Abstract
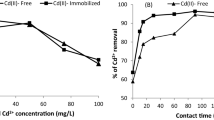
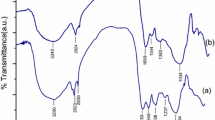
The development of heavy metal treatment technologies plays a crucial role in avoiding contamination of water bodies. Nannochloropsis oculata biomass was used for Pb2+ and Cd2+ biosorption. The biosorption capacity (g metal × g biomass−1) was determined by evaluating the effects of pH and biomass amount. The maximum biosorption capacities for Pb2+ and Cd2+ were 1087.20 ± 9.12 mg g−1 and 934.44 ± 12.84 mg g−1, respectively. The pH for the highest biosorption of Pb2+ was 5.0, and it was 4.0 for Cd2+. The optimal amount of biomass needed to remove 100 ppm Pb2+ was 0.05 g, and it was 0.3 g for 100 ppm Cd2+, which suggests that microalgae showed greater capacity for removal of Pb2+ than Cd2+. The sorption rates for Cd2+ and Pb2+ were fitted with the pseudo-second-order kinetic model, and qe values of 94.33 mg g−1 and 88.49 mg g−1, respectively, were obtained. Cd2+ reached equilibrium in the medium faster than Pb2+. The mechanism for adsorption of Pb2+ and Cd2+ is not controlled by intraparticular or film diffusion. FTIR results showed that Pb2+ and Cd2+ occupy the same carboxyl, amide, and hydroxyl functional groups.
Highlights
-
Lead was adsorbed at higher pH than cadmium.
-
Cadmium and lead were adsorbed in the same functional groups.
-
A loss in adsorption efficiency phenomenon was produced with increasing biomass of N. oculata concentration.
-
The pseudo-second-order kinetic model best described lead and cadmium removal.
-
Chemical adsorption was the rate-controlling step on lead and cadmium sorption.






Similar content being viewed by others
Data Availability
The data that support the findings of this study include raw data, samples, and records and are available from the corresponding author upon reasonable request.
References
Abdel-Aty, A. M., Ammar, N. S., Abdel Ghafar, H. H., & Ali, R. K. (2013). Biosorption of cadmium and lead from aqueous solution by fresh water alga Anabaena sphaerica biomass. Journal of Advanced Research, 4(4), 367–374. https://doi.org/10.1016/j.jare.2012.07.004
Aksu, Z. (1998). Biosorption of heavy metals by microalgae in batch and continuous systems. Wastewater Treatment with Algae, (Ii), 37–53. https://doi.org/10.1007/978-3-662-10863-5_3
AOAC. (1995). Official Methods of Analysis. by Patricia A. Cunniff (Ed.), Aoac Intl, William Horwitz, 16th Edition, Association of Official Analytical Chemists, Washington DC.
AOAC. (2005). Association of Official Analytical Chemist, Official Methods of Analysis. In W. Horwitz & G. W. Latimer (Eds.), AOAC International (18th ed., pp. 20877–2417). AOAC International.
Bayramoǧlu, G., Tuzun, I., Celik, G., Yilmaz, M., & Arica, M. Y. (2006). Biosorption of mercury (II), cadmium (II) and lead (II) ions from aqueous system by microalgae Chlamydomonas reinhardtii immobilized in alginate beads. International Journal of Mineral Processing, 81(1), 35–43.
Brinza, L., Dring, M. J., & Gavrilescu, M. (2007). Marine micro and macro algal species as biosorbents for heavy metals. Environmental Engineering and Management Journal, 6(3), 237–251. https://doi.org/10.30638/eemj.2007.029
Bulgariu, D., & Bulgariu, L. (2012). Equilibrium and kinetics studies of heavy metal ions biosorption on green algae waste biomass. Bioresource Technology, 103(1), 489–493. https://doi.org/10.1016/j.biortech.2011.10.016
Bulgariu, D., & Bulgariu, L. (2016). Potential use of alkaline treated algae waste biomass as sustainable biosorbent for clean recovery of cadmium(II) from aqueous media: Batch and column studies. Journal of Cleaner Production, 112, 4525–4533. https://doi.org/10.1016/j.jclepro.2015.05.124
Campaña-Torres, A., Porchas-Cornejo, M. A., Martínez-Córdova, L. R., López-Elías, J. A., & Martínez-Porchas, M. (2012). Productive response of Nannochloropsis oculata, cultured in different media and their efficiency as food for the rotifer Brachionus rotundiformis. Phyton, 81, 45–50.
Caviedes Rubio, D. I., Muñoz Calderón, R. A., Perdomo Gualtero, A., Rodríguez Acosta, D., & Sandoval Rojas, I. J. (2015). Tratamientos para la remoción de metales pesados comúnmente presentes en aguas residuales industriales Una Revisión. Ingeniería y Región, 13(1), 73. https://doi.org/10.25054/22161325.710
Chojnacka, K., Chojnacki, A., & Górecka, H. (2005). Biosorption of Cr3+, Cd2+ and Cu2+ ions by blue-green algae Spirulina sp.: Kinetics, equilibrium and the mechanism of the process. Chemosphere, 59(1), 75–84. https://doi.org/10.1016/j.chemosphere.2004.10.005
Chu, W. L., & Phang, S. M. (2019). Biosorption of heavy metals and dyes from industrial effluents by microalgae. Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment. https://doi.org/10.1007/978-981-13-2264-8_23
Cunniff, P., & Chemists, A. of O. A. (1995). Official methods of analysis of AOAC international. Association of Official Analytical Chemists.
Das, N. (2010). Recovery of precious metals through biosorption – A review. Hydrometallurgy, 103(1–4), 180–189. https://doi.org/10.1016/j.hydromet.2010.03.016
Dirbaz, M., & Roosta, A. (2018). Adsorption, kinetic and thermodynamic studies for the biosorption of cadmium onto microalgae Parachlorella sp. Journal of Environmental Chemical Engineering, 6(2), 2302–2309. https://doi.org/10.1016/j.jece.2018.03.039
DuBois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3), 350–356. https://doi.org/10.1021/ac60111a017
Edris, G., Alhamed, Y., & Alzahrani, A. (2014). Biosorption of cadmium and lead from aqueous solutions by Chlorella vulgaris biomass: Equilibrium and kinetic study. Arabian Journal for Science and Engineering, 39(1), 87–93. https://doi.org/10.1007/s13369-013-0820-x
Fraile, A., Penche, S., González, F., Blázquez, M. L., Muñoz, J. A., & Ballester, A. (2005). Biosorption of copper, zinc, cadmium and nickel by Chlorella vulgaris. Chemistry and Ecology, 21(1), 61–75. https://doi.org/10.1080/02757540512331334933
Gilca, E., Tabaselia, C., Miclean, M., & Roman, C. (2017). Removal of Cu2+ Ions From Aqueous Solutions Using Nannochloropsis Oculata Biomass. Agricultura, 99(3–4), 64–68. https://doi.org/10.15835/agrisp.v99i3-4.12679
Gong, R., Ding, Y., Liu, H., Chen, Q., & Liu, Z. (2005). Lead biosorption and desorption by intact and pretreated Spirulina maxima biomass. Chemosphere, 58(1), 125–130. https://doi.org/10.1016/j.chemosphere.2004.08.055
Gu, S., & Lan, C. Q. (2021). Biosorption of heavy metal ions by green alga Neochloris oleoabundans: Effects of metal ion properties and cell wall structure. Journal of Hazardous Materials, 418(April), 126336. https://doi.org/10.1016/j.jhazmat.2021.126336
Guevara-García, Á. A., Lara, F. P., Juárez, L. K., & Herrera-Estrella, L. R. (2017). Heavy metal adaptation. eLS. John Wiley & Sons, Ltd.
Gupta, V. K., & Rastogi, A. (2008). Equilibrium and kinetic modelling of cadmium(II) biosorption by nonliving algal biomass from Oedogonium sp. aqueous phase. Journal of Hazardous Materials, 153(1–2), 759–766. https://doi.org/10.1016/j.jhazmat.2007.09.021
Inthorn, D., Sidtitoon, N., Silapanuntakul, S., & Incharoensakdi, A. (2002). Sorption of mercury, cadmium and lead by microalgae. ScienceAsia, 28(3), 253. https://doi.org/10.2306/scienceasia1513-1874.2002.28.253
Kaparapu, J., & Prasad, K. M. (2018). Equilibrium, kinetics and thermodynamic studies of cadmium(II) biosorption on Nannochloropsis oculata. Applied Water Science, 8(6), 1–9. https://doi.org/10.1007/s13201-018-0810-y
Khan, T. A., Mukhlif, A. A., Khan, E. A., & Sharma, D. K. (2016). Isotherm and kinetics modeling of Pb(II) and Cd(II) adsorptive uptake from aqueous solution by chemically modified green algal biomass. Modeling Earth Systems and Environment, 2(3), 1–13. https://doi.org/10.1007/s40808-016-0157-z
Kim, E. J., Park, S., Hong, H. J., Choi, Y. E., & Yang, J. W. (2011). Biosorption of chromium (Cr(III)/Cr(VI)) on the residual microalga Nannochloropsis oculata after lipid extraction for biodiesel production. Bioresource Technology, 102(24), 11155–11160. https://doi.org/10.1016/j.biortech.2011.09.107
Kumar, S. K., Dahms, H. U., Won, E. J., Lee, J. S., & Shin, K. H. (2015). Microalgae – A promising tool for heavy metal remediation. Ecotoxicology and Environmental Safety, 113, 329–352. https://doi.org/10.1016/j.ecoenv.2014.12.019
Leong, Y. K., & Chang, J. S. (2020). Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresource Technology. https://doi.org/10.1016/j.biortech.2020.122886
Liao, Q., Chang, H.-X.X., Fu, Q., Huang, Y., Xia, A., Zhu, X., & Zhong, N. (2018). Physiological-phased kinetic characteristics of microalgae Chlorella vulgaris growth and lipid synthesis considering synergistic effects of light, carbon and nutrients. Bioresource Technology, 250, 583–590. https://doi.org/10.1016/j.biortech.2017.11.086
Manzoor, F., Karbassi, A., & Golzary, A. (2019). Removal of heavy metal contaminants from wastewater by using Chlorella vulgaris Beijerinck: A review. Current Environmental Management, 6(3), 174–187. https://doi.org/10.2174/2212717806666190716160536
Martínez-Macias, R., Meza-Escalante, E., Serrano-Palacios, D., Gortáres-Moroyoqui, P., Ruíz-Ruíz, P. E., & Ulloa-Mercado, G. (2018). Effect of fed-batch and semicontinuous regimen on Nannochloropsis oculata grown in different culture media to high-value products. Journal of Chemical Technology and Biotechnology, 93(2), 585–590. https://doi.org/10.1002/jctb.5405
Martínez-Macias, M. R., Correa-murrieta, M. A., & Villegas-peralta, Y. (2019). Uptake of copper from acid mine drainage by the microalgae Nannochloropsis oculata. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-018-3963-1
Mehta, S. K., & Gaur, J. P. (2001). Characterization and optimization of Ni and Cu sorption from aqueous solution by Chlorella vulgaris. Ecological Engineering, 18(1), 1–13. https://doi.org/10.1016/S0925-8574(00)00174-9
Mehta, S. K., & Gaur, J. P. (2005). Use of algae for removing heavy metal ions from wastewater: Progress and prospects. Critical Reviews in Biotechnology, 25(3), 113–152. https://doi.org/10.1080/07388550500248571
Mirghaffari, N., Moeini, E., & Farhadian, O. (2015). Biosorption of Cd and Pb ions from aqueous solutions by biomass of the green microalga, Scenedesmus quadricauda. Journal of Applied Phycology, 27(1), 311–320. https://doi.org/10.1007/s10811-014-0345-z
Moreno-Rivas, S. C., & Ramos-Clamont Montfort, G. (2018). Descontaminación de arsénico, cadmio y plomo en agua por biosorción con Saccharomyces cerevisiae. TIP Revista Especializada en Ciencias Químico-Biológicas, 21, 51. https://doi.org/10.22201/fesz.23958723e.2018.0.155
Murdock, J. N., & Wetzel, D. L. (2009). FT-IR microspectroscopy enhances biological and ecological analysis of algae. Applied Spectroscopy Reviews, 44(4), 335–361. https://doi.org/10.1080/05704920902907440
Nateras-Ramírez, O., Martinez-Macias, M. R., Sánchez-Machado, D. I., López-Cervantes, J., & Aguilar-Ruiz, R. J. (2022). An overview of microalgae for Cd2+ and Pb2+ biosorption from wastewater. Bioresource Technology Reports, 17(September 2021), 100932. https://doi.org/10.1016/j.biteb.2021.100932
Papageorgiou, S. K., Katsaros, F. K., Kouvelos, E. P., Nolan, J. W., Le Deit, H., & Kanellopoulos, N. K. (2006). Heavy metal sorption by calcium alginate beads from Laminaria digitata. Journal of Hazardous Materials, 137(3), 1765–1772. https://doi.org/10.1016/j.jhazmat.2006.05.017
Rangabhashiyam, S., & Balasubramanian, P. (2019). Characteristics, performances, equilibrium and kinetic modeling aspects of heavy metal removal using algae. Bioresource Technology Reports, 5, 261–279. https://doi.org/10.1016/j.biteb.2018.07.009
Rangsayatorn, N., Upatham, E. S., Kruatrachue, M., Pokethitiyook, P., & Lanza, G. R. (2002). Phytoremediation potential of Spirulina (Arthrospira) platensis: Biosorption and toxicity studies of cadmium. Environmental Pollution, 119(1), 45–53. https://doi.org/10.1016/S0269-7491(01)00324-4
Renaud, S. M., Parry, D. L., Van Thinh, L., Kuo, C., Padovan, A., & Sammy, N. (1991). Effect of light intensity on the proximate biochemical and fatty acid composition of Isochrysis sp. and Nannochloropsis oculata for use in tropical aquaculture. Journal of Applied Phycology, 3(1), 43–53. https://doi.org/10.1007/BF00003918
Sánchez-Machado, D. I., López-Cervantes, J., Lopez-Hernandez, J., & Paseiro-Losada, P. (2004). Fatty acids, total lipid, protein, and ash contents of processed edible seaweeds. Food Chemistry, 85, 439–444. https://doi.org/10.1016/j.foodchem.2003.08.001
Sánchez-Torres, H., Juscamaita-Morales, J., Vargas-Cárdenas, J., & Oliveros-Ramos, R. (2008). Producción de la microalga Nannochloropsis oculata (Droop) Hibberd en medios enriquecidos con ensilado biológico de pescado. Ecología Aplicada, 7(1,2), 149–158.
Schmitt, D., Müller, A., Csögör, Z., Frimmel, F. H., & Posten, C. (2001). The adsorption kinetics of metal ions onto different microalgae and siliceous earth. Water Research, 35(3), 779–785. https://doi.org/10.1016/S0043-1354(00)00317-1
Gupta, S. S., & Bhattacharyya, K. G. (2011). Kinetics of adsorption of metal ions on inorganic materials: A review. Advances in Colloid and Interface Science. https://doi.org/10.1016/j.cis.2010.12.004.
Shen, Y., Li, H., Zhu, W., Ho, S. H., Yuan, W., Chen, J., & Xie, Y. (2017). Microalgal-biochar immobilized complex: A novel efficient biosorbent for cadmium removal from aqueous solution. Bioresource Technology, 244, 1031–1038. https://doi.org/10.1016/j.biortech.2017.08.085
Singh, A., Mehta, S. K., & Gaur, J. P. (2007). Removal of heavy metals from aqueous solution by common freshwater filamentous algae. World Journal of Microbiology and Biotechnology, 23(8), 1115–1120. https://doi.org/10.1007/s11274-006-9341-z
Suganya, S., Saravanan, A., Kumar, P. S., Yashwanthraj, M., Rajan, P. S., & Kayalvizhi, K. (2017). Sequestration of Pb(II) and Ni(II) ions from aqueous solution using microalga Rhizoclonium hookeri: Adsorption thermodynamics, kinetics, and equilibrium studies. Journal of Water Reuse and Desalination, 7(2), 214–227. https://doi.org/10.2166/wrd.2016.200
Sukarni, S., Hamidi, N., Yanuhar, U., & Wardana, I. N. G. (2014). Potential and properties of marine microalgae Nannochloropsis oculata as biomass fuel feedstock. International Journal of Energy and Environmental Engineering, 5(4), 279–290. https://doi.org/10.1007/s40095-014-0138-9
Tüzün, I., Bayramoǧlu, G., Yalçin, E., Başaran, G., Çelik, G., & Arica, M. Y. (2005). Equilibrium and kinetic studies on biosorption of Hg(II), Cd(II) and Pb(II) ions onto microalgae Chlamydomonas reinhardtii. Journal of Environmental Management, 77(2), 85–92. https://doi.org/10.1016/j.jenvman.2005.01.028
Kuyucak, N., & Volesky, B. (1988). Biosorbents for recovery of metals from industrial solutions. Biotechnology Letters 10, 137–142. https://doi.org/10.1007/BF01024641
Wang, J., & Chen, C. (2009). Biosorbents for heavy metals removal and their future. Biotechnology Advances, 27(2), 195–226. https://doi.org/10.1016/j.biotechadv.2008.11.002
Zeraatkar, A. K., Ahmadzadeh, H., Talebi, A. F., Moheimani, N. R., & McHenry, M. P. (2016). Potential use of algae for heavy metal bioremediation, a critical review. Journal of Environmental Management, 181, 817–831. https://doi.org/10.1016/j.jenvman.2016.06.059
Zhou, J. L., Huang, P. L., & Lin, R. G. (1998). Sorption and desorption of Cu and Cd by macroalgae and microalgae. Environmental Pollution, 101(1), 67–75. https://doi.org/10.1016/S0269-7491(98)00034-7
Zhu, C. J., & Lee, Y. K. (1997). Determination of biomass dry weight of marine microalgae. Journal of Applied Phycology, 9(2), 189–194. https://doi.org/10.1023/A:1007914806640
Acknowledgements
This work was supported by the Institute Technologic of Sonora through project promotion and supporting research development (PROFAPI, 2020).
Author information
Authors and Affiliations
Contributions
Writing – original draft preparation, methodology, and formal analysis and investigation: Omar Nateras-Ramírez; conceptualization, funding acquisition, review, and editing: Maria del Rosario Martinez-Macias; resources, review, and editing: Jaime López-Cervantes; critical supervision and revising of important intellectual content: Dalia Isabel Sánchez-Machado; review, editing, formal analysis, and investigation: Rocio Janeth Aguilar-Ruiz.
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies performed with human participants by any of the authors.
Competing Interests
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nateras-Ramírez, O., López-Cervantes, J., Sánchez-Machado, D. et al. Kinetic Modeling of Cd(II) and Pb(II) Biosorption from Aqueous Solution by Inactive Biomass of Nannochloropsis oculata Microalgae. Water Air Soil Pollut 233, 184 (2022). https://doi.org/10.1007/s11270-022-05636-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05636-3