Abstract
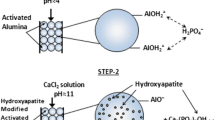
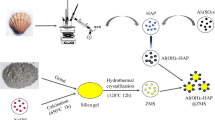
Fluoride retention from water is nowadays a serious health problem. This study reports the potential of a newly developed nano-hydrotalcite/hydroxyapatite (n-HT/HAp) composite, and its constituent materials, hydrotalcite (HT) and hydroxyapatite (HAp), in fluoride removal. Calcined hydrotalcites (cHT) showed a remarkable fluoride removal ability from water through memory effect mechanism. HAp, the mineral compound of bones, adsorbs fluoride as well but through ion exchange mechanism. Fluoride substitutes hydroxyls to produce fluorapatite. Among the tested calcined hydrotalcites, cHT Mg-Al (4:1) sample, composed of magnesium divalent cation to aluminum ratio of 4, was identified as the best-performing hydrotalcite. The differences among cHT samples in fluoride removal capacities are attributed to hydrotalcite composition as well as to particle size. The performance of these materials is compared with that of n-HT/HAp composite whose main features are basic acidic material and not yet tested in fluoride retention. Interestingly, n-HT/HAp also performs best, 98 %, slightly higher than the best cHT Mg-Al (4:1) sample with 97 % fluoride removal efficiency from such a high initial fluoride solution of 20 mg/L at 10 g/L dose, yielding the final residual fluoride concentrations of 0.36 and 0.6 mg/L, respectively; both meet the WHO standard for drinking water. Besides, the uncalcined hydrotalcite constituent added virtue to the advantage of using n-HT/HAp in fluoride removal as the efficiency was compensated by the nanometric size of the hydrotalcite particle.




Similar content being viewed by others
References
Abe, I., Iwasaki, S., Tokimoto, T., Kawasaki, N., Nakamura, T., & Tanada, S. (2004). Journal of Colloid and Interface Science, 275, 35–39.
Badillo-Almaraz, V. E., Flores, J. A., Arriola, H., López, F. A., & Ruiz-Ramírez, L. (2007). Journal of Radioanalytical and Nuclear Chemistry, 271, 741–744.
Bengtsson, Å., Shchukarev, A., Persson, P., & Sjöberg, S. (2009). Langmuir, 25, 2355–2362.
Chen, N., Zhang, Z., Feng, C., Li, M., Zhu, D., Chen, R., & Sugiura, N. (2010). Journal of Hazardous Materials, 183, 460–465.
Chinoy, N. J., Rao, M. V., Narayana, M. V., & Neelakanta, E. (1991). Reproductive Toxicology, 5, 505–512.
Christoffersen, J., Christoffersen, M. R., Arends, J., & Leonardsen, E. S. (1995). Caries Research, 29, 223–230.
de Roy, A., Forano, C., & Besse, J. P. (2006). Layered double hydroxides: synthesis and post-synthesis modification. In V. Rives (Ed.), Layered double hydroxides: present and future. New York: Nova Science Publishers Inc.
Díaz-Barriga, F., Navarro-Quezada, A., Grijalva, M. I., Grimaldo, M., Loyola-Rodríguez, J. P., & Deogracias-Ortiz, M. (1997). Fluoride, 30, 233–239.
Erickson, K. L., Bostrom, T. E., & Frost, R. L. (2004). Materials Letters, 59, 226–229.
Feng, L., Xu, W., Liu, T., & Liu, J. (2012). Journal of Hazardous Materials, 221–222, 228–235.
Fetter, G., Hernández, F., Maubert, M., Lara, V. H., & Bosch, P. (1997). Journal of Porous Materials, 4, 27–30.
Galicia Chacón, L., Molina Frechero, N., Oropeza, A., Gaona, E., & Juárez López, L. (2011). Revista Internacional de Contaminacion Ambiental, 27, 283–289.
Gao, S., Sun, R., Wei, Z. G., Zhao, H. Y., Li, H. X., & Hu, F. (2009). Journal of Fluorine Chemistry, 130, 550–556.
Gómez-Hortigüela, L., Pérez-Pariente, J., Chebude, Y., & Díaz, I. (2014). RSC Advances, 4, 7998–8003.
Hammari, L. E. L., Laghzizil, A., Barboux, P., Lahlil, K., & Saoiabi, A. (2004). Journal of Hazardous Materials, B114, 41–44.
Hichour, M., Persin, F., Sandeaux, J., & Gavach, C. (2000). Separation and Purification Technology, 18, 1–11.
Jagtap, S., Yenkie, M. K., Labhsetwar, N., & Rayalu, S. (2012). Chemical Reviews, 112, 2454–2466.
Jiménez-Núñez, M. L., Olguín, M. T., & Solache-Ríos, M. (2007). Separation Science and Technology, 42, 3623–3639.
Jiménez-Reyes, M., & Solache-Ríos, M. (2010). Journal of Hazardous Materials, 180, 297–302.
Kloos, H., & Tekle Haimanot, R. (1999). Distribution of fluoride and fluorosis in Ethiopia and prospects for control. Tropical Medicine and International Health, 4, 355–364.
Leach, S. A. (1959). British Dental Journal, 106, 133–142.
Liang, L., Jing, H., Min, W., & Xue, D. (2006a). Industrial and Engineering Chemistry Research, 45, 8623–8628.
Liang, L., Jing, H., Min, W., Evans, D. G., & Xue, D. (2006b). Journal of Hazardous Materials, B133, 119–128.
Longanathan, P., Vigneswaran, S., Kandasamy, J., & Naidu, R. (2013). Journal of Hazardous Materials, 248–249, 1–19.
McCann, H. G. (1953). Journal of Biological Chemistry, 201, 247–259.
Miyata, S. (1983). Clay Minerals, 31, 305–311.
M. Mourabet, A. El Rhilassi, H. El Boujaady, M. Bennani-Ziatni, R. El Hamri, A. Taitai, J. Saudi Chem. Society (2012). doi:10.1016/j.jscs.2012.03.003.
Mullen, J. (2005). British Dental Journal, 199, 1–4.
Pandi, K., & Viswanathan, N. (2014). Carbohydrate Polymers, 112, 662–667.
Poinern, G. E. J., Ghosh, M. K., Ng, Y.-J., Issa, T. B., Anand, S., & Singh, P. (2011). Journal of Hazardous Materials, 185, 29–37.
Reimann, C., Bjorvatn, K., Frengstad, B., Melaku, Z., Tekle-Haimanot, R., & Siewers, U. (2003). Science of the Total Environment, 311, 65–80.
Rivera, J. A., Fetter, G., Baños, L., Guzman, J., & Bosch, P. (2009a). Journal of Porous Materials, 16, 401–408.
Rivera, J. A., Fetter, G., & Bosch, P. (2009b). Journal of Porous Materials, 16, 409–418.
Sato, T., Fujita, H., Endo, T., & Shimada, M. (1988). Solid, 5, 219–228.
Sternitzke, V., Kaegi, R., Jean-Nicolas, A., Lewin, E., Hering, J. G., & Johnson, C. A. (2012). Science and Technology, 46, 802–809.
Sundaram, S. C., Viswanathan, N., & Meenakshi, S. (2008a). Journal of Hazardous Materials, 155, 206–215.
Sundaram, C. S., Viswanathan, N., & Meenakshi, S. (2008b). Bioresource Technology, 99, 8226–8230.
Sundaram, C. S., Viswanathan, N., & Meenakshi, S. (2009). Journal of Hazardous Materials, 172, 147–151.
Tekle-Haimanot, R., Fekadu, A., Bushera, B., Mekonnen, Y. (1995). Fluoride levels in water and endemic fluorosis in Ethiopian Rift valley. 1st International Workshop on Fluorosis Prevention and Defluoridation of Water. Int. Soc. Fluoride Res. eds E. Dahi & H. Bregnhoj. Ngurdoto, Tanzania, October (1995) 18–22.
The World Health Organization. (1984). Guidelines for drinking water quality, WHO, volume 1, recommendations. Geneva, Switzerland.
The World Health Organization. (1996). Guidelines for drinking water quality, WHO, volume 2, health criteria and other information, second edition. Geneva, Switzerland.
UNICEF’s Position on Water Fluoridation: http://www.nofluoride.com/Unicef_fluor.cfm; consulted on march 31, (2014).
Viswanathan, N., & Meenakshi, S. (2010). Applied Clay Science, 48, 607–611.
Wang, H., Chen, J., Cai, Y., Ji, J., Liu, L., & Teng, H. H. (2007). Applied Clay Science, 35, 59–66.
Zhang, D., Luo, H., Zheng, L., Wang, K., Li, H., Wang, Y., & Feng, H. (2012). Journal of Hazardous Materials, 241–242, 418–426.
Acknowledgments
PB deeply appreciates Universidad Autónoma Nacional de Mexico for supporting him on his sabbatical leave at AAU. ID is grateful to CSIC for her research leave at AAU. The financial support from the Spanish Government MINECO (project MAT2012-31127) and Mexican Government CONACYT is acknowledged. The Chemistry Department, Addis Ababa University, is also acknowledged for the financial support (TR/008/2011). Taju Sani thanks DU for his study leave at AAU.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sani, T., Adem, M., Fetter, G. et al. Defluoridation Performance Comparison of Nano-hydrotalcite/Hydroxyapatite Composite with Calcined Hydrotalcite and Hydroxyapatite. Water Air Soil Pollut 227, 90 (2016). https://doi.org/10.1007/s11270-016-2786-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-2786-2