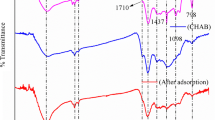
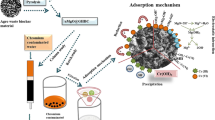
Abstract
Biochars, derived from rambutan (Nepheliumlappaceum) peel through slow pyrolysis, were characterised and investigated as potential adsorbent for the removal of copper ion, Cu(II) from aqueous solution. Characteristics of five biochars of rambutan peel with different pyrolytic temperatures ranging from 300 to 700 °C (B300, B400, B500, B600, B700) were studied, and adsorption abilities of respective biochars were evaluated. Adsorption experiments were carried out by varying adsorbent dosage (0.2, 0.4, 0.8, 1.0, 2.0, and 4.0 g/L) and initial copper ion, Cu(II) concentrations (50 and 100 mg/L) to determine the optimum pyrolytic temperature of biochar with high adsorption affinity. The adsorption kinetics were best described by the pseudo-second order model for all the tested biochars, while the adsorption equilibrium best fitted by Langmuir isotherm. The overall results showed that biochar derived at 600 °C can be used as an effective adsorbent for removal of Cu(II) from aqueous solutions. Furthermore, feedforward artificial neural network (FFBP) modelling was performed to compare the simulated results with experimental output data of Thermogravimetric analysis (TGA) and atomic absorption spectroscopy (AAS) analysis which were trained using Levenberg-Marquardt (LM) backpropagation algorithm. The FFBP structure for pyrolysis process comprised of TGA temperature as input and biomass final weight as output. The adsorption modelling was simulated using adsorption time, temperature, biochar dosage and initial Cu(II) concentration as input data, while final Cu(II) concentration was used as output data to the network. Finally, modelling structure of 1-9-1 and 4-8-1 gave best performance with regression, R 2 value of 0.9999 and 0.9547 for TGA and AAS analysis, respectively.










Similar content being viewed by others
References
Agrafioti, E., et al. (2013). Biochar production by sewage sludge pyrolysis. Journal of Analytical and Applied Pyrolysis, 101, 72–78.
Ahmad, M., et al. (2012). Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresource Technology, 118, 536–544
Ahmad, M. A., & Alrozi, R. (2011a). Optimization of rambutan peel based activated carbon preparation conditions for Remazol Brilliant Blue R removal. Chemical Engineering Journal, 168(1), 280–285.
Ahmad, M. A., & Alrozi, R. (2011b). Removal of malachite green dye from aqueous solution using rambutan peel-based activated carbon: equilibrium, kinetic and thermodynamic studies. Chemical Engineering Journal, 171(2), 510–516.
Aksu, Z., & İşoğlu, İ. A. (2005). Removal of copper(II) ions from aqueous solution by biosorption onto agricultural waste sugar beet pulp. Process Biochemistry, 40(9), 3031–3044.
Ali, R. M., et al. (2016). Potential of using green adsorbent of heavy metal removal from aqueous solutions: adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecological Engineering, 91, 317–332.
Aman, T., et al. (2008). Potato peels as solid waste for the removal of heavy metal copper(II) from waste water/industrial effluent. Colloids and Surfaces, B: Biointerfaces, 63(1), 116–121.
Arumugasamy, S. K., & Selvarajoo, A. (2015). Feedforward neural network modeling of biomass pyrolysis process for biochar production. Chemical Engineering Transactions, 45, 1681–1686.
Aydin, H., Bulut, Y., & Yerlikaya, Ç. (2008). Removal of copper (II) from aqueous solution by adsorption onto low-cost adsorbents. Journal of Environmental Management, 87(1), 37–45.
Benaïssa, H., & Elouchdi, M. A. (2007). Removal of copper ions from aqueous solutions by dried sunflower leaves. Chemical Engineering and Processing: Process Intensification, 46(7):614–622
Chithra, S., et al. (2016). A comparative study on the compressive strength prediction models for High Performance Concrete containing nano silica and copper slag using regression analysis and Artificial Neural Networks. Construction and Building Materials, 114, 528–535.
Conesa, J. A., Caballero, J. A., & Reyes-Labarta, J. A. (2004). Artificial neural network for modelling thermal decompositions. Journal of Analytical and Applied Pyrolysis, 71(1), 343–352.
Demirbas, A., & Arin, G. (2002). An overview of biomass pyrolysis. Energy Sources, 24(July 2015), 471–482.
El-Ashtoukhy, E. S. Z., Amin, N. K., & Abdelwahab, O. (2008). Removal of lead (II) and copper (II) from aqueous solution using pomegranate peel as a new adsorbent. Desalination, 223(1–3), 162–173.
Erenturk, S., & Malkoc, E. (2006). Removal of lead(II) by adsorption onto Viscumalbum L.: Effect of temperature and equilibrium isotherm analyses. Applied Surface Science, 253(10), 4727–4733.
Gai, X., et al. (2014). Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PloS One, 9(12), 1–19.
Iftikhar, A. R., et al. (2009). Kinetic and thermodynamic aspects of Cu(II) and Cr(III) removal from aqueous solutions using rose waste biomass. Journal of Hazardous Materials, 161(2–3), 941–947.
Imamoglu, M., & Tekir, O. (2008). Removal of copper (II) and lead (II) ions from aqueous solutions by adsorption on activated carbon from a new precursor hazelnut husks. Desalination, 228(1–3), 108–113.
Jia, M., et al. (2013). Effects of pH and metal ions on oxytetracycline sorption to maize-straw-derived biochar. Bioresource Technology, 136, 87–93.
Jiang, S., et al. (2016). Copper and zinc adsorption by softwood and hardwood biochars under elevated sulphate-induced salinity and acidic pH conditions. Chemosphere, 142, 64–71.
Kiani, M. K. D., & Ghobadian, B. (2012). Artificial neural networks approach for the prediction of Thermal balance of SI engine using ethanol-gasoline blends, multidisciplinary research and practice for information systems. Lecture Notes in Computer Science, 7465, 31–43.
Kiliç, M., et al. (2013). Adsorption of heavy metal ions from aqueous solutions by bio-char, a by-product of pyrolysis. Applied Surface Science, 283, 856–862.
Komnitsas, K., et al. (2015). Assessment of pistachio shell biochar quality and its potential for adsorption of heavy metals. Waste and Biomass Valorization, 6(5), 805–816.
Kumar, U., & Bandyopadhyay, M. (2006). Sorption of cadmium from aqueous solution using pretreated rice husk. Bioresource Technology, 97(1), 104–109.
Langmuir, I. (1918). The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemical Society, 40(9), 1361–1403.
Lee, Y., Eum, P. R. B., et al. (2013a). Characteristics of biochar produced from slow pyrolysis of Geodae-Uksae 1. Bioresource Technology, 130, 345–350.
Lee, Y., Park, J., et al. (2013b). Comparison of biochar properties from biomass residues produced by slow pyrolysis at 500 °C. Bioresource Technology, 148, 196–201.
Lehmann, J. A. J. (2015). Biochar for environmental management: science, technology and implementation. Abingdon: Routledge s.l.:s.n.
Li, F., & Shen, K. (2016). Preparation and characterization of biochars from Eichornia crassipes for cadmium removal in aqueous solutions. PLoS One, 11(12), 1–13.
Li, M., et al. (2013). Cu(II) removal from aqueous solution by Spartina alterniflora derived biochar. Bioresource Technology, 141, 83–88.
Mahmoud, D. K., et al. (2011). Batch adsorption of basic dye using acid treated kenaf fibre char: Equilibrium, kinetic and thermodynamic studies. Chemical Engineering Journal, 181–182, 449–457.
Maurya, R., et al. (2014). Biosorption of methylene blue by de-oiled algal biomass: equilibrium, kinetics and artificial neural network modelling. PloS One, 9(10), 1–13.
Mohan, D., et al. (2014). Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—a critical review. Bioresource Technology, 160, 191–202.
Moustafa, A. A. (2011). Performance evaluation of artificial neural networks for spatial data analysis. Contemporary Engineering Sciences, 4, 149–163.
Ozcimen, D., & Ersoy-Mericboyu, A. (2010). Characterization of biochar and bio-oil samples obtained from carbonization of various biomass materials. Renewable Energy, 35(6), 1319–1324.
Park, S. H., et al. (2016). Removal of copper(II) in aqueous solution using pyrolytic biochars derived from red macroalga Porphyra tenera. Journal of Industrial and Engineering Chemistry, 36, 314–319.
Pellera, F. M., et al. (2012). Adsorption of Cu(II) ions from aqueous solutions on biochars prepared from agricultural by-products. Journal of Environmental Management, 96(1), 35–42.
Shariff, A., Aziz, N. S. M., & Abdullah, N. (2014). Slow pyrolysis of oil palm empty fruit bunches for biochar production and characterisation. Journal of Physical Science, 25(2), 97–112.
Subramanyam, B., & Das, A. (2009). Linearized and non-linearized isotherm models comparative study on adsorption of aqueous phenol solution in soil. International Journal of Environmental Science & Technology, 6(4), 633–640.
Sun, H., et al. (2012). Multiple controls on the chemical and physical structure of biochars. Industrial and Engineering Chemistry Research, 51(9), 3587–3597.
Sun, L., Wan, S., & Luo, W. (2013). Biochars prepared from anaerobic digestion residue, palm bark, and eucalyptus for adsorption of cationic methylene blue dye: characterization, equilibrium, and kinetic studies. Bioresource Technology, 140, 406–413.
Tsai, W. T., & Chen, H. R. (2013). Adsorption kinetics of herbicide paraquat in aqueous solution onto a low-cost adsorbent, swine-manure-derived biochar. International journal of Environmental Science and Technology, 10, 1349.
Uchimiya, M., et al. (2011). Influence of pyrolysis temperature on biochar property and function as a heavy metal sorbent in soil. Journal of Agricultural and Food Chemistry, 59(6), 2501–2510.
Xu, R., et al. (2011). Adsorption of methyl violet from aqueous solutions by the biochars derived from crop residues. Bioresource Technology, 102(22), 10293–10298.
Yang, Y., et al. (2014). Evaluation of adsorption potential of bamboo biochar for metal-complex dye: equilibrium, kinetics and artificial neural network modeling. International Journal of Environmental Science & Technology, 11(4), 1093–1100.
Yao, H., et al. (2012). Adsorption of fluoroquinolone antibiotics by wastewater sludge biochar: Role of the sludge source. Chemosphere, 55(6), 829–883.
Zheng, W., et al. (2008). Adsorption removal of cadmium and copper from aqueous solution by areca-a food waste. Journal of Hazardous Materials, 157(2–3), 490–495.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Selvanathan, M., Yann, K.T., Chung, C.H. et al. Adsorption of Copper(II) Ion from Aqueous Solution Using Biochar Derived from Rambutan (Nepheliumlappaceum) Peel: Feedforward Neural Network Modelling Study. Water Air Soil Pollut 228, 299 (2017). https://doi.org/10.1007/s11270-017-3472-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3472-8