Abstract
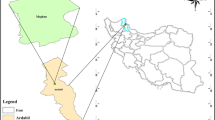
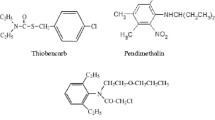
Toxicological effects of herbicides on the non-target microorganisms in the soil that play important roles in degrading organic matter, nutrient cycling, and decomposition should be considered if they were applied at higher concentrations. This study was conducted to reveal the toxicity of three modern herbicides (dicamba + tritosulfuron; DT, isoxinutole + thlencarbuone-methyl + cyprosulfamide; ITC and nicosulfuron; N) on soil carbon mineralization for short term. Recommended concentration (25 g/day for DT1, 30 ml/day for ITC1, and 125 ml/day N1), its 5 (DT5, ITC5, and N5), and 10 (DT10, ITC10, and N10) folds of herbicides were applied on a clay soil with no pesticide application history. Soil + herbicide mixes and soil without herbicide as control were incubated under constant temperature (28 °C) and moisture (80% of soil field capacity) for 42 days. In general, all doses of herbicides were significantly decreased soil carbon mineralization in short term (P < 0.05). Cumulative carbon mineralization was declined for 3.4% by DT1, for 15.0% by DT5, for 22.4% by DT10, for 13.7% by ITC1, for 14.2% by ITC5, for 18.6% by ITC10, for 4.7% by N1, for 10.0% by N5, and for 21.0% by N10, respectively, compared to control. Significant decreases were observed in the rates of carbon mineralization except DT1 and N1. Results suggested that higher doses of these herbicides negatively affected on soil microbial respiration.




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Adejoro, S. A. (2019). Biostimulatory and carbon sequestration potentials of neem seed-based fertilizer formulation in nicosulfuron contaminated soil. Journal of Agricultural Science and Practice, 4(3), 76–85. https://doi.org/10.31248/JASP2019.132
Adejoro, S. A., Adegaye, A. C., & Sonoiki, D. S. (2018). Soil microbial community response to compost addition to nicosulfuron contaminated soil. Journal of Agricultural Studies, 6(4), 49–63. https://doi.org/10.5296/jas.v6i4.13945
Aka Sagliker, H. (2018). Carbon mineralisation in orange grove soils treated with different doses of glyphosate-amine salt. Journal of Environmental Protection and Ecology, 19(3), 1102–1110. https://doi.org/10.38001/ijlsb.636695
Alef, K. (1995). Soil respiration. In K. Alef & P. Nannipieri (Eds.), Methods in Soil Microbiology and Biochemistry (pp. 214–219). Academic Press Inc. https://doi.org/10.1016/B978-0-12-513840-6.X5014-9
Bowles, T. M., Acosta-Martínez, V., Calderón, F., & Jackson, L. E. (2014). Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape. Soil Biology and Biochemistry, 68, 252–262. https://doi.org/10.1016/j.soilbio.2013.10.004
Brown, H. M., & Kearney, P. C. (1991). Plant biochemistry, environmental properties and global impact of sulfonylurea herbicides. In D. R. Baker, J. G. Fenyes, & W. K. Moberg (Eds.), Synthesis and chemistry of agrochemicals II (pp. 32–49). American Chemical Society.
Bunch, T. R; Gervais, J. A.; Buhl, K.; Stone, D. 2012. Dicamba general fact sheet. National Pesticide Information Center, Oregon State University Extension Services. Retrieved August 18, 2021 from http://npic.orst.edu/factsheets/dicamba_gen.html.
Burke, D. J., Weintraub, M. N., Hewins, C. R., & Kalisz, S. (2011). Relationship between soil enzyme activities, nutrient cycling and soil fungal communities in a northern hardwood forest. Soil Biology and Biochemistry, 43(4), 795–803. https://doi.org/10.1016/j.soilbio.2010.12.014
Denef, K., Plante, A., & Six, J. (2009). Characterization of soil organic matter. In W. L. Kutsch, M. Bahn, & A. Heinemeyer (Eds.), Soil carbon dynamics-An integrated methodology (pp. 91–126). Cambridge University Press. https://doi.org/10.1017/CBO9780511711794.007
Derksen, D. A., Anderson, R. L., Blackshaw, R. E., & Maxwell, B. (2002). Weed dynamics and management strategies for cropping systems in the northern great plains. Agronomy Journal, 94(2), 174–185. https://doi.org/10.2134/agronj2002.1740
Eser, F., Sağliker, H. A., & Darici, C. (2007). The effects of glyphosate isopropylamine and trifluralin on the carbon mineralization of olive tree soils. Turkish Journal of Agriculture and Forestry, 31(5), 297–302.
European Food Safety Authority (EFSA). (2008). Opinion on the toxicological relevance of the soil and groundwater metabolite TBSA of tritosulfuron in the context of the human risk assessment-scientific opinion of the panel on plant protection products and their residues. EFSA Journal, 6(1), 621.
European Food Safety Authority (EFSA). (2013). Conclusion on the peer review of the pesticide risk assessment of the active substance thiencarbazone-methyl. EFSA Journal, 11(7), 3270. https://doi.org/10.2903/j.efsa.2013.3270
European Food Safety Authority (EFSA). (2016). Peer review of the pesticide risk assessment of the active substance isoxaflutole. EFSA Journal, 14(2), 4416. https://doi.org/10.2903/j.efsa.2016.4416
Gul, P., Ahmad, K. S., & Gul, M. M. (2020). Herbicide thiencarbazone-methyl pedospheric disposition through sorption and degradation mechanisms in heterogenous soils. Environmental Earth Sciences, 79(20), 1–19. https://doi.org/10.1007/s12665-020-09179-w
Hechmi, S., Hamdi, H., Mokni-Tlili, S., Zoghlami, I. R., Khelil, M. N., Benzarti, S., Hassen, A., & Jedidi, N. (2020). Carbon mineralization, biological indicators, and phytotoxicity to assess the impact of urban sewage sludge on two light-textured soils in a microcosm. Journal of Environmental Quality, 49(2), 460–471. https://doi.org/10.1002/jeq2.20011
Hossain, M. B., Rahman, M. M., Biswas, J. C., Miah, M. M. U., Akhter, S., Maniruzzaman, M., Choudhury, A. K., Ahmed, F., Shiragi, M. H. K., & Kalra, N. (2017). Carbon mineralization and carbon dioxide emission from organic matter added soil under different temperature regimes. International Journal of Recycling of Organic Waste in Agriculture, 6(4), 311–319. https://doi.org/10.1007/s40093-017-0179-1
Kacar, B. (2009). Toprak Analizleri. Nobel Yayın Dağıtım.
Kocak, B., & Darici, C. (2016). Priming effects of leaves of Laurus nobilis L. and 1, 8-cineole on carbon mineralization. Chilean journal of agricultural research, 76(1), 100–104. https://doi.org/10.4067/s0718-58392016000100014
Kumar, A., Nayak, A. K., Shukla, A. K., Panda, B. B., Raja, R., Shahid, M., Tripathi, R., Mohanty, S., & Rath, P. C. (2012). Microbial biomass and carbon mineralization in agricultural soils as affected by pesticide addition. Bulletin of Environmental Contamination and Toxicology, 88(4), 538–542. https://doi.org/10.1007/s00128-012-0538-6
Ljungdahl, L. G., & Eriksson, K. E. (1985). Ecology of microbial cellulose degradation. In K. C. Marshall (Ed.), Advances in Microbial Ecology (pp. 237–299). Springer.
McFadden, M. E., & Hladik, M. L. (2021). Cyprosulfamide: Analysis of the herbicide safener and two of its degradates in surface water and groundwater from the midwestern United States. ACS Agricultural Science & Technology, 1(4), 355–361. https://doi.org/10.1021/acsagscitech.1c00050
Obriot, F., Stauffer, M., Goubard, Y., Cheviron, N., Peres, G., Eden, M., Revallier, A., Vieuble-Gonod, L., & Houot, S. (2016). Multi-criteria indices to evaluate the effects of repeated organic amendment applications on soil and crop quality. Agriculture, Ecosystems & Environment, 232, 165–178. https://doi.org/10.1016/j.agee.2016.08.004
Pallett, K. E., Little, J. P., Sheekey, M., & Veerasekaran, P. (1998). The mode of action of isoxaflutole: I. Physiological effects, metabolism, and selectivity. Pesticide Biochemistry and Physiology, 62(2), 113–124. https://doi.org/10.1006/pest.1998.2378
Perucci, P., Vischetti, C., & Battistoni, F. (1999). Rimsulfuron in a silty clay loam soil: Effects upon microbiological and biochemical properties under varying microcosm conditions. Soil Biology and Biochemistry, 31, 195–204. https://doi.org/10.1016/S0038-0717(98)00087-X
Petkova, M., Naydenov, M., Mitkov, A., Neshev, N., Yanev, M., & Tonev, T. (2020). Isolation and characterization of soil microorganisms degrading the herbicide isoxaflutol. Scientific Papers Series a. Agronomy, 63(1), 709–714.
Petric, I., Karpouzas, D. G., Bru, D., Udikovic-Kolic, N., Kandeler, E., Djuric, S., & Martin-Laurent, F. (2016). Nicosulfuron application in agricultural soils drives the selection towards NS-tolerant microorganisms harboring various levels of sensitivity to nicosulfuron. Environmental Science and Pollution Research, 23(5), 4320–4333. https://doi.org/10.1007/s11356-015-5645-6
Radivojević, L., Gašić, S., Šantrić, L., Gajić-Umiljendić, J., & Marisavljević, D. (2012). Short-time effects of the herbicide nicosulfuron on the biochemical activity of Chernozem soil. Journal of the Serbian Chemical Society, 77(6), 845–855. https://doi.org/10.2298/JSC110825004R
Regitano, J. B., & Koskinen, W. C. (2008). Characterization of nicosulfuron availability in aged soils. Journal of Agricultural and Food Chemistry, 56(14), 5801–5805.
Riaz, M., Jamil, M., & Mahmood, T. Z. (2007). Yield and yield components of maize as affected byvarious weed control methods under rain fed conditions of Pakistan. International Journal of Agriculture and Biological Sciences, 9(1), 152–155.
Rivas, F. J., & Solís, R. R. (2018). Chloride promoted oxidation of tritosulfuron by peroxymonosulfate. Chemical Engineering Journal, 349, 728–736. https://doi.org/10.1016/j.cej.2018.05.117
Rosinger, C., & Schulte, W. (2019). Safeners for herbicides. In P. Jeschke, M. Witschel, W. Kramer, & U. Schirmer (Eds.), Modern Crop Protection Compounds (pp. 425–450). John Wiley & Sons Inc.
Santel, H. J. (2012). Thiencarbazone-methyl (TCM) and Cyprosulfamide (CSA)-A new herbicide and a new safener for use in corn. Julius-Kühn-Archiv, 2(434), 499–505. https://doi.org/10.5073/jka.2012.434.062
Šantric, L. J., Radivojevic, L. J., Gajic-Umiljendic, J., Saric-Krsmanovic, M., & Ðurovic-Pejcev, R. (2018). The effects of nicosulfuron and glyphosate on microbial activity of different soils. Planta Daninha, 36https://doi.org/10.1590/S0100-83582018360100103
Serim, A. T., & Maden, S. (2012). Soil persistence of tritosulfuron+ dicamba in the central Anatolia region in Turkey. In International Symposium: Current Trends in Plant Protection-Proceedings. Institute for Plant Protection and Environment, Belgrade (Serbia).
Sinsabaugh, R. L., Hill, B. H., & Follstad Shah, J. J. (2010). Erratum: Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment (Nature (2009) 462 (795–798)). Nature, 468(7320), 122. https://doi.org/10.1038/nature09548
Sofi, J. A., Lone, A. H., Ganie, M. A., Dar, N. A., Bhat, S. A., Mukhtar, M., Dar, M. A., & Ramzan, S. (2016). Soil microbiological activity and carbon dynamics in the current climate change scenarios: A review. Pedosphere, 26(5), 577–591. https://doi.org/10.1016/S1002-0160(15)60068-6
Stephenson, D. O., & Bond, J. A. (2012). Evaluation of thiencarbazone-methyl–and isoxaflutole-based herbicide programs in corn. Weed Technology, 26(1), 37–42. https://doi.org/10.1614/WT-D-11-00053.1
Tomkiel, M., Baćmaga, M., Borowik, A., Kucharski, J., & Wyszkowska, J. (2019). Effect of a mixture of flufenacet and isoxaflutole on population numbers of soil-dwelling microorganisms, enzymatic activity of soil, and maize yield. Journal of Environmental Science and Health, Part B, 54(10), 832–842. https://doi.org/10.1080/03601234.2019.1636601
Torstensson, L., & Stenström, J. (1986). “Basic” respiration rate as a tool for prediction of pesticide persistence in soil. Toxicity Assessment, 1(1), 57–72. https://doi.org/10.1002/tox.2540010106
Tyler, H. L. (2020). Foliar dicamba application has no lasting effects on microbial activities in the soybean rhizosphere. American Journal of Plant Sciences, 11(11), 1706–1713. https://doi.org/10.4236/ajps.2020.1111122
United States Environmental Protection Agency (USEPA) (2006). Reregistration eligibility decision for dicamba and associated salt, Washington, DC. Retrived August 18, 2021, from https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P10049M3.txt
Ushio, M., Miki, T., & Balser, T. C. (2013). A coexisting fungal-bacterial community stabilizes soil decomposition activity in a microcosm experiment. PLoS ONE, 8(11), e80320. https://doi.org/10.1371/journal.pone.0080320
Wolters, V. (2000). Invertebrate control of soil organic matter stability. Biology and Fertility of Soils, 31(1), 1–19. https://doi.org/10.1007/s003740050618
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koçak, B., Cenkseven, S. Effects of Three Commonly Used Herbicides in Maize on Short-Term Soil Organic Carbon Mineralization. Water Air Soil Pollut 232, 386 (2021). https://doi.org/10.1007/s11270-021-05337-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05337-3