Abstract
In the present report, the oxidative degradation of an endocrine-disrupting compound triadimenol has been investigated using the photo-Fenton reaction. Different reaction conditions that affected the degradation kinetics were examined including the iron (Fe2+) ions and hydrogen peroxide (H2O2) concentrations, the initial concentration of triadimefon, and the type of iron salt. The degradation rates proved to be strongly influenced by the above parameters. The reaction conditions in terms of Fe2+ and H2O2 concentrations were systematically studied and optimized using an experimental design through central composite design. The kinetics of the process can be characterized as pseudo-first-order. As iron and hydrogen peroxide concentrations increase, the degradation rate of triadimenol accelerates and complete degradation can be achieved after a short time of illumination. The effectiveness of the five different iron salts on the degradation performance were also investigated. Experiments with longer time illumination can lead to complete mineralization and detoxification of triadimenol solutions. The properties of the proposed oxidative degradation scheme make it a promising candidate for wastewater treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Environmental pollution is a pervasive problem with widespread ecological consequences. For this reason, many substances have been characterized by US EPA as priority pollutants (Malato et al., 2009). In the last decades, one of the most important class of pollutants, endocrine-disrupting chemicals, has received a lot of attention (Chihiro et al., 2003). An endocrine-disrupting chemical is an exogenous substance that causes adverse health effects in an organism, or its progeny, and can damage the endocrine function (Voulvoulis et al., 2008). In this class belong a wide range of substances such as pesticides, fungicides, and phthalates.
Fungicides are a group of chemicals which are used to protect crops such as soft fruits from fungal attack (Albanis et al., 2000). In the last years, large amounts of fungicides have been applied in the Mediterranean region. In Greece, for example in 1987, they used 3,600,000 kg of fungicides while in USA each year more than 73 million pounds is applied (Baird & DeLorenzo, 2010). As a result of their extensive use, fungicide residues can be found in surface waters such as rivers. Lately, some fungicides have found use as alternatives in paints, in order to protect boats from algae and seaweeds.
One main class of fungicides is triazoles. Conazoles are systemic fungicides which belong to the family of triazoles. These compounds are characterized by a five-membered nitrogen heterocyclic ring (Baird & DeLorenzo, 2010). Conazoles protect crops by inhibiting the biosynthesis of ergosterol, which role is to maintain the fluidity in fungal cells. From the family of conazole fungicides, the two most important members with wide use are triadimefon [1-(4-chlorophenoxy)-3,3-dimethyl-1-(1H-1,2,4,-triazol-1-yl)-2-butanone] and triadimenol [β-(4-chlorophenoxy)-a-(1–1-dimethyl-1H-1,2,4,-triazole-1-ethanol] (Fig. 1). These compounds have chiral centers and consist of one and two pairs of enantiomers (Del Nozal et al., 2003). Triadimefon blocks fungal attack through his biotransformation into triadimenol.
Triadimenol is a metabolite of triadimefon and has curative and eradicative effect (Da Silva et al., 2001). It is typically used to control the powdery mildews in cereals, fruits, tomatoes, and ornamentals (Du et al., 2007).
Traditional methods of water disinfection typically involved treatment methods which are often chemically, energetically, and operationally intensive, and have considerable cost, engineering expertise, and infrastructure, all of which precludes their worldwide use. Additionally, chemical treatments (i.e., using NH3, chlorine compounds, HCl, NaOH, O3, permanganate, ferric salts, anti-scalants, corrosion control chemicals, and ion exchange resins) and their residuals (sludge, toxic waste) can increase the problems regarding to the contamination and salting of freshwater sources (Malato et al., 2009). In order to remove or to degrade these substances, new methods were applied, known as advanced oxidation processes (AOPs).
Advanced oxidation processes can be used for water decontamination comprising organic pollutants, diversified to bio-recalcitrant and/or for disinfection removing current and emerging pathogens. These methods rely on the formation of highly reactive chemical species which degrade even the most recalcitrant molecules into biodegradable compounds. More specifically, they are based on the formation of hydroxyl radicals (·OH), which can act as high oxidative species (oxidation potential 2.80 V) and can be generated through different pathways (Byrne et al., 2015; Malato et al., 2009). Some systems which belong to AOPs are Fenton reaction (Fe2+/H2O2), photo-Fenton reaction (Fe3+/H2O2/hv), UV/H2O2, O3/H2O2, and O3/UV (Cavalcanti et al., 2021; de Oliveira Marques Cavalcanti et al., 2022; Santana et al., 2021; Von Sonntag, 2008).
In Fenton reaction, hydrogen peroxide is decomposed in water and oxygen in the presence of iron ions in aqueous solutions which produces ·OH stoichiometrically and results in oxidation of the Fe(II) to Fe(III) (Malato et al., 2009). The reaction which takes place is described below:
The main benefit of Fenton’s reagent over other HO· systems is its lower cost compared to TiO2 particles or O3 generators. The use of light-induced reactions in water treatments has drawn increasing attention recently. The photo-assisted Fenton reaction naturally provides faster kinetics and an increased mineralization level than the thermal reaction in the absence of light. In photo-Fenton reaction, the use of light (λ < 500 nm) accelerates the formation of hydroxyl radicals through the following reaction that regenerates Fe2+ ions and turns the process to catalytic:
In the present study, the oxidative degradation of triadimenol using photo-Fenton reaction is investigated here for the first time. The photo-Fenton reaction has been used for the homogeneous treatment of polluted aqueous solutions. Various parameters (such as Fe2+ and H2O2 concentration, the type of iron salt) that affected the degradation kinetics were systematically examined and optimized using central composite design. The main goals of the present study were (i) to investigate the kinetics of the fungicide disappearance in aqueous solution and (ii) to study the degree of mineralization.
2 Experimental
2.1 Reagents, solutions, and materials
Triadimenol was of analytical grade (> 95%) and purchased from Sigma-Aldrich (Chemie GmbH, Steinheim, Germany). HPLC-grade solvents, acetonitrile, and water were supplied by Merck. Hydrogen peroxide (H2O2, 30%) was supplied by Panreac (Quimica S.L.U., Castellar del Vallès, Barcelona, Spain). Iron salts including Fe2(SO4)3, FeCl3·6H2O, and Fe(NO3)3·9H2O were supplied by Merck, while FeSO4 and Fe(NH4)2(SO4)2 by Panreac (Quimica S.L.U., Castellar del Vallès, Barcelona, Spain) and Fe(ClO4)3 by Sigma0Aldrich (Chemie GmbH, Steinheim, Germany). Stock solutions of triadimenol at concentration of 50 mg L−1 and all solutions for the experiments were prepared in deionized ultrapure water protected from the light and stored at 4 °C. Water was produced by Direct-Q Millipore purification water system (Bedford, MA).
2.2 Analytical Instrumentation and Conditions
All analyses were carried out on Waters Acquity UPLC-PDA System (Waters, Manchester, UK) equipped with a thermostated autosampler and column oven. The analytical column consisted of a Waters Acquity UPLC BEH C18 column (50 × 2.1 mm, 1.7 μm) (Milford, MA, USA) using a flow rate of 0.35 mL min−1. The elution of the compounds was performed isocratically using a mixture of water:CH3OH, 60:40% v/v, respectively. The column temperature was set constant at 25 °C. The injection volume was 5 μL and the detection of the analyte was performed at 224 nm.
The dissolved organic carbon (DOC) was determined by Shimadzu V-csh TOC analyzer (Kyoto, Japan) and for the examination of the toxic properties of the sample’s measurements were carried out using MICROTOX test measuring the luminescence of bacteria V. fisheri at 5 and 15 min of time exposure. For this test, the salinity must be 2% and for this reason it was necessary the adjustment of the pH (7.0 ± 1.0) and of the osmotic pressure.
2.3 Experimental Procedure
The experiments were performed using a 500-mL Pyrex UV reactor equipped with a 125-W high-pressure mercury lamp surrounded by a Pyrex filter blocking wavelengths below 290 nm. A tap water cooling circuit was employed to kept the temperature at the range of 30–35 °C. There was no extra purge of air or oxygen in the reactor. The initial triadimenol concentration was 20 mg L−1. Samples were withdrawn from the reactor at certain time intervals and the triadimenol concentration was determined by UHPLC-PDA using a C18 BEH analytical column (50 × 2.1 mm, 1.7 μm). The elution of the compound was performed using a mixture of H2O:MeOH, 60:40% v/v.
In the preliminary experiments, four systems namely (i) UV irradiation, (ii) Fe3+ combined with UV-A, (iii) H2O2 combined with UV-A, and (iv) Fe3+, H2O2 combined with UV-A, were investigated (Chiou et al., 2006). The concentrations of Fe3+ and H2O2 were 1 and 20 mg L−1, respectively.
3 Results and Discussion
3.1 Primary Degradation—Kinetics
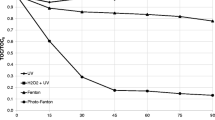
The photodegradation of triadimenol in the presence of the semiconductors is shown in Fig. 2. Four different well-known systems (UV, Fe(III)/UV, H2O2/UV, and Fe(III)/H2O2/UV) were studied. As derived from Fig. 2, photo-Fenton system provided the best performance, because almost complete degradation of the initial compound after 30 min of light exposure is achieved. In photo-Fenton system, the use of light increases the generation of hydroxyl radicals, which are the main oxidizing species as already mentioned above.
Observed degradation rate constants were estimated by fitting the plots (concentrations vs time) to a pseudo-first-order kinetic model, as described by the following equation:
where C (mol L−1) is the concentration of the triadimenol, Co is the initial concentration, and kobs (min−1) is the observed reaction rate constant. The apparent first order constant k was found to be 0.35% min−1, while half-time (t1/2) was estimated to 2.0 min (R2 = 0.93).
3.2 Effect of Iron Salt Type
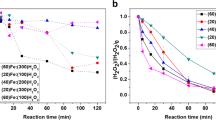
Different iron salts can be used as the source of Fe3+ for the photo-Fenton reaction, and the effect of the anions to degradation process was examined. It should be underlined that the regulation of pH of the solution was performed with the acid containing the same anion as the salt used (i.e., in the case of Fe(NO3)3, the acidification was made by HNO3). Experiments with different iron salts were performed and the results are illustrated in Fig. 3. The experiments indicated that faster decomposition was achieved with iron nitrate, Mohr’s salt, and iron sulfate, because in the case of chloride salt, chloride anions can act as hydroxyl radical scavengers. The effectiveness of the different salts is given below:
According to the literature, the temperature is a vital parameter of the photo-Fenton process efficiency (Pignatello et al., 2007). Generally, the efficiency is progressively enhanced at elevated temperature resulting to faster regeneration of ferrous allowing Fe2+ available to produce ·OH. However, higher temperatures led to a detrimental loss of iron due its precipitation (Zapata et al., 2009). To avoid the above issue, the temperature was controlled about 30–35 °C during the process.
3.3 Effect of Iron and Hydrogen Peroxide Concentrations—Optimization Using Central Composite Design
Two of the most important parameters affecting the efficiency of photo-Fenton system are iron and hydrogen peroxide concentration. For this purpose, preliminary experiments were conducted with different concentrations of iron ions and hydrogen peroxide. The results are shown in Fig. 4. Obviously, as the iron concentration increases, the degradation rate of triadimenol is faster. This behavior could be expected, since the concentration of hydroxyl radicals, which is the main oxidizing agent, is directly combined to the concentration of Fe3+ (Bizani et al., 2014). An apparent optimal concentration of iron ions would be the 1 mg L−1. As the major disadvantage of photo-Fenton system is the iron removal at the end of the treatment, the optimum approach would have the lowest residual concentration of the pesticide with the least iron concentration. As hydrogen peroxide is concerned, the degradation rate is faster as its concentration of increases up to concentration level of 20 mg L− and leveled-off. Thereafter, a slight decrease was observed due to that hydrogen peroxide can act as hydroxyl radical’s scavenger under these experimental conditions (Parra et al., 2000).
In order to further investigate and optimize both concentrations of iron and H2O2 on photo-Fenton efficiency, a central composite design (CCD) was applied. The CCD is a widely used design typically for quadratic models fitting that combines a two-level factorial design with axial points (star points) and at least one point at the center of the experimental region in order to fit quadratic polynomials (Hibbert, 2012; Stalikas et al., 2009). This design has some characteristics (Bezerra et al., 2008; Manousi et al., 2022; Tsanaktsidou et al., 2022; Zacharis & Vastardi, 2018): (i) it requires a number of experiments according to the equation of N = k2 + 2 k + Cp, where k is the number of the factors and Cp is the central point replicates, (ii) α-values depend on the number of variables and can be calculated from the type α = 2(k-p)/4, and (iii) all factors must be studied in five levels (− α, − 1, 0, + 1, + α). Center points are usually repeated to get a good estimation of experimental error (pure error). The value of “a” needed to ensure orthogonality and rotatability can be calculated from the following equation. In our case, eleven experiments were carried out. The dependent variables are the remaining percent of compound and the dissolve organic carbon (DOC%) remaining after 1 and 60 min of illumination, respectively. The concentration range of iron was set between 0.5 and 5 mg L−1 and for hydrogen peroxide 5–120 mg L−1, respectively. The Design Expert 7.1.6. (trial version, Stat Ease) software statistic tool was used to analyze the CCD and to plot the response surfaces. The factorial design points are tabulated in Table 1.
Figure 5 shows the response surfaces and the contour plots built up with the experimental data of Table 1. These plots show the effect of hydrogen peroxide and iron ion concentration on the compound decomposition and DOC removal after 1 and 60 min of illumination, respectively. According to Fig. 5A, it can be concluded as iron concentration increases less compound remains. On the other hand, when iron and hydrogen peroxide concentration increase further than optimal values, a negative effect appears on the remaining percent of DOC (Fig. 5B). According to CCD for the decomposition of triadimenol, the proposed model is linear and for DOC removal is quadratic. The calculated R2 of the models was higher than 0.7477 indicating that the predicted models can explain adequately the responses. The models were statistically validated using ANOVA (Online resource 1). The p-value of “lack-of-fit” (LOF) was higher than 0.05 and therefore non-significant associated to the pure error (at 95% probability level). Diagnostic plots (i.e., normal probability plot of residuals, plot of residuals against to the predicted values) are shown in Fig. 6. The data were monotonously dispersed around the line; thus, there is a good correlation between the predicted and actual responses.
As far as the disappearance of triadimenol is concerned, the proposed model can be described as:
The optimum concentrations suggested from the statistical analysis are 5 and 120 mg L−1 for [Fe2+] and [H2O2], respectively. The estimated remaining triadimenol % (after 1 min of illumination) was 13.43%.
In the case of DOC removal, the corresponding model can be expressed by the following equation:
The optimum conditions for the DOC removal were 1.2 and 35 mg L−1 for the concentration of iron and H2O2, respectively. Under these experimental conditions, the remaining DOC % after 60 min of illumination was calculated to be 8.8%.
3.4 Mineralization—Toxicity Evaluation
Two of the main goals of AOPs are the mineralization of the substance and the decomposition into less toxic by-products. In order to evaluate the degree of mineralization, longer period experiments were performed, in which dissolved organic carbon (DOC) was measured. For the estimation of toxicity, the luminescent bacteria V. fisheri was used. The results are shown in Fig. 7. After 90 min of illumination, complete removal of triadimenol was achieved, while DOC removal was 80% but after 3 h complete mineralization could be accomplished. The estimation of toxicity shows that triadimenol is not a very toxic compound in the beginning and after 2 h of illumination formulation of by-products with no toxicity was observed.
4 Conclusions
In this study, the degradation of the endocrine-disrupting compound triadimenol using the photo-Fenton reaction was investigated. The conclusions of this research can be summarized as follows:
-
i)
Photo-Fenton system is very effective for the decomposition of triadimenol. Almost complete degradation after 30 min of illumination was achieved.
-
ii)
Iron and hydrogen peroxide concentration are two main parameters affecting the efficiency of photo-Fenton system. As iron concentration increases, the degradation rate is faster and it is better to add adequate quantity of H2O2 in order to keep it stable.
-
iii)
Central composite design shows that incensement of iron and hydrogen peroxide concentration up to a point accelerates the degradation rate of photo-Fenton reaction. Same conclusion arises when these parameters were investigated with univariate analysis. The best model for the remaining percent of compound is the linear while for the remaining percent of DOC is the quadratic. Both univariate and multivariate analyses show that iron concentration is a more important parameter than hydrogen peroxide concentration.
-
iv)
Experiments with different salts as iron source were carried out. Best results were got with Fe(NO3)3, because chloride ions can act as hydroxyl radical scavengers and sulfate ions can make complexes with iron ions.
-
v)
Triadimenol is not a very toxic compound. After 3 h of illumination, formation of no toxic by-products and complete mineralization of target compound is observed.
The present photo-oxidative method is considered acceptable and efficient for the treatment of triadimenol-contaminated water by reducing the operating costs and the prolonged duration of the treatment.
Data Availability
All the data associated with this paper are presented in the form of tables and figures.
References
Albanis T. A., Lambropoulou D. A. & Konstantinou I. K. (2000). Determination of fungicides in natural waters using solid-phase microextraction and gas chromatography coupled with electron-capture and mass spectrometric detection. Journal of Chromatography A, 893, 143–156. https://doi.org/10.1016/s0021-9673(00)00750-0
Baird, T. D., & DeLorenzo, M. E. (2010). Descriptive and mechanistic toxicity of conazole fungicides using the model test alga Dunaliella tertiolecta (chlorophyceae). Environmental Toxicology, 25(3), 213–220. https://doi.org/10.1002/TOX.20493
Bezerra, M. A., Santelli, R. E., Oliveira, E. P., Villar, L. S., & Escaleira, L. A. (2008). Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta, 76(5), 965–977. https://doi.org/10.1016/j.talanta.2008.05.019
Bizani, E., Lambropoulou, D., Fytianos, K., & Poulios, I. (2014). Photocatalytic degradation of molinate in aqueous solutions. Environmental Science and Pollution Research, 21(21), 12294–12304. https://doi.org/10.1007/S11356-014-3086-2/FIGURES/7
Byrne, J. A., Dunlop, P. S. M., Hamilton, J. W. J., Fernández-Ibáñez, P., Polo-López, I., Sharma, P. K., & Vennard, A. S. M. (2015). A review of heterogeneous photocatalysis for water and surface disinfection. Molecules (basel, Switzerland), 20(4), 5574–5615. https://doi.org/10.3390/MOLECULES20045574
Cavalcanti, V. de O. M., Santana, R. M. R., Neves, N. S. da C. S., de Lucena, A. L. A., de Oliveira, M. A. S., do Nascimento, G. E., & Napoleão, D. C. (2021). Treatment of the drugs atenolol and propranolol by advanced oxidation processes, a kinetic approach, toxicity effects on seeds, and chromatographic analysis. Chemical Papers, 75(8), 4391–4403https://doi.org/10.1007/S11696-021-01667-Y
Chiou, C. S., Chen, Y. H., Chang, C. T., Chang, C. Y., Shie, J. L., & Li, Y. S. (2006). Photochemical mineralization of di-n-butyl phthalate with H2O2/Fe3+. Journal of Hazardous Materials, 135(1–3), 344–349. https://doi.org/10.1016/J.JHAZMAT.2005.11.072
Chihiro O., Hisao Y., Masakazu H., Kenzi S. & Tadashi H. (2003). Adsorptive and photocatalytic performance of TiO2 pillared montmorillonite in degradation of endocrine disruptors having different hydrophobicity. Applied CatalysisB: Environmental, 41, 313–321. https://doi.org/10.1016/S0926-3373(02)00169-8
Da Silva, J. P., Da Silva, A. M., Khmelinskii, I. V., Martinho, J. M. G., & Vieira Ferreira, L. F. (2001). Photophysics and photochemistry of azole fungicides: Triadimefon and triadimenol. Journal of Photochemistry and Photobiology a: Chemistry, 142(1), 31–37. https://doi.org/10.1016/S1010-6030(01)00489-0
de Oliveira Marques Cavalcanti, V., da Rocha Santana, R. M., Silva, F. S., de Lucena, A. L. A., Lima, V. E., de Melo Neto, A. A., et al. (2022). Degradation of mixtures of pressure-regulating drugs present in different matrices using magnetite/Fenton. Chemical Papers https://doi.org/10.1007/S11696-022-02304-Y
Del Nozal, M. J., Toribio, L., Bernal, J. L., & Castaño, N. (2003). Separation of triadimefon and triadimenol enantiomers and diastereoisomers by supercritical fluid chromatography. Journal of Chromatography A, 986(1), 135–141. https://doi.org/10.1016/S0021-9673(02)01920-9
Du, F., Luo, X., Jiang, G., Hou, S., Liu, G., Ren, L., et al. (2007). Determination of triadimenol based on the quenching effect on resonance light scattering from the triadimenol-deoxyribonucleic acid-hydrochloric acid system. Analytical and Bioanalytical Chemistry, 388(2), 489–493. https://doi.org/10.1007/S00216-007-1218-Y
Hibbert, D. B. (2012). Experimental design in chromatography: A tutorial review. Journal of Chromatography B, 910, 2–13. https://doi.org/10.1016/j.jchromb.2012.01.020
Malato, S., Fernández-Ibáñez, P., Maldonado, M. I., Blanco, J., & Gernjak, W. (2009). Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catalysis Today, 147(1), 1–59. https://doi.org/10.1016/J.CATTOD.2009.06.018
Manousi, N., Vlachaki, A., Kika, F. S., Markopoulou, C. K., Tzanavaras, Paraskevas, D., & Zacharis, C. K. (2022). Salting‐out homogeneous liquid liquid microextraction for the determination of azole drugs in human urine: Validation using total error concept. Journal of Separation Science https://doi.org/10.1002/jssc.202100942
Parra, S., Sarria, V., Malato, S., Péringer, P., & Pulgarin, C. (2000). Photochemical versus coupled photochemical–biological flow system for the treatment of two biorecalcitrant herbicides: Metobromuron and isoproturon. Applied Catalysis b: Environmental, 27(3), 153–168. https://doi.org/10.1016/S0926-3373(00)00151-X
Pignatello, J. J., Oliveros, E., & MacKay, A. (2007). Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. 36(1), 1–84 10.1080/10643380500326564
Santana, R. M. R., Napoleão, D. C., dos Santos Júnior, S. G., Gomes, R. K. M., de Moraes, N. F. S., Zaidan, L. E. M. C., et al. (2021). Photo-Fenton process under sunlight irradiation for textile wastewater degradation: Monitoring of residual hydrogen peroxide by spectrophotometric method and modeling artificial neural network models to predict treatment. Chemical Papers, 75(6), 2305–2316. https://doi.org/10.1007/S11696-020-01449-Y
Stalikas, C., Fiamegos, Y., Sakkas, V., & Albanis, T. (2009). Developments on chemometric approaches to optimize and evaluate microextraction. Journal of Chromatography A, 1216(2), 175–189. https://doi.org/10.1016/j.chroma.2008.11.060
Tsanaktsidou, E., Markopoulou, C. K., Tzanavaras, P. D., & Zacharis, C. K. (2022). Homogeneous liquid phase microextraction using hydrophilic media for the determination of fluoroquinolones in human urine using HPLC-FLD. Microchemical Journal, 172(PA), 106906. https://doi.org/10.1016/j.microc.2021.106906
Voulvoulis N., McKinlay R., Plant J. A. & Bell J. N. B. (2008). Endocrine disrupting pesticides: Implications for risk assessment. Environment International , 34, 168–183. https://doi.org/10.1016/j.envint.2007.07.013
Von Sonntag, C. (2008). Advanced oxidation processes: Mechanistic aspects. Water Science and Technology : A Journal of the International Association on Water Pollution Research, 58(5), 1015–1021. https://doi.org/10.2166/WST.2008.467
Zacharis, C. K., & Vastardi, E. (2018). Application of analytical quality by design principles for the determination of alkyl p-toluenesulfonates impurities in Aprepitant by HPLC. Validation using total-error concept. Journal of Pharmaceutical and Biomedical Analysis, 150, 152–161. https://doi.org/10.1016/J.JPBA.2017.12.009
Zapata, A., Oller, I., Bizani, E., Sánchez-Pérez, J. A., Maldonado, M. I., & Malato, S. (2009). Evaluation of operational parameters involved in solar photo-Fenton degradation of a commercial pesticide mixture. Catalysis Today, 144(1–2). https://doi.org/10.1016/j.cattod.2008.12.030
Acknowledgements
The authors wish to thank the “Stavros Niarchos Foundation” for funding the acquisition of the Acquity UPLC-PDA instrument.
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gkorgkolia, C., Fytianos, K. Degradation of the Endocrine-Disrupting Compound Triadimenol Using Photo-Fenton Reaction System and Experimental Design. Water Air Soil Pollut 234, 57 (2023). https://doi.org/10.1007/s11270-023-06078-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06078-1