Abstract
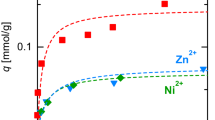
In this study, the competitive adsorption of Ni(II)–Pb(II) and Ni(II)–Zn(II) on oxidized activated carbon fiber (ACF-Ox) from aqueous solutions was studied. The experimental competitive adsorption data were interpreted by the following multicomponent adsorption isotherms: non-modified, extended, and modified Langmuir; non-modified and modified Redlich-Peterson; extended Freundlich; and Sheindorf-Rebuhn-Sheintuch. The extended Freundlich multicomponent isotherm best fitted the adsorption data for both systems Ni(II)–Pb(II) and Ni(II)–Zn(II) onto ACF-Ox. The single metal adsorption on ACF-Ox showed that the Ni(II) adsorption capacity was 1.12 times greater than that of Zn(II); however, in the competitive adsorption, the Zn(II) presented an intense antagonism to the adsorption of Ni(II) and Ni(II) to Zn(II) too. The single-adsorption isotherms of Pb(II) and Ni(II) on ACF-Ox revealed that the selectivity of Pb(II) towards ACF-Ox was slightly higher than that of the Ni(II). Nevertheless, in the competitive adsorption, the affinity of Pb(II) towards the ACF-Ox was far greater than that of the Ni(II).








Similar content being viewed by others
Abbreviations
- a i :
-
Redlich-Peterson isotherm constant for metal i (L/g)
- a ij :
-
Competition coefficient of component i by component j
- b i :
-
Redlich-Peterson isotherm constant for metal i (L/meq)βι
- C i :
-
Concentration of metal i in aqueous solution at equilibrium (meq/L)
- C i,exp :
-
Experimental concentration of metal i (meq/L)
- C i,F :
-
Final concentration of metal i in aqueous solution at equilibrium (meq/L)
- C i,0 :
-
Initial concentration of metal i in aqueous solution (meq/L)
- %D :
-
Average percentage deviation
- K i :
-
Langmuir isotherm constant of metal i (L/meq)
- K E,i :
-
Extended Langmuir multicomponent isotherm constant for component i (L/meq)
- k i :
-
Freundlich isotherm constant for metal i (meq(1–1/n) L(1/n)/g)
- m :
-
Mass of ACF-Ox (g)
- N :
-
Number of metals in solution
- N m :
-
Number of experiment data
- n i :
-
Freundlich isotherm constant for metal i
- q i :
-
Uptake of metal i adsorbed per mass of ACF-Ox (meq/g)
- q i,j,cal :
-
Uptake of metal of i corresponding to data number j calculated with the multicomponent adsorption isotherm (meq/g)
- q i,j,exp :
-
Experimental uptake of metal i corresponding to data number j (meq/g)
- q m,i :
-
Langmuir isotherm constant for metal i (meq/g)
- q max :
-
Constant of extended Langmuir multicomponent isotherm (meq/g)
- V 0 :
-
Initial volume of solution (L)
- x i, y i, z i :
-
Extended Freundlich multicomponent isotherm constants for component i
- β i :
-
Redlich-Peterson isotherm constant for metal i
- η i :
-
Interaction factor for metal i
References
Álvarez-Merino, M. A., López-Ramón, V., & Moreno-Castilla, C. (2005). A study of the static and dynamic adsorption of Zn(II) ions on carbon materials from aqueous solutions. Journal of Colloid and Interface Science, 288(2), 335–341.
Arcibar-Orozco, J. A., Rangel-Mendez, J. R., & Diaz-Flores, P. E. (2014). Simultaneous adsorption of Pb(II)-Cd(II), Pb(II)-phenol, and Cd(II)-phenol by activated carbon cloth in aqueous solution. Water, Air, and Soil Pollution, 226(1).
Babic, B. M., Milonjic, S. K., Polovina, M. J., & Kaludierovic, B. V. (1999). Point of zero charge and intrinsic equilibrium constants of activated carbon cloth. Carbon, 37(3), 477–481.
Babic, B. M., Milonjic, S. K., Polovina, M. J., Cupic, S., & Kaludjerovic, B. V. (2002). Adsorption of zinc, cadmium and mercury ions from aqueous solutions on an activated carbon cloth. Carbon, 40(7), 1109–1115.
Berber-Mendoza, M. S., Leyva-Ramos, R., Cerino-Cordoba, F. J., Mendoza-Barron, J., Garcia, H. J. A., & Flores-Cano, J. V. (2013). Role of carboxylic sites in the adsorption of nickel (II) and zinc (II) onto plain and oxidized activated carbon fibers. Water, Air, and Soil Pollution, 224(7).
Boehm, H. P. (1994). Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon, 32(5), 759–769.
Faur, C., Métivier-Pignon, H., & Le Cloirec, P. (2005). Multicomponent adsorption of pesticides onto activated carbon fibers. Adsorption, 11(5–6), 479–490.
Faur-Brasquet, C., Kadirvelu, K., & Le Cloirec, P. (2002). Removal of metal ions from aqueous solution by adsorption onto activated carbon cloths: adsorption competition with organic matter. Carbon, 40(13), 2387–2392.
Futalan, C. M., Kan, C. C., Dalida, M. L., Hsien, K. J., Pascua, C., & Wan, M. W. (2011). Comparative and competitive adsorption of copper, lead, and nickel using chitosan immobilized on bentonite. Carbohydrate Polymers, 83(2), 528–536.
Kadirvelu, K., Faur-Brasquet, C., & Le Cloirec, P. (2000). Removal of Cu(II), Pb(II), and Ni(II) by adsorption onto activated carbon cloths. Langmuir, 16(22), 8404–8409.
Kang, K. C., Kim, S. S., Choi, J. W., & Kwon, S. K. (2008). Sorption of Cu2+ and Cd2+ onto acid- and base-pretreated granular activated carbon and activated carbon fiber samples. Journal of Industrial and Engineering Chemistry, 14, 131–135.
Leyva, R., Flores, J. V., Díaz, P. E., & Berber, M. S. (2008). Adsorción de cromo (VI) en solución acuosa sobre fibra de carbón activado. Informacion Tecnologica, 19(5), 27–36.
Leyva-Ramos, R., Berber-Mendoza, M. S., Salazar-Rabago, J., Guerrero-Coronado, R. M., & Mendoza-Barron, J. (2011). Adsorption of lead(II) from aqueous solution onto several types of activated carbon fibers. Adsorption, 17(3), 515–526.
Liu, Z., Zhou, L., Wei, P., Zeng, K., Wen, C., & Lan, H. (2008). Competitive adsorption of heavy metal ions on peat. Journal of China University of Mining and Technology, 18(2), 255–260.
Mahamadi, C., & Nharingo, T. (2010). Competitive adsorption of Pb2+, Cd2+ and Zn2+ ions onto Eichhornia crassipes in binary and ternary systems. Bioresource Technology, 101(3), 859–864.
Masson, S., Gineys, M., Delpeux-Ouldriane, S., Reinert, L., Guittonneau, S., Béguin, F., & Duclaux, L. (2016). Single, binary, and mixture adsorption of nine organic contaminants onto a microporous and a microporous/mesoporous activated carbon cloth. Microporous and Mesoporous Materials, 234, 24–34.
Mena Aguilar, K. M., Amano, Y., & Machida, M. (2016). Ammonium persulfate oxidized activated carbon fiber as a high capacity adsorbent for aqueous Pb(II). Journal of Environmental Chemical Engineering, 4, 4644–4652.
Padilla-Ortega, E., Leyva-Ramos, R., & Flores-Cano, J. V. (2013). Binary adsorption of heavy metals from aqueous solution onto natural clays. Chemical Engineering Journal, 225, 536–546.
Papageorgiou, S. K., Katsaros, F. K., Kouvelos, E. P., & Kanellopoulos, N. K. (2009). Prediction of binary adsorption isotherms of Cu2+, Cd2+ and Pb2+ on calcium alginate beads from single adsorption data. Journal of Hazardous Materials, 162(2–3), 1347–1354.
Park, S. J., Shin, J. S., Shim, J. W., & Ryu, S. K. (2004). Effect of acidic treatment on metal adsorptions of pitch-based activated carbon fibers. Journal of Colloid and Interface Science, 275(1), 342–344.
Rangel-Mendez, J. R., & Streat, M. (2002). Adsorption of cadmium by activated carbon cloth: influence of surface oxidation and solution pH. Water Research, 36(5), 1244–1252.
Sheindorf, C., Rebhun, M., & Sheintuch, M. (1981). A Freundlich-type multicomponent isotherm. Journal of Colloid and Interface Science, 79(1), 136–142.
Shim, J. W., Park, S. J., & Ryu, S. K. (2001). Effect of modification with HNO3 and NaOH on metal adsorption by pitch-based activated carbon fibers. Carbon, 39(11), 1635–1642.
Srivastava, V. C., Mall, I. D., & Mishra, I. M. (2006a). Equilibrium modelling of single and binary adsorption of cadmium and nickel onto bagasse fly ash. Chemical Engineering Journal, 117(1), 79–91.
Srivastava, V. C., Mall, I. D., & Mishra, I. M. (2006b). Modelling individual and competitive adsorption of cadmium(II) and zinc(II) metal ions from aqueous solution onto bagasse fly ash. Separation Science and Technology, 41(12), 2685–2710.
Srivastava, V. C., Mall, I. D., & Mishra, I. M. (2008). Removal of cadmium(II) and zinc(II) metal ions from binary aqueous solution by rice husk ash. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 312(2–3), 172–184.
Taşar, Ş., Kaya, F., & Özer, A. (2014). Biosorption of lead(II) ions from aqueous solution by peanut shells: equilibrium, thermodynamic and kinetic studies. Journal of Environmental Chemical Engineering, 2(2), 1018–1026.
Yang, R. T. (1987). Gas separation by adsorption processes, Elsevier Ltd. All rights reserved, Imprint Butterworth-Heinemann.
Zhu, Y., Hu, J., & Wang, J. (2012). Competitive adsorption of Pb(II), Cu(II) and Zn(II) onto xanthate-modified magnetic chitosan. Journal of Hazardous Materials, 221–222, 155–161.
Funding
This work was funded by Fondo de Ciencia Basica SEP-CONACyT through grant no. SEP-61537 and Fondo de apoyo a la Investigación (FAI) C12-FAI-03-48.48.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berber-Mendoza, M.S., Martínez-Costa, J.I., Leyva-Ramos, R. et al. Competitive Adsorption of Heavy Metals from Aqueous Solution onto Oxidized Activated Carbon Fiber. Water Air Soil Pollut 229, 257 (2018). https://doi.org/10.1007/s11270-018-3906-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-3906-y