionic dyes), easy desorption steps, steady performance and multiple cycles (10 times). In addition, the adsorption process of MB by microspheres is consistent with the proposed second-order kinetic model (R2 = 0.999) and Langmuir isothermal model (RL2 = 0.997). With the assistance of FT-IR, XRD, XPS, BET, SEM, and TG-DTG techniques, it was demonstrated that the great pore size (8.15 nm) and large specific surface area (267.97 m2/g) of the microspheres could promote the contact between the active site and MB, thus achieving efficient removal of MB by using hydrogen bonding and electrostatic attraction. Furthermore, the unique spherical mesoporous structure, and the Si-O-Ti bond formed by the Ti doping rather than via simple wrapping and adhesion modification, which also ensured the stability of the microspheres during multiple cycles and the desorption process.





Similar content being viewed by others
Data Availability
All the data generated or analyzed during this study are included in this published article [and its Supplementary Information files].
References
Ade, I. A., Tran, H. N., Zhang, J.-W., Wang, Y.-C., Dat, N. D., Nguyen, D. T., & Chao, H.-P. (2022). Adsorption characteristics of lead, copper, cadmium, methylene blue, phenol, and toluene in water using composite synthesized from titanium dioxides and carbon spheres through hydrothermal method. Journal of Water Process Engineering, 50, 103221. https://doi.org/10.1016/j.jwpe.2022.103221
Aldegs, Y., Elbarghouthi, M., Elsheikh, A., & Walker, G. (2008). Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes and Pigments, 77(1), 16–23. https://doi.org/10.1016/j.dyepig.2007.03.001
Bai, X., Sun, H., Sun, J., & Zhu, Z. (2022). Efficient removal of sixteen priority polycyclic aromatic hydrocarbons from textile dyeing sludge using electrochemical Fe2+-activated peroxymonosulfate oxidation-A green pretreatment strategy for textile dyeing sludge toxicity reduction. Journal of Hazardous Materials, 435, 129087. https://doi.org/10.1016/j.jhazmat.2022.129087
Bhatnagar, A., Sillanpää, M., & Witek-Krowiak, A. (2015). Agricultural waste peels as versatile biomass for water purification – A review. Chemical Engineering Journal, 270, 244–271. https://doi.org/10.1016/j.cej.2015.01.135
Bilal, M., Ihsanullah, I., Hassan Shah, M. U., Bhaskar Reddy, A. V., & Aminabhavi, T. M. (2022). Recent advances in the removal of dyes from wastewater using low-cost adsorbents. Journal of Environmental Management, 321, 115981. https://doi.org/10.1016/j.jenvman.2022.115981
Cheng, Z., Luo, S., Li, X., Zhang, S., Thang Nguyen, T., Guo, M., & Gao, X. (2021). Ultrasound-assisted heterogeneous Fenton-like process for methylene blue removal using magnetic MnFe2O4/biochar nanocomposite. Applied Surface Science, 566, 150654. https://doi.org/10.1016/j.apsusc.2021.150654
Din Mir, N. U., Shahwaz Ahmad, M., Khan, S., Yasir Khan, M., Vakil, F., Saraswat, S., & Shahid, M. (2022). Simpler is better: A heterometallic (Mn-Na) metal organic framework (MOF) with a rare myc topology synthesized from bench chemicals for selective adsorption and separation of organic dyes. Inorganic Chemistry Communications, 146, 110046. https://doi.org/10.1016/j.inoche.2022.110046
Ferreira-Neto, E. P., Ullah, S., Martinez, V. P., Yabarrena, J. M. S. C., Simões, M. B., Perissinotto, A. P., et al. (2021). Thermally stable SiO2@TiO2 core@shell nanoparticles for application in photocatalytic self-cleaning ceramic tiles. Materials Advances, 2(6), 2085–2096. https://doi.org/10.1039/D0MA00785D
Fujishima, M., Takatori, H., & Tada, H. (2011). Interfacial chemical bonding effect on the photocatalytic activity of TiO2–SiO2 nanocoupling systems. Journal of Colloid and Interface Science, 361(2), 628–631. https://doi.org/10.1016/j.jcis.2011.06.024
Ghaedi, S., Seifpanahi-Shabani, K., & Sillanpää, M. (2022). Waste-to-resource: New application of modified mine silicate waste to remove Pb2+ ion and methylene blue dye, adsorption properties, mechanism of action and recycling. Chemosphere, 292, 133412. https://doi.org/10.1016/j.chemosphere.2021.133412
Gollakota, A. R. K., Munagapati, V. S., Shadangi, K. P., Reddy, G. M., Wen, J.-C., & Shu, C.-M. (2021). Encapsulating toxic rhodamine 6G dye, and Cr (VI) metal ions from liquid phase using AlPO4-5 molecular sieves. Preparation, characterization, and adsorption parameters. Journal of Molecular Liquids, 336, 116549. https://doi.org/10.1016/j.molliq.2021.116549
Hachemaoui, M., Boukoussa, B., Adel, M., Mekki, A., & Hamacha, R. B. (2020). Dyes adsorption, antifungal and antibacterial properties of metal loaded mesoporous silica: Effect of metal and calcination treatment. Materials Chemistry and Physics, 256, 123704.
Hadi, S., Taheri, E., Amin, M. M., Fatehizadeh, A., & Gardas, R. L. (2021). Empirical modeling and kinetic study of methylene blue removal from synthetic wastewater by activation of persulfate with heterogeneous Fenton-like process. Journal of Molecular Liquids, 328, 115408. https://doi.org/10.1016/j.molliq.2021.115408
Hara, T., Nabei, H., & Kyuka, A. (2020). Activated carbon/titanium dioxide composite to adsorb volatile organic compounds associated with human body odor. Heliyon, 6(11), e05455.
Ho, Y. S. (1999). Pseudo-second order model for sorption processes. Process biochemistry, 34(5), 451–465.
Hou, J., Chen, Y., Shi, W., Bao, C., & Hu, X. (2020). Graphene oxide/methylene blue composite membrane for dyes separation: Formation mechanism and separation performance. Applied Surface Science, 505, 144145. https://doi.org/10.1016/j.apsusc.2019.144145
Ihaddaden, S., Aberkane, D., Boukerroui, A., & Robert, D. (2022). Removal of methylene blue (basic dye) by coagulation-flocculation with biomaterials (bentonite and Opuntia ficus indica). Journal of Water Process Engineering, 49, 102952. https://doi.org/10.1016/j.jwpe.2022.102952
Javadian, H., & Taghavi, M. (2014). Application of novel polypyrrole/thiol-functionalized zeolite Beta/MCM-41 type mesoporous silica nanocomposite for adsorption of Hg2+ from aqueous solution and industrial wastewater: Kinetic, isotherm and thermodynamic studies. Applied Surface Science, 289, 487–494.
Jia, Z., Han, C., Wu, L., Zhang, D., & Li, M. (2022). Biotemplated synthesis of hollow nickel silicate fiber for organic dye contaminants and its selective adsorption. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 648, 129219. https://doi.org/10.1016/j.colsurfa.2022.129219
Jiang, Q., Huang, J., Ma, B., Yang, Z., Zhang, T., & Wang, X. (2020). Recyclable, hierarchical hollow photocatalyst TiO2@SiO2 composite microsphere realized by raspberry-like SiO2. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 602, 125112. https://doi.org/10.1016/j.colsurfa.2020.125112
Jian-wen, S., Shao-hua, C., Shu-mei, W., et al. (2009). Favorable recycling photocatalyst TiO2/CFA: Effects of loading method on the structural property and photocatalytic activity. Journal of Molecular Catalysis A: Chemical, 303(1–2), 141–147.
Kang, S., Liu, W., Wang, Y., Wang, Y., Wu, S., Chen, S., et al. (2022). Starch-derived flocculant with hyperbranched brush architecture for effectively flocculating organic dyes, heavy metals and antibiotics. Journal of the Taiwan Institute of Chemical Engineers, 135, 104383. https://doi.org/10.1016/j.jtice.2022.104383
Khan, M., & Cao, W. (2013). Preparation of Y-doped TiO2 by hydrothermal method and investigation of its visible light photocatalytic activity by the degradation of methylene blue. Journal of Molecular Catalysis A Chemical, 376, 71–77.
Khanna, S., Gotipamul, P. P., Rajan, K. D., Kumar, G. M., Chidambaram, S., & Rathinam, M. (2022). Rapid selective adsorption of hazardous dyes using charge controlled NiO layers encapsulated SiO2 core-shell nanostructures. Ceramics International, 48(19), 28969–28979. https://doi.org/10.1016/j.ceramint.2022.04.169
Lan, D., Zhu, H., Zhang, J., Li, S., Chen, Q., Wang, C., et al. (2022). Adsorptive removal of organic dyes via porous materials for wastewater treatment in recent decades: A review on species, mechanisms and perspectives. Chemosphere, 293, 133464. https://doi.org/10.1016/j.chemosphere.2021.133464
Lei, C., Bian, Y., Zhi, F., Hou, X., Lv, C., & Hu, Q. (2022). Enhanced adsorption capacity of cellulose hydrogel based on corn stalk for pollutants removal and mechanism exploration. Journal of Cleaner Production, 375, 134130. https://doi.org/10.1016/j.jclepro.2022.134130
Liang Mian, Y., Di, Z., Chong, H., & Ying, L. L. (2019). Adsorption and photodegradation of methylene blue by titanium dioxide - silica composites. Water Supply Technology, 13(6), 5.
Liang, Z., Zhao, Z., Sun, T., Shi, W., & Cui, F. (2017). Enhanced adsorption of the cationic dyes in the spherical CuO/meso-silica nano composite and impact of solution chemistry. Journal of Colloid and Interface Science, 485, 192–200. https://doi.org/10.1016/j.jcis.2016.09.028
Lin, R., Liang, Z., Yang, C., Zhao, Z., & Cui, F. (2020). Selective adsorption of organic pigments on inorganically modified mesoporous biochar and its mechanism based on molecular structure. Journal of Colloid and Interface Science, 573, 21–30. https://doi.org/10.1016/j.jcis.2020.03.112
Liu, J., Li, M., Wang, J., Song, Y., Jiang, L., Murakami, T., & Fujishima, A. (2009). Hierarchically macro-/mesoporous Ti−Si oxides photonic crystal with highly efficient photocatalytic capability. Environmental Science & Technology, 43(24), 9425–9431.
Ma, J., Wei, Y., Liu, W. X., & Cao, W. B. (2009). Preparation of nanocrystalline Fe-doped TiO2 powders as a visible-light-responsive photocatalyst. Research on Chemical Intermediates, 35(3), 329–336.
Mahanta, U., Khandelwal, M., & Deshpande, A. S. (2022). TiO2@SiO2 nanoparticles for methylene blue removal and photocatalytic degradation under natural sunlight and low-power UV light. Applied Surface Science, 576, 151745.
Mais, L., Vacca, A., Mascia, M., Usai, E. M., Tronci, S., & Palmas, S. (2020). Experimental study on the optimisation of azo-dyes removal by photo-electrochemical oxidation with TiO2 nanotubes. Chemosphere, 248, 125938.
Oninla, V. O., Awokoya, K. N., Babalola, J. O., Balogun, K. I., & Ismail, O. S. (2022). Optimization of synthesis conditions for graft copolymerization of methacrylic acid onto Garcinia kola pods and use in the sequestration of cationic dyes from simulated wastewaters Biomass Conversion and Biorefinery. 2022:1-8.
Qin, P., Chen, D., Li, M., Li, D., Gao, Y., Zhu, S., et al. (2022). Melamine/MIL-101(Fe)-derived magnetic carbon nanotube-decorated nitrogen-doped carbon materials as sorbent for rapid removal of organic dyes from environmental water sample. Journal of Molecular Liquids, 359, 119231. https://doi.org/10.1016/j.molliq.2022.119231
Raza, N., Raza, W., Gul, H., Azam, M., & Kim, K. H. (2020a). Solar-light-active silver phosphate/titanium dioxide/silica heterostructures for photocatalytic removal of organic dye. Journal of Cleaner Production, 254, 120031.
Raza, N., Raza, W., Gul, H., Azam, M., Lee, J., Vikrant, K., & Kim, K.-H. (2020b). Solar-light-active silver phosphate/titanium dioxide/silica heterostructures for photocatalytic removal of organic dye. Journal of Cleaner Production, 254, 120031. https://doi.org/10.1016/j.jclepro.2020.120031
Riaz, M., Khan, N., Khan, S. A., Ahmad, Z., Khan, M. A., Iqbal, M., Hemeg, H. A., Bakhsh, E. M., & Khan, S. B. (2022). Enhanced catalytic reduction/degradation of organic pollutants and antimicrobial activity with metallic nanoparticles immobilized on copolymer modified with NaY zeolite films. Journal of Molecular Liquids, 359, 119246. https://doi.org/10.1016/j.molliq.2022.119246
Roghanizad, A., Abdolmaleki, M. K., Ghoreishi, S. M., & Dinari, M. (2020). One-pot synthesis of functionalized mesoporous fibrous silica nanospheres for dye adsorption: isotherm, kinetic, and thermodynamic studies. Journal of Molecular Liquids, 300, 112367.
Ruan, C., Ma, Y., Shi, G., He, C., Du, C., Jin, X., et al. (2022). Self-assembly cellulose nanocrystals/SiO2 composite aerogel under freeze-drying: Adsorption towards dye contaminant. Applied Surface Science, 592, 153280. https://doi.org/10.1016/j.apsusc.2022.153280
Ruello, J. L. A., & Kim, H. (2022). Microwave-induced fabrication of fiber-reinforced adsorbent from waste cardboard and chitosan for effective dye removal. Journal of Environmental Chemical Engineering, 10(6), 108724. https://doi.org/10.1016/j.jece.2022.108724
Sboui, N., Agougui, H., Jabli, M., & Boughzala, K. (2022). Synthesis, physico-chemical, and structural properties of silicate apatites: Effect of synthetic methods on apatite structure and dye removal. Inorganic Chemistry Communications, 142, 109628. https://doi.org/10.1016/j.inoche.2022.109628
Sharma, S., Sharma, G., Kumar, A., TS, A. G., Naushad, M., ZA, A. L. O., & Stadler, F. J. (2022). Adsorption of cationic dyes onto carrageenan and itaconic acid-based superabsorbent hydrogel: Synthesis, characterization and isotherm analysis. Journal of Hazardous Materials, 421, 126729. https://doi.org/10.1016/j.jhazmat.2021.126729
Shu, X., Chen, Y., Yuan, H., Gao, S., & Xiao, D. (2007). H2O2 sensor based on the room-temperature phosphorescence of nano TiO2/SiO2 composite. Analytical Chemistry, 79(10), 3695–3702. https://doi.org/10.1021/ac0624142
Tang, Z., Hu, X., Ding, H., Li, Z., Liang, R., & Sun, G. (2021). Villi-like poly(acrylic acid) based hydrogel adsorbent with fast and highly efficient methylene blue removing ability. Journal of Colloid and Interface Science, 594, 54–63. https://doi.org/10.1016/j.jcis.2021.02.124
Veregue, F. R., Lima, H., Ribeiro, S. C., Almeida, M. S., & Rinaldi, A. W. (2020). MCM-41/chondroitin sulfate hybrid hydrogels with remarkable mechanical properties and superabsorption of methylene blue. Carbohydrate Polymers, 247, 116558.
Vilar, V. J. P., Botelho, C. M. S., & Boaventura, R. A. R. (2005). Influence of pH, ionic strength and temperature on lead biosorption by Gelidium and agar extraction algal waste. Process Biochemistry, 40(10), 3267–3275. https://doi.org/10.1016/j.procbio.2005.03.023
Wang, Q., Ju, J., Tan, Y., Hao, L., Ma, Y., Wu, Y., et al. (2019). Controlled synthesis of sodium alginate electrospun nanofiber membranes for multi-occasion adsorption and separation of methylene blue. Carbohydrate Polymers, 205, 125–134. https://doi.org/10.1016/j.carbpol.2018.10.023
Wang, W., Tian, G., Wang, D., Zhang, Z., Kang, Y., Zong, L., & Wang, A. (2016). All-into-one strategy to synthesize mesoporous hybrid silicate microspheres from naturally rich red palygorskite clay as high-efficient adsorbents. Scientific Reports, 6(1), 39599.
Xiao, W., Jiang, X., Liu, X., Zhou, W., Garba, Z. N., Lawan, I., et al. (2021). Adsorption of organic dyes from wastewater by metal-doped porous carbon materials. Journal of Cleaner Production, 284, 124773. https://doi.org/10.1016/j.jclepro.2020.124773
Xiao, Z., Wu, R., Shu, T., Wang, Y., & Li, L. (2023). Synthesis of Co-doped Fe metal–organic framework MIL-101(Fe,Co) and efficient degradation of organic dyes in water. Separation and Purification Technology, 304, 122300. https://doi.org/10.1016/j.seppur.2022.122300
Zhang, H., Peng, B., Liu, Q., Wu, C., & Li, Z. (2022). Preparation of porous biochar from heavy bio-oil for adsorption of methylene blue in wastewater. Fuel Processing Technology, 238, 107485. https://doi.org/10.1016/j.fuproc.2022.107485
Zhao, L., Wang, D., Gao, J. S., & Xu, C. M. (2005). Quantum chemical study of TiO2-SiO2 composite oxide structure and infrared spectra. Journal of Catalysis, 026(001), 15–19.
Zhao, Y., Gong, B., Liu, D., et al. (2017). Synthesis of chitosan-functionalized MCM-41-A and its performance in Pb(II) removal from synthetic water. Journal of the Taiwan Institute of Chemical Engineers, 71, 537–545.
Zhou, C., Gao, Q., Luo, W., Zhou, Q., Wang, H., Yan, C., & Duan, P. (2015). Preparation, characterization and adsorption evaluation of spherical mesoporous Al-MCM-41 from coal fly ash. Journal of the Taiwan Institute of Chemical Engineers, 52, 147–157. https://doi.org/10.1016/j.jtice.2015.02.014
Funding
This study was financially supported by the Natural Science Foundation of Chongqing (cstc2020jscx-msxmX0059) and the domestic waste resource treatment, provincial and ministerial, co-construction Collaborative Innovation Center Project of Chongqing University of Science and Technology (shljzyh2021-08).
Author information
Authors and Affiliations
Contributions
Huishan Cai: methodology, investigation, writing—original draft, writing—review and editing. Lin Zhang: methodology, writing—review and editing. Chengwei Zuo: writing—review and editing. Yuan Wei: writing—review and editing. Hao Wang: writing—review and editing. Zhenfu Jia: funding acquisition and supervision. Xiaodong Su: conceptualization, writing—review and editing, funding acquisition, and supervision.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cai, H., Zhang, L., Zuo, C. et al. One-Step Synthesis of Recyclable Ti-Doped Porous SiO2 Microspheres with Superior Structural Stability for Cationic Dye Adsorption. Water Air Soil Pollut 234, 345 (2023). https://doi.org/10.1007/s11270-023-06375-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06375-9