Abstract
Ozone (O3) decomposition in the troposphere is a very important process which prevents excessive O3 accumulation in the air. It is particularly significant on warm summer days which are marked by a high risk of photochemical smog. We used Spearman’s rank correlation test to determine relationships between the drop in O3 concentrations over time (-ΔO3), nitrogen oxide (NO), nitrogen dioxide (NO2), and total nitrogen oxide (NOx) concentrations and meteorological factors (1-h average) in low-polluted urban area in Olsztyn (north-eastern Poland). Nitrogen oxide concentrations were measured continuously by the chemiluminescence method, and O3 concentrations were determined by the UV photometric method. The obtained results suggest that the rate of decomposition of tropospheric O3 is affected mostly by the presence of NOx, high temperature, and air humidity (positive correlation) as well as by wind speed (negative correlation). Maximum correlation coefficient values were reported between –ΔO3 and air temperature, –ΔO3 and absolute air humidity when NOx concentrations were low (below 1.0 microgram per cubic meter), reaching 0.271 and 0.243, respectively. These results indicate that O3 also reacted with air components other than NO and NO2. Precipitation at average temperature of < 0 °C did not significantly contribute to a drop in O3 concentrations at night-time. In the warm season, precipitation slowed down the rate of O3 decomposition, mostly because NOx were scrubbed by rain. An analysis of seasonal and daily –ΔO3 fluctuations revealed that –ΔO3 values were highest in the summer and shortly after sunset in the diurnal cycle.
Similar content being viewed by others
1 Introduction
High ozone (O3) concentrations in the tropospheric layer of the Earth’s atmosphere pose a threat to the health of humans and land animals and have an adverse effect on plants (de Wit et al. 2015; Harmens et al. 2016). Episodes of high concentrations of O3, particulate matter (PM10, PM2.5), and nitrogen dioxide (NO2) are major current problems related to air pollution. However, it should be stressed that PM10, PM2.5, and NO2 are found mostly in the air in urban and industrial regions, whereas O3 is present not only in urban but also in suburban and rural areas. The maximum daily 8-h mean concentration of O3 should not exceed 120 μg m−3 (long-term objective) or 120 μg m−3 on more than 25 days per calendar year (target value) (EU 2008). In Europe, 41% of all stations showed O3 concentrations above the target value for the protection of human health in 2015, which is considerably more stations than over the previous 5 years. In addition, only 13% of air quality monitoring stations fulfilled the long-term objective (no exceedance of the threshold level). Eighty-eight percent of the stations with values above the long-term objective were background stations (EEA 2017). Tropospheric O3 is also a greenhouse gas which contributes to global warming (IPCC 2007). In addition to its adverse effect on human health and the natural environment, tropospheric O3 also contributes to significant economic losses by damaging crops, lowering crop yield and causing damage to certain materials, in particular rubber. The annual losses resulting from the above have been estimated at around €6.7 billion in 47 countries in Europe (Holland et al. 2006).
Large quantities of O3 are formed in the stratosphere. A part of this naturally formed stratospheric O3 goes down to troposphere. However, photochemical processes involving nitrogen oxides (NOx), volatile organic compounds (VOCs), and carbon monoxide (CO) are a more important source of O3 in ground-level ambient air (Sharma et al. 2017). Photochemical smog is the most dangerous phenomenon related to the formation of O3 as a result of the above chemical reactions. In addition to O3, a smoggy atmosphere comprises other characteristic components such as organic oxidizers (including peroxyacetyl nitrate, PAN), aldehydes, NOx, hydrogen peroxide, and free radicals (Kanaya et al. 2007). Photochemical smog is one of the most adverse contributors to the quality of urban air (EEA 2017). Its formation is supported by the following meteorological conditions: high insolation, high temperature, temperature inversion and dry, stagnant air masses (Manahan 2007; Hosoi et al. 2011). This environment triggers intense reactions with sunlight irradiation which produce O3 and other secondary air pollutants. The most important phase of those changes is the NO–NO2–O3 inter-conversion cycle which takes place in line with the below equations (Crutzen et al. 1999; Warmiński and Bęś 2009):
where
- M:
-
molecules which absorb excess energy and determine the stability of O3 molecules (usually N2 or O2 molecules)
- hν :
-
photon energy (with electromagnetic wave length of λ = 290–430 nm)
R1-R3 reactions establish a photostationary state equilibrium which is described by the following equation (Leighton 1961):
where
- \( {J}_{N{O}_2} \) :
-
nitrogen dioxide (NO2) photolysis rate constant (rate constant of R1 reaction)
- k 3 :
-
nitrogen oxide (NO) oxidation rate constant (rate constant of R3 reaction)
The above equation demonstrates that all other reactions which reduce NO concentrations in favor of NO2 increase O3 concentrations. Those reactions are inclusive of photochemical oxidation of CO, methane (CH4), and non-methane VOC (Warmiński and Rogalski 2007; Kalbarczyk and Kalbarczyk 2017), namely,
Atmospheric O3 is a relatively unstable gas. It participates in a variety of redox reactions in the atmosphere. Atmospheric O3 reacts with both organic and inorganic compounds (mainly with NO, SO2, CO, H2O) which are in plentiful supply in ground-level air (Crutzen et al. 1999; Hosoi et al. 2011). For this reason, cyclic, diurnal changes are observed in tropospheric O3 levels where maximum concentration values are reported in the afternoon and minimum concentrations are noted in the hours directly preceding sunrise (Mazzeo et al. 2005; Kanaya et al. 2007; Warmiński and Rogalski 2007; Kalbarczyk et al. 2016). It has been suggested that the most important night-time reaction takes place between O3 and NO. As demonstrated by Nicholson et al. (2001), the drop in O3 concentrations in urban air which is characterized by high NO levels is more pronounced in the summer than in the winter. According to our previous research, the NO oxidation rate constant (k3) in the city of Olsztyn (north-eastern Poland) ranged from 22.5 to 27.7 ppm−1 min−1 in July and from 14.1 to 15.5 ppm−1 min−1 in January (Warmiński and Rogalski 2007). According to Mazzeo et al. (2005), the k3 constant in Buenos Aires was 21.5 to 23.0 ppm−1 min−1 in wintertime. The reported differences are due to various thermal conditions because k3 is a function of air temperature as demonstrated by the equation (Seinfeld and Pandis 2016):
where T—air temperature (K).
Other redox reactions involving O3 may be predominant at low NOx concentrations, subject to the locally occurring gaseous pollutants, aerosols, and water vapor. Therefore, we hypothesized that the rate of decrease in tropospheric O3 is also influenced by factors other than nitrogen oxide concentrations and temperature. The main motivation for the present study was to assess the sensitivity of night-time ozone concentrations to meteorological parameters at different NOx levels. The results will be useful for modeling tropospheric ozone concentrations. According to Wałaszek et al. (2018), modeling errors are predominantly systematic and result from chemical mechanisms, meteorological fields, and initial and boundary conditions, including background ozone levels. Reliable estimation of initial ozone concentrations is crucial for reducing ozone modeling errors. Ozone decomposition in the lower atmospheric layer is a very important phenomenon which prevents excessive accumulation of this gas in ambient air, in particular on warm summer days.
2 Materials and Methods
2.1 Location of the Monitoring Site
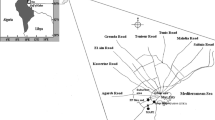
The study was conducted at the air quality monitoring station of the University of Warmia and Mazury in Olsztyn. The station’s location and the mode of collecting representative air samples are consistent with the requirements set forth in the Regulation of the Polish Minister of Environment of September 3, 2012 and Directive 2008/50/EC of the European Parliament and of the Council of May 21, 2008 (EU 2008). The city of Olsztyn is situated in north-eastern Poland, in a region which is popularly known as the Green Lungs of Poland (Fig. 1). The region is characterized by a low degree of industrialization and by exceptional environmental values. The main sources of air pollution in Olsztyn are road transport and household gas emissions. The air quality monitoring station is situated in the south-western part of Olsztyn, on Lake Kortowskie. The monitoring site has the following geographic coordinates: 53° 45′ 34.3″ N, 20° 27′ 09.5″ E. The nearest sources of pollution are Warszawska street which is a part of national road No. 16 (east-bound, at a distance of approx. 500 m), residential estates in Słoneczny Stok composed of single-family houses equipped with coal-fired boilers (1000 m), Dajtki (1400 m), Brzeziny (1600 m), and the city center (2200 m).
The results of daily measurements were compared with data from selected air quality monitoring stations of the Chief Inspectorate for Environmental Protection (GIOŚ) to determine spatiotemporal trends in tropospheric O3 and NOx levels. The selected monitoring stations are situated in the Region of Warmia and Mazury (north-eastern Poland), the Warsaw Metropolitan Area (capital of Poland, central Poland), and the Silesian Metropolitan Area (highly industrialized region in southern Poland). The stations in north-eastern Poland are situated in the cities of Olsztyn, Elbląg, Ostróda, Mrągowo, and Gołdap. Data were also obtained from a rural background monitoring station in the Borecka Forest (PL0005R Diabla Gora) which is a part of the European Monitoring and Evaluation Program (EMEP). In the Warsaw Metropolitan Area, O3 concentrations were presented as the average value from five monitoring stations in Warsaw, and the results from the Silesian Metropolitan Area were averaged based on the data from the cities of Katowice, Zabrze, Tychy, and Dąbrowa Górnicza. The concentrations of NOx were measured in nine monitoring stations in each region. Detailed information about monitoring stations and measurements is provided by GIOŚ at http://powietrze.gios.gov.pl.
2.2 Measurements and Instrumentation
NO and NO2 concentrations were measured continuously by the chemiluminescence method, and O3 levels were determined by the UV photometric method (API 200E and API 400E analyzers, Teledyne Advanced Pollution Instrumentation, Inc. USA). The applied methods are consistent with the ISO 7996:1985 and ISO 13964:1998 standards (ISO 1985; ISO 1998), and the analyzers have US EPA certification (USEPA RFNA-1194-099 and USEPA EQOA-0992-087). All analyzers met the requirements of minimum data capture (DC), stipulated by Directive 2008/50/EC (EU 2008). Minimum DC for O3 and NOx is 90% in summer and 75% in winter. In our study, conducted in 2006, minimum DC was 98.6 and 76.1% for O3 and 98.5 and 91.1% for NOx in summer and winter, respectively. Air temperature and humidity were measured with the DMA580 digital thermo-hygrometer, wind direction and speed measurements were performed using the DNA502 gonio-anemometer and the DNA511 tacho-anemometer, and precipitation was measured with the DQA031 automated rain gauge (LSI s.p.a., Italy). Instantaneous data (5-s) were registered, validated, and averaged to 1 h in a datalogger with the CS5 v. 5.3 system (CSMS, Poland). Temporal trends in ground-level O3 and NOx concentrations in ambient air in low-polluted urban area of the city of Olsztyn are presented in Fig. 2. The concentrations of O3, NO2, and NO and meteorological parameters were measured throughout 2006, but this study accounts only for data collected at night-time, when photochemical processes cease and when only reactions leading to O3 decomposition take place.
2.3 Data Analysis
We determined the drop in O3 concentrations (–ΔO3) in hour C with the use of the equation:
where xAB, xBC—average O3 concentration (μg m−3) based on instantaneous measurements between hour A to hour B and hour B to hour C; tC, tB—time (h) at hour C and B from the beginning of the measurement series.
Validated NO, NO2, NOx, and O3 concentrations and meteorological parameters (ambient air temperature, relative and absolute air humidity, wind speed and direction, total atmospheric precipitation) were subjected to a preliminary statistical analysis to verify the hypothesis on the goodness of fit of value distribution to normal distributions. Kolmogorov-Smirnov and chi-square tests invalidated the above hypothesis. Therefore Spearman’s rank correlation test was employed to determine relationships between –ΔO3 and the investigated factors.
Calculations were performed in several time series: (i) throughout the year (all night-time hours); (ii) hours without atmospheric precipitation; (iii) cool season (period with average weekly temperature of ambient air at night-time < 0 °C; in this study, this criterion was fulfilled over a period of 12 weeks, from 01 Jan. 2006 to 25 Mar. 2006); (iv) warm season (12 warmest weeks in the year, from week 25 to 36, i.e. from 11 Jun. 2006 to 09 Sept. 2006; the average night-time temperature of ambient air in the above period was higher than 13.9 °C); (v) only hours during which specific NOx concentrations were recorded (≤ 1.0, ≤ 2.0, ≤ 3.0, and > 3.0 μg m−3).
Coefficients of correlation were calculated, and seasonal and diurnal variations in –ΔO3 were analyzed. The average weekly value of –ΔO3 was adopted as the dependent variable and the number of the week in the year as the independent variable. Diurnal variation was analyzed separately for the cool and warm season, and the time of the day (independent variable, x) was transformed in line with the following rules: (i) from sunset to midnight, the values of x for 4 p.m., 5 p.m., 6 p.m., etc. were set at 4, 5, 6, etc.; (ii) at midnight, the value of × was set at 12; (iii) from midnight to sunrise, the values of x for 1 a.m., 2 a.m., 3 a.m., etc. were set at 13, 14, 15, etc. The above transformation was performed to ensure the continuity of x value (hours) from sunset to sunrise. The obtained data were verified statistically in STATISTICA 10 software (StatSoft, Inc.).
3 Results and Discussion
3.1 Spatiotemporal Trends in Tropospheric Ozone and Nitrogen Oxide Concentrations in Poland
The average concentration of tropospheric O3 in all air quality monitoring stations in the Region of Warmia and Mazury (north-east Poland) was lowest in 2016 and highest in 2006 at 48.7 and 56.9 μg m−3, respectively (Fig. 3a). A clear decreasing trend in the above parameter was observed in north-east Poland. In our study, the concentration of tropospheric ozone on Lake Kortowskie in Olsztyn decreased from 61.3 μg m−3 in 2006 to 54.2 μg m−3 in 2008 and exceeded the regional average by 2.4 μg m−3. A similar trend was noted in the rural background monitoring station in the Borecka Forest (EMEP station). In contrast, in the monitoring station in downtown Olsztyn, O3 concentration was highest in 2007 at 61.5 μg m−3. A comparison of average values for 2006–2008 indicates that O3 levels were around 1 μg m−3 lower in central Olsztyn than on Lake Kortowskie. The above was related to differences in the concentration of NOx which was around 8 μg m−3 higher in central Olsztyn (Fig. 3b). O3 and NOx concentrations are measured simultaneously in many locations in Poland and around the world. A negative correlation is observed between ozone and NOx levels due to the reaction between O3 and NO (Nicholson et al. 2001; Kalbarczyk et al. 2016). For this reason, ozone levels in industrialized urban areas characterized by higher NOx concentrations, such as Warsaw and the Silesian Metropolitan Area, are approximately 10–11 μg m−3 lower than in north-eastern Poland. However, a rising trend in the concentrations of both ozone and nitrogen oxides has been noted in those locations. Kalbarczyk et al. (2016), Karagiannidis et al. (2015), and Mazzeo et al. (2005) reported similar correlations between O3 and NOx levels.
3.2 Correlations between –ΔO3 vs. Meteorological Factors and Nitrogen Oxide Concentration
The key factors which determined the rate of drop in O3 concentrations at night-time were wind speed, total atmospheric precipitation, ambient air temperature, absolute humidity of ambient air, NO2, and NOx concentrations (Table 1). Except for wind speed and total atmospheric precipitation, the remaining factors were positively correlated with –ΔO3. If data from hours with atmospheric precipitation are omitted in the analysis, the influence of chemical factors on the determination of the dependent variable (–ΔO3) decreases, while the impact of meteorological factors increases.
The obtained results supported our assumptions that temperature significantly contributes to a decrease in O3 concentrations. However, no statistically significant correlation was found between the rate of O3 concentration drop and input O3 and NO concentrations. It could probably be explained by the multifactorial nature of the phenomenon. Moreover, NO concentrations determined at air quality monitoring stations are not input concentrations but the concentrations of NO which has not yet been oxidized to NO2. At measuring stations situated at least several hundred meters away from NOx sources, NO is largely oxidized to NO2 before it reaches the monitoring station with the air masses (Nicholson et al. 2001; Warmiński and Rogalski 2006). Therefore, NOx concentrations are proportional to initial NO concentration levels. For this reason, no statistically significant dependencies were found between –ΔO3 and NO levels, but only between –ΔO3 and NO2 and NOx concentrations (Table 1). The formation of NO3 radicals in dark hours is a well-described reaction which leads to a drop in O3 concentrations (Zheng et al. 2017):
NO3 radicals are determined practically only at night-time because they rapidly photolyze to NO2 and O (Atkinson and Arey 2003, Stutz et al. 2010, Wang et al. 2013, Akimoto 2016).
The highest correlation was found between wind speed and –ΔO3, and the determined dependency was negative. Strong winds cause intense mixing of masses of polluted and clean air; therefore, the higher the wind speed, the greater the drop in NOx levels (Table 2). The negative correlation between NOx concentrations and wind speed has been documented in the works of, among others, Delaney and Dowding (1998), Tang et al. (2011), Karagiannidis et al. (2015), and Valotto and Varin (2016). For this reason, we determined lower NOx concentrations and –ΔO3 values under strong wind conditions (Tables 1 and 2). A negative correlation between O3 concentrations and wind speed was also reported by Banta et al. (2011) and Kalbarczyk et al. (2016).
A positive correlation between –ΔO3 and absolute humidity could be due to the reaction between O3 and water in the gaseous state. However, this mechanism of O3 breakdown at night-time is doubtful due to a very low value of the correlation coefficient at 0.133 (Table 1). Other mechanisms could be involved in the decomposition of ground-level ozone, but the reaction with NO (R3) appears to be predominant.
Ozone and particles adsorbed on aerosol surface also react at a faster rate in high humidity air. Chughtai et al. (2003) proposed the following relationship between the drop in O3 concentrations (−d[O3]/dt) caused by its reaction with soot aerosol and absolute humidity of air:
where
- [O3]:
-
ozone concentrations (ppm)
- p :
-
absolute humidity (Pa)
- k″:
-
reaction constant determined by temperature and the type of aerosol surface
Equation 4 indicates that the higher the air humidity, the higher the rate of O3 reaction with soot components. Sakamoto et al. (2004) also noted that the presence of water vapor in air speeds up the oxidation of SO2 through O3 on the surface of yellow sand particles.
Buckley and Birks (1995) also pointed to the possibility of visible-light photolysis of O3∙H2O cluster molecules (Van der Waals complexes) as well as reaction without the involvement of solar radiation:
Reaction R7 is energetically possible for light with a wavelength under 665 nm, while reaction R8 is exothermic and it takes place even without light absorption, i.e., at night. Those reactions could also be responsible for higher –ΔO3 values observed at night-time when air humidity is high.
Air pollutants, in particular the highly reactive O3, can also undergo dry deposition on the surface of soil, plants, buildings, etc. During the day, in vegetated areas, both stomatal fluxes and non-stomatal fluxes play an important role in the dry deposition of O3. At night, the dry deposition of O3 is dominated by non-stomatal fluxes (Fares et al. 2010; El-Madany et al. 2017). The total dry deposition of O3 is several-fold or 10- to 20-fold lower at night than during the day (Padro 1996; El-Madany et al. 2017). According to the literature, stomatal flux is proportional to air humidity (Zapletal et al. 2012; Fares et al. 2013). The night-time concentrations of O3 decrease steadily due to the absence of sunlight and redox reactions.
Total atmospheric precipitation was negatively correlated with –ΔO3 (Table 1). Although the Spearman’s rank correlation coefficient was statistically significant (p < 0.01), the precipitation factor in the analysis of annual data did not produce satisfactory results (RS = − 0.058). We propose a hypothesis that this is due to the types of precipitation at various times of the year. The analysis of annual data accounts for total precipitation which is not broken into different types of precipitation, i.e., rain, snow, or hail. For this reason, we performed an additional analysis of correlation covering two seasons: (i) cool (marked by ambient air temperature below 0 °C; coldest 12 weeks in 2006) and (ii) warm (12 warmest weeks in 2006).
The correlation between –ΔO3 and total atmospheric precipitation was higher in the warm period (RS = − 0.113, p < 0.001) in comparison with data covering the entire year (Table 3). A negative correlation rank coefficient may seem puzzling in this context. It indicates that O3 concentrations decrease at a slower rate as precipitation intensity grows. An analysis of data showed that at times of precipitation over the warm season, –ΔO3 reaches 1.43 μg m−3 h−1 on average, while it is threefold higher in hours without precipitation (Fig. 4). This is probably due to lower NOx concentrations at times of precipitation and, as indicated earlier, NO significantly contributes to a reduction in tropospheric O3 levels. It should be noted that the value of Rs was very low, although statistically significant. A stronger correlation between –ΔO3 and NO2 (NOx) concentrations and wind speed was determined in the warm season than in the cool season (Table 3). However, statistically significant correlations between –ΔO3 and air temperature and between –ΔO3 and air humidity were not found in either of the analyzed periods.
3.3 Influence of NOx Concentrations on the Results of Correlation Analysis
Nitrogen oxide concentrations proved to be one of the most important factors determining the rate of the drop in O3 concentrations at night-time. In view of the above, an additional Spearman’s rank correlation test was performed for night-time data, and the obtained average NOx concentrations were in the range of ≤ 1.0, ≤ 2.0, ≤ 3.0, and > 3.0 μg m−3. An analysis of correlation test results in Table 4 indicates that selected meteorological factors have a growing impact on –ΔO3 values as NOx concentrations decrease from > 3.0 to ≤ 1.0 μg m−3. It applies primarily to temperature and absolute humidity and, to a lesser extent, to wind speed. A reverse dependency was reported for NO2 and NOx concentrations.
3.4 Seasonal and Diurnal Variations of –ΔO3
Tables 1, 3, and 4 present Spearman’s rank correlation coefficients (RS) and significance levels (p) determined not only between –ΔO3 and chemical and meteorological factors, but also between –ΔO3 and the hour of the day and the week of the year. In most cases, those coefficients were statistically significant. Seasonal variations in –ΔO3 (weekly data) are presented in Fig. 5. Maximum –ΔO3 values were noted in the summer, whereas minimum ΔO3 values (near zero) were observed at the beginning and end of the year. The noted difference is due mostly to seasonal variations in air temperature.
In the cool period with temperatures of < 0 °C, diurnal changes in –ΔO3 values ranged from around − 1.0 to 4.0 μg m−3 h−1 (Fig. 6a). Higher fluctuations in –ΔO3 values were observed in the warm period, from around 1.0 to more than 8.0 μg m−3 h−1 (Fig. 6b). The highest –ΔO3 values were recorded between 4 p.m. and 9 p.m. in the cool season and between 9 p.m. and 10 p.m. in the warm season. The highest drop in O3 concentrations is observed in the hours directly after sunset, when air temperature is still relatively high but photochemical processes have already ceased. In the following hours, –ΔO3 values gradually decrease. OH and HO2 radical concentrations also begin to decrease as of sunset (Arias and Hastie 1996; Cantrell et al. 1996; Commane et al. 2010).
4 Conclusions
Ozone decomposition reactions in the troposphere are an important stage of the O3 cycle. The higher the rate of those reactions, the lower the risk of photochemical smog. The analysis of changes in concentration levels of O3 and other air parameters in the lower parts of the troposphere at night-time supported the preliminary identification of factors which significantly contribute to the rate of those reactions in low-polluted urban area. We conclude that the rate of decomposition of tropospheric ozone (–ΔO3) was affected mainly by the presence of NOx and wind speed. It should be noted, however, that NOx concentrations were largely determined by wind speed. High wind speed causes intense mixing of air masses, leading to a drop in the concentration levels of pollutants in urban areas, including NOx. Therefore, wind speed indirectly influenced –ΔO3. Other significant determinants included air temperature and absolute humidity which were positively correlated with –ΔO3. Maximum correlation coefficients for these parameters were observed at low NOx concentrations in the air (≤ 1.0 μg m−3), which reached 0.271 and 0.243, respectively. Both values of RS were similar to the value of RS for NOx. It should be noted that correlation coefficients were low, but statistically significant. The above could suggest that ozone also reacts with air components other than NOx. The observation that the decrease in ozone concentrations was not correlated with relative humidity, but was correlated with absolute humidity, in particular when NOx levels were very low, is a crucial finding. The effect of absolute humidity on –ΔO3 decreased with a rise in NOx levels. Precipitation in the cool season with temperatures of < 0 °C did not contribute to a drop in O3 concentrations at night-time. In the warm season, rainfalls slowed down the rate at which O3 concentrations decreased, compared with dry spells. This could result from effective removal of NOx, in particular the highly water-soluble NO2, by rain. O3 is sparingly water-soluble, and its wet deposition takes longer relative to NO2. Seasonal and daily fluctuations in temperature were responsible for –ΔO3 variations. The highest –ΔO3 values were reported in the warmest period of the year and shortly after sunset in the daily cycle. The presence of high concentration levels of OH and HO2 radicals directly after sunset could also be a cause of the observed variations in –ΔO3 values at that time.
To conclude, it can be postulated that some factors which significantly affect O3 formation during day-time (nitrogen oxides, high air temperature, low wind speed) also contribute to the rate of O3 decomposition at night-time. For this reason, daily O3 concentration amplitudes are much higher in the summer than at other times of the year. We are also of the opinion that low air humidity during the summer continental high slows down the drop in O3 concentrations at night-time. Day-time weather in the same period is marked by high temperatures and insolation, which supports O3 formation in photochemical processes. Due to the above, O3 concentrations may exceed target value for protection of human health after only several hot summer days and nights with low air humidity.
Our results can be useful for improving tropospheric ozone models. Such models account for ozone forming parameters as well as initial ozone levels (Wałaszek et al. 2018). As demonstrated by the study, selected meteorological parameters and NOx levels have a small, but statistically significant effect on initial ozone levels.
References
Akimoto, H. (2016). Atmospheric reaction chemistry. Tokyo: Springer Japan.
Arias, M., & Hastie, D. (1996). Radical chemistry at the SONTOS site in rural Ontario. Atmospheric Environment, 30(12), 2167–2175.
Atkinson, R., & Arey, J. (2003). Gas-phase tropospheric chemistry of biogenic volatile organic compounds. A review. Atmospheric Environment, 37(Supplement 2), 197–219.
Banta, R., Senff, C., Alvarez, R., Langford, A., Parrish, D., Trainer, M., et al. (2011). Dependence of daily peak O3 concentrations near Houston, Texas on environmental factors: wind speed, temperature, and boundary-layer depth. Atmospheric Environment, 45(1), 162–173.
Buckley, P., & Birks, J. (1995). Evaluation of visible-light photolysis of ozone-water cluster molecules as a source of atmospheric hydroxyl radical and hydrogen peroxide. Atmospheric Environment, 29(18), 2409–2415.
Cantrell, C., Shetter, R., & Calvert, J. (1996). Peroxy radical chemistry during FIELDVOC 1993 in Brittany, France. Atmospheric Environment, 30(23), 3947–3957.
Chughtai, A., Kim, J., & Smith, D. (2003). The effect of temperature and humidity on the reaction of ozone with combustion soot: implications for reactivity near the tropopause. Journal of Atmospheric Chemistry, 45(3), 231–243.
Commane, R., Floquet, C., Ingham, T., Stone, D., Evans, M., & Heard, D. (2010). Observations of OH and HO2 radicals over West Africa. Atmospheric Chemistry and Physics, 10(18), 8783–8801.
Crutzen, P., Lawrence, M., & Pöschl, U. (1999). On the background photochemistry of tropospheric ozone. Tellus B: Chemical and Physical Meteorology, 51(1), 123–146.
de Wit, H., Hettelingh, J.-P., & Harmens, H. (2015). Trends in ecosystem and health responses to long-range transported atmospheric pollutants. ICP waters report 125/2015. Norway: Norwegian Institute for Water Research. Oslo.
Delaney, C., & Dowding, P. (1998). The relationship between extreme nitrogen oxide (NOx) concentrations in Dublin’s atmosphere and meteorological conditions. Environmental Monitoring and Assessment, 52(1/2), 159–172.
EEA. (2017). Air quality in Europe—2017 report. EEA report, no 13/2017. Luxembourg: European Environment Agency, Publications Office of the European Union.
El-Madany, T., Niklasch, K., & Klemm, O. (2017). Stomatal and non-stomatal turbulent deposition flux of ozone to a managed peatland. Atmosphere, 8(12), 175.
EU. (2008). Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe. Official Journal of the European Union, L 152/1.
Fares, S., McKay, M., Holzinger, R., & Goldstein, A. (2010). Ozone fluxes in a Pinus ponderosa ecosystem are dominated by non-stomatal processes: evidence from long-term continuous measurements. Agricultural and Forest Meteorology, 150(3), 420–431.
Fares, S., Matteucci, G., Scarascia Mugnozza, G., Morani, A., Calfapietra, C., Salvatori, E., et al. (2013). Testing of models of stomatal ozone fluxes with field measurements in a mixed Mediterranean forest. Atmospheric Environment, 67, 242–251.
Harmens, H., Mills, G., Hayes, F., Sharps, K., & Frontasyeva, M. (2016). Air pollution and vegetation. ICP Vegetation Annual Report 2015/2016. Bangor: Centre for Ecology and Hydrology.
Holland, M., Kinghorn, S., Emberson, L., Cinderby, S., Ashmore, M., Mills, G., et al. (2006). Development of a framework for probabilistic assessment of the economic losses caused by ozone damage to crops in Europe. ICP Vegetation Report. Bangor: Centre for Ecology and Hydrology.
Hosoi, S., Yoshikado, H., Gaidajis, G., & Sakamoto, K. (2011). Study of the relationship between elevated concentrations of photochemical oxidants and prevailing meteorological conditions in the North Kanto area, Japan. Water, Air, & Soil Pollution, 215(1–4), 105–116.
IPCC. (2007). Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the IPCC. Intergovernmental Panel on Climate Change. Cambridge and New York.
ISO. (1985). Ambient air—determination of the mass concentration of nitrogen oxides—chemiluminescence method. ISO 7996:1985. International Organisation for Standardisation. Geneva.
ISO. (1998). Air quality—determination of ozone in ambient air—ultraviolet photometric method. ISO 13964:1998. International Organisation for Standardisation. Geneva.
Kalbarczyk, R., & Kalbarczyk, E. (2017). Variability and temporal structure of concentrations of carbon monoxide in Poznań (Central-Western Poland). Journal of Elementology, 22(2), 697–711.
Kalbarczyk, R., Sobolewski, R., & Kalbarczyk, E. (2016). Biometeorological determinants of the tropospheric ozone concentration in the suburban conditions of Wroclaw, Poland. Journal of Elementology, 21(3), 729–744.
Kanaya, Y., Tanimoto, H., Matsumoto, J., Furutani, H., Hashimoto, S., Komazaki, Y., et al. (2007). Diurnal variations in H2O2, O3, PAN, HNO3 and aldehyde concentrations and NO/NO2 ratios at Rishiri Island, Japan: potential influence from iodine chemistry. The Science of the Total Environment, 376(1–3), 185–197.
Karagiannidis, A., Poupkou, A., Giannaros, T., Giannaros, C., Melas, D., & Argiriou, A. (2015). The air quality of a Mediterranean urban environment area and its relation to major meteorological parameters. Water, Air, & Soil Pollution, 226(1), 2239.
Leighton, P. (1961). Photochemistry of air pollution. New York: Academic Press.
Manahan, S. (2007). Environmental science and technology. A sustainable approach to green science and technology. Boca Raton: CRC Press/Taylor & Francis.
Mazzeo, N., Venegas, L., & Choren, H. (2005). Analysis of NO, NO2, O3 and NOx concentrations measured at a green area of Buenos Aires City during wintertime. Atmospheric Environment, 39(17), 3055–3068.
Nicholson, J., Weston, K., & Fowler, D. (2001). Modelling horizontal and vertical concentration profiles of ozone and oxides of nitrogen within high-latitude urban areas. Atmospheric Environment, 35(11), 2009–2022.
Padro, J. (1996). Summary of ozone dry deposition velocity measurements and model estimates over vineyard, cotton, grass and deciduous forest in summer. Atmospheric Environment, 30(13), 2363–2369.
Sakamoto, K., Takada, H., & Sekiguchi, K. (2004). Influence of ozone, relative humidity, and flow rate on the deposition and oxidation of sulfur dioxide on yellow sand. Atmospheric Environment, 38(40), 6961–6967.
Seinfeld, J., & Pandis, S. (2016). Atmospheric chemistry and physics: From air pollution to climate change. Hoboken: Wiley.
Sharma, S., Sharma, P., & Khare, M. (2017). Photo-chemical transport modelling of tropospheric ozone: a review. Atmospheric Environment, 159, 34–54.
Stutz, J., Wong, K., Lawrence, L., Ziemba, L., Flynn, J., Rappenglück, B., et al. (2010). Nocturnal NO3 radical chemistry in Houston, TX. Atmospheric Environment, 44(33), 4099–4106.
Tang, L., Rayner, D., & Haeger-Eugensson, M. (2011). Have meteorological conditions reduced NO2 concentrations from local emission sources in Gothenburg? Water, Air, & Soil Pollution, 221(1–4), 275–286.
Valotto, G., & Varin, C. (2016). Characterization of hourly NOx atmospheric concentrations near the Venice international airport with additive semi-parametric statistical models. Atmospheric Research, 167, 216–223.
Wałaszek, K., Kryza, M., & Werner, M. (2018). The role of precursor emissions on ground level ozone concentration during summer season in Poland. Journal of Atmospheric Chemistry, 75, 181–204.
Wang, S., Shi, C., Zhou, B., Zhao, H., Wang, Z., Yang, S., et al. (2013). Observation of NO3 radicals over Shanghai, China. Atmospheric Environment, 70, 401–409.
Warmiński, K., & Bęś, A. (2009). Diurnal and seasonal variations in the NO2 photolysis rate constant, NO titration rate constant and the NO2/NO ratio in ambient air in the City of Olsztyn. Ecological Chemistry and Engineering A, 16(8), 1029–1037.
Warmiński, K., & Rogalski, L. (2006). Wind direction as a determinant of nitric oxide and nitrogen dioxide imission. Zeszyty Problemowe Postepow Nauk Rolniczych, 513, 517–525 (in polish).
Warmiński, K., & Rogalski, L. (2007). Analysis of photochemical reactions of ozone and its precursors in the troposphere in the summer and winter periods. Polish Journal of Environmental Studies, 16(3B), 497–502.
Zapletal, M., Pretel, J., Chroust, P., Cudlín, P., Edwards-Jonášová, M., Urban, et al. (2012). The influence of climate change on stomatal ozone flux to a mountain Norway spruce forest. Environmental Pollution, 169, 267–273.
Zheng, S., Singh, R., Wu, Y., & Wu, C. (2017). A comparison of trace gases and particulate matter over Beijing (China) and Delhi (India). Water, Air, & Soil Pollution, 228(5), 181.
Acknowledgments
The authors would like to thank Professor Leszek J. Rogalski who initiated an air pollution research program in the city of Olsztyn and promoted the idea of building an air quality monitoring station on the University campus. This study was supported by the Ministry of Science and Higher Education of Poland as part of statutory activities (No. 20.610.027-300).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Warmiński, K., Bęś, A. Atmospheric Factors Affecting a Decrease in the Night-Time Concentrations of Tropospheric Ozone in a Low-Polluted Urban Area. Water Air Soil Pollut 229, 350 (2018). https://doi.org/10.1007/s11270-018-4012-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-4012-x