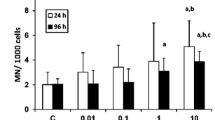
Abstract
Both polychlorinated biphenyls (PCBs) and cadmium (Cd) can be frequently found in marine ecosystems and have detrimental effects on marine organisms, especially on filter-feeding marine mussels. Although biological responses to single metal or PCB exposure in mussels have been well-studied, information about oxidative stress is still limited, especially in different tissues in mussels. Considering the variety of contaminants existing in the actual marine environment, the exposures of the marine mussel Mytilus coruscus to Cd2+ alone (0.194, 0.388, and 0.775 mg/L) and Aroclor 1254 alone (0.005, 0.010, and 0.050 mg/L) and the co-exposures of the marine mussel Mytilus coruscus to Cd2+ (0.194 and 0.388 mg/L) and Aroclor 1254 (0.005 and 0.010 mg/L) were tested in an 8-day exposure experiment followed by a 7-day acclimation experiment. The alterations in superoxide dismutase, catalase, glutathione peroxidase, and malondialdehyde levels in the gills of the mussels were assessed. The effects of the depressed antioxidant were induced by the exposures of Cd2+ and Aroclor 1254 and their co-exposures. All exposures resulted in an initial increase and then a reduction in antioxidant enzyme activities. The range and rate of the antioxidant enzyme activities were positively correlated with stress duration and the concentration of the stress material. The effect of combined stress was stronger than that of each individual stressor. The valuable information for future investigations of stress response mechanisms, especially in relation to tissue functions in marine organisms, has been provided by the results and experimental model. The study of combined pollution effects has more scientific significance for marine pollution monitoring.




Similar content being viewed by others
References
Benedetti, M., Lanzoni, I., Nardi, A., d'Errico, G. M., Carlo Di Fattorini, D., Nigro, M., & Regoli, F. (2016). Oxidative responsiveness to multiple stressors in the key Antarctic species Adamussium colbecki: Interactions between temperature acidification and cadmium exposure. Marine Environmental Research, 121, 20–30.
Beyer, J., Green, W., Norman, S. B., Allan Ian, J., Anders, R., Tânia, G., Bråte Inger Lise, N., & Merete, S. (2017). Blue mussels (Mytilus edulis spp.) as sentinel organisms in coastal pollution monitoring: A review. Marine Environmental Research, 130, 338–365.
Boukadida, K., Cachot, J., Clerandeaux, C., Gourves, P. Y., & Banni, M. (2017). Early and efficient induction of antioxidant defense system in Mytilus galloprovincialis embryos exposed to metals and heat stress. Ecotoxico Environ Saf, 138, 105–112.
Burmester, V., Jorge, N., & Claudia, W. (2012). Adaptation of freshwater mussels to cyanobacterial toxins: Response of the biotransformation and antioxidant enzymes. Ecotoxicology and Environmental Safety, 78, 296–309.
Casalino, E., Azaretti, G., Sblano, C., & Clemente, L. (2002). Molecular inhibitory mechanisms of antioxidant enzymes in rat liver and kidney by cadmium. Toxicology, 179(1–2), 37–50.
Chelikani, P., Fita, I., & Loewen, P. C. (2004). Diversity of structures and properties among catalases. Cellular and Molecular Life Sciences, 61, 192–208.
Chen, S. Y., Qu, M. J., Ding, J. W., Zhang, Y. F., & Di, Y. N. (2018). BaP-metals co-exposure induced tissue-specific antioxidant defense in marine mussels Mytilus coruscus. Chemosphere, 205, 286–296.
Deng, S. P., Zhao, Y. T., Zhu, C. H., Zeng, M. S., Fu, S. F., & Li, G. L. (2012). Effect of cadmium on the antioxidant enzyme activity and lipid peroxidation in Sanguinolaria acuta. Acta Hydrobiologica Sinica, 36, 689–695.
Dong, S. H., Yang, Y. Y., Cheng, B., Ren, C. B., & Yan, F. (2019). Responses of antioxidant defenses in the clam Mactra veneriformis to 2,2′,4,4′tetrabromodiphenyl ether exposure. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 217, 98–105.
Eertman, R. H. M., Zurburg, W., Schipper, C. A., Ben, S., & Smaal Aad, C. (1996). Effects of PCB126 and cadmium on the anaerobic metabolism of the mussel Mytilus edulis L. Comparative Biochemistry & Physiology Part C: Pharmacogy, Toxicology & Endocrinology, 113(2), 267–272.
Fernández, B., Campillo, J. A., & Martínez-Gómez, C. (2012). Assessment of the mechanisms of detoxification of chemical compounds and antioxidant enzymes in the digestive gland of mussels, Mytilus galloprovincialis, from Mediterranean coastal sites. Chemosphere, 87(11), 1235–1245.
Fernández, B., Campillo, J. A., Martínez-Gómez, C., & Benedicto, J. (2010). Antioxidant responses in gills of mussel (Mytilus galloprovincialis) as biomarkers of environmental stress along the Spanish Mediterranean coast. Aquatic Toxicology, 99, 186–197.
Feyk Lori, A., Giesy John, P., & Lambert George, H. (2009). Relationship between polychlorinated biphenyl 126 treatment and cytochrome p4501a activity in chickens, as measured by in vivo caffeine and ex vivo ethoxyresorufin metabolism. Environmental Toxicology and Chemistry, 18, 2013–2022.
Gerber, R., Smit, N. J., & Johan, H. (2018). Biomarkers in tigerfish (Hydrocynus vittatus) as indicators of metal and organic pollution in ecologically sensitive subtropical rivers. Ecotoxicology and Environmental Safety, 157, 307–317.
Gonzalez, P., Baudrimont, M., Boudou, A., & Bourdineaud, J. P. (2006). Comparative effects of direct cadmium contamination on gene expression in gills, liver, skeletal muscles and brain of the zebrafish (Danio rerio). Biometals, 19, 225–235.
Ighodaro, O. M., & Akinloye, O. A. (2018). First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria Journal of Medicine, 54, 287–293.
Järup, L., & Agneta, Å. (2009). Current status of cadmium as an environmental health problem. Toxicology and Applied Pharmacology, 238(3), 201–208.
Jeong, C.-B., Hye-Min, K., Lee, M.-C., Eunjin, B., & Lee, J.-S. (2019). Effects of polluted seawater on oxidative stress, mortality, and reproductive parameters in the marine rotifer Brachionus koreanus and the marine copepod Tigriopus japonicus. Aquatic Toxicology, 208, 39–46.
Ji ZH, Zhang YX, Tian J, Wang FB, Song, MY, Li H (2018) Oxidative stress and cytotoxicity induced by tetrachlorobisphenol a in Saccharomyces cerevisiae cells. Ecotoxicology and Environmental Safety161: 1–7 .
Jia, X. Y., Zhang, H. J., & Liu, X. X. (2011). Low levels of cadmium exposure induce DNA damage and oxidative stress in the liver of Oujiang colored common carp Cyprinus carpio var color. Fish Physiology and Biochemistry, 37, 97–103.
Jiang, J. H., Zhu, L. Z., & Zhang, M. (2006). Concentration and sources of typical organic contaminants in seawater, sediment and organisms in Iiaojiang Bay. Environmental Chemistry, 25, 546–549.
Jiang, W. W., Fang, J. G., Gao, Y. P., Du, M. R., & Jiang, Z. J. (2019). Biomarkers responses in Manila clam, Ruditapes philippinarum after single and combined exposure to mercury and benzo[a]pyrene. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 20, 1–8.
Kadiiska, M. B., Gladen, B. C., Baird, D. D., Germolec, D., Graham, B. L., Parker, C. E., & Nyska, A. (2005). Biomarkers of oxidative stress study II: Are oxidation products of lipids, proteins, and DNA markers of CCl(4) poisoning. Free Radical Biology & Medicine, 38(6), 698–710.
Kryndushkin D, Rao VA (2016) Comparative effects of metal-catalyzed oxidizing systems on carbonylation and integrity of therapeutic proteins. Pharm res (N.Y.) 33: 526-539.
Kappus H (1985) Lipid peroxidation: Mechanisms, analysis, enzymology, and biological relevance. In “oxidative stress”, H.Sies ed., academic press Inc., London; 273.
Letcher R J, Klasson-Wehler E, Bergman Å. (2000) The Handbook of Environmental Chemistry , vol . 3 , Part K. New Types of Persistent Halogenated Compounds. Heidelberg : Springer 315–359.
Liu, H. M., Dong, Y. H., Huo, L. H., Lin, Z. H., & Wang, Z. P. (2010). Acute toxicity of Cu2+ and its effects on antioxidant enzymes in Sinonovacula constricta juvenile. Journal of Fishery Sciences of China, 19(1), 182–187.
Liu, Q., Liao, Y. B., & S L. (2018). Concentration and potential health risk of heavy metals in seafoods collected from Sanmen Bay and its adjacent areas, China. Marine Pollution Bulletin, 131, 356–364.
Liu, R. M., Men, C., Liu, Y. Y., Yu, W. W., & Shen, Z. Y. (2016). Spatial distribution and pollution evaluation of heavy metals in Yangtze estuary sediment. Marine Pollution Bulletin, 110, 564–571.
Livingstone, D. R. (2001). Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organ-isms. Marine Pollution Bulletin, 4, 656–666.
Livingstone, D. R., Archibald, S., Chipman, J. K., et al. (1992). Antioxidant enzymes in liver of dab Limanda limanda from the North Sea. Marine Ecology Progress Series, 91, 97–104.
Maria, V. L., Gomes, T., Barreira, L., & Bebianno, M. J. (2013). Impact of benzo(a)pyrene, cu and their mixture on the proteomic response of Mytilus galloprovincialis. Aquatic Toxicology, 144-145, 284–295.
Narasimhan, T. R., Kim, H. L., & Safe, S. H. (1991). Effects of hydroxylated polychlorinated biphenyls on mouse liver mitochondrial oxidative phosphorylation. Journal of Biochemical Toxicology, 6, 229–236.
Nguyen, D. D., Tsai, C. L., Hsu, Y. C., Chen, Y. W., Weng, Y. M., & Chang, M. B. (2017). PCDD/Fs and dl-PCBs concentrations in water samples of Taiwan. Chemosphere, 173, 603–611.
Olsvik PA, Kristensen T, Waagbø R, Rosselan BO, Tollefsen K.-E., Baeverfjord G, Berntssen MHG (2005) mRNA expression of antioxidant enzymes (SOD, CAT and GSH-Px) and lipid peroxidative stress in liver of Atlantic salmon (Salmo salar) exposed to hyperoxic water during smoltification. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 141: 314–323.
Peng L, Zeng JN, Chen QZ,Huang W, Du P (2015) Effects of cadmium on antioxidant enzyme activity in gill and acute toxicity of cadmium on Mytilus coruscus. Environmental Science & Technology 38(2):13–18.
Pillet M , Castaldo G, Weggheleire S.De , Bervoets L, Blust R, Boeck G.De (2019) Limited oxidative stress in common carp (Cyprinus carpio, L., 1758) exposed to a sublethal tertiary (cu, cd and Zn) metal mixture. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 218:70–80.
Prasad, S., Gupta, S. C., & Tyagi, A. K. (2017). Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Canc Lett, 387, 95–105.
Rainbow, P. S. (2007). Trace metal bioaccumulation: Models, metabolic availability and toxicity. Environment International, 33(4), 576–582.
Rihab, B. A., Sabrine, B. O., Lina, C., Imed, M., Hatem, B. O., & Ali, O. (2017). Cadmium effect on physiological responses of the tolerant Chlorophyta specie Picocystis sp. isolated from Tunisian wastewaters. Environ. Sci Pollut Res Int, 24, 1803–1810.
Xiu, M., Pan, L., & Jin, Q. (2014). Bioaccumulation and oxidative damage in juvenile scallop Chlamys farreri exposed to benzo[a]pyrene, benzo[b]fluoranthene and chrysene. Ecotoxicology and Environmental Safety, 107, 103–110.
Xu, Z. H., Regenstein, J. M., Xie, D. D., Lu, W. J., & Mao, L. C. (2018). The oxidative stress and antioxidant responses of Litopenaeus vannamei to low temperature and air exposure. Fish & Shellfish Immunology, 72, 564–571.
Saddick Salina, Afifi Mohamed, Osama A. Abu Zinada (2017) Effect of zinc nanoparticles on oxidative stress-related genes and antioxidant enzymes activity in the brain of Oreochromis niloticus and Tilapia zillii. Saudi Journal of Biological Sciences 24: 1672–1678.
Schieber, M., & Chandel Navdeep, S. (2014). ROS function in redox signaling and oxidative stress. Current Biology, 24, 453–462.
Shi, J. C., Li, X., He, T. T., Wang, Z., Li, P., Lai, Y. Z., Edmond, S., & Liu, W. H. (2018). Integrated assessment of heavy metal pollution using transplanted mussels in eastern Guangdong, China. Environmental Pollution, 243, 601–609.
Sun, D. Q., Li, A. W., Li, J., Li, D. G., Li, Y. X., Feng, H., & Gong, M. Z. (2009). Changes of lipid peroxidation in carbon disulfide-treated rat nerve tissues and serum. Chemico-Biological Interactions, 179, 110–117.
Ullah, S., Zhongqiu, L., Zaigham, H., Shahid, U. K., & Shah, F. (2018). Malathion induced oxidative stress leads to histopathological and biochemical toxicity in the liver of rohu (Labeo rohita, Hamilton) at acute concentration. Ecotoxicology and Environmental Safety, 161, 270–280.
Valko, M., Morris, H., & Cronin, M. T. D. (2005). Metals, toxicity and oxidative stress. Current Medicinal Chemistry, 12(10), 1161–1208.
Vellingera, C., Gismondib, E., Feltena, V., Kahina, R. P. M., Marc, P., & Philippe, U.-P. (2013). Single and combined effects of cadmium and arsenate in Gammarus pulex (Crustacea, Amphipoda): Understanding the links between physiological and behavioural responses. Aquatic Toxicology, 140–141, 106–116.
Víg, É., & Nemcsók, J. (1989). The effects of hypoxia and paraquat on the superoxide dismutase activity in different organs of carp, Cyprinus carpio L. Journal of Fish Biolog, 35(1), 23–25.
Wang, L., Li, J. J., Li, J. G., & Liu, Z. P. (2010). Effects of lead and/or cadmium on the oxidative damage of rat kidney cortex mitochondria. Biological Trace Element Research, 137, 69–78.
Wang, X., Xu, H., Zhou, Y., Wu, C., & Kanchanopas-Barnette, P. (2016). Spatial distribution and sources of polychlorinated biphenyls in surface sediments from the Zhoushan archipelago and Xiangshan Harbor, East China Sea. Marine Pollution Bulletin, 105(1), 385–392.
Wang Y,Fang J, Leonard Stephen S, Rao Krishna K. Murali (2004) Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radical Biology & Medicine 36: 1434–1443.
Wilhelm Filho, D., Torres, M. A., Zaniboni-Filho, E., & Pedrosa, R. C. (2005). Effect of different oxygen tension on weight gain, feed conversion, and antioxidant status in piapara, Leporinus elongatus(Valenciennes,1847). Aquaculture, 244, 349–357.
Yu, Q. Y., Wang, Y., & Xu, Y. (2013). Effects of cadmium and lead on the lipid peroxidation and levels of antioxidant enzymes in Ruditapes philippinarum. Asian Journal of Ecotoxicology, 8, 504–512.
Zhang, J., Liu, F., Chen, R. B., Feng, T., Dong, S. J., & Shen, H. Q. (2012). Levels of polychlorinated biphenyls and organochlorine pesticides in edible shellfish from Xiamen (China) and estimation of human dietary intake. Food and Chemical Toxicology, 50, 4285–4291.
Zhou, Y. X., & Zhang, Z. S. (1989). Toxicity test methods for aquatic organisms. Agricultural press, Beijing, 52–143.
Zuykov, M., Emilien, P., & Harper David, A. T. (2013). Bivalve mollusks in metal pollution studies: From bioaccumulation to biomonitoring. Chemosphere, 93, 201–208.
Acknowledgment
We are grateful to all anonymous editors and reviewers for providing comments on this manuscript.
Funding
We also appreciate the generous financial support of this work provided by the Zhejiang Qingshan Lake Innovation Platform for Marine Science and Technology (No. 2017E80001), the Scientific Research Fund of the Second Institute of Oceanography, MNR (No. JG1910), the National Natural Science Foundation of China (No. 41601560), the National Natural Science Foundation of China (No. 41806136), the National Key Technology Research and Development Program of the Ministry of Science and Technology of the China (2015BAD08B01), and the Project of State Key Laboratory of Satellite Ocean Environment Dynamics (No. SOEDZZ1902).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peng, L., Zeng, J., Liu, Q. et al. Single and Combined Effects of Cadmium and Aroclor 1254 on Oxidative Stress in Gills of Mytilus coruscus. Water Air Soil Pollut 231, 51 (2020). https://doi.org/10.1007/s11270-020-4397-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-4397-1