Abstract
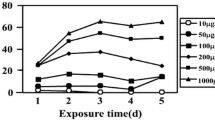
To investigate the intrinsic mechanism of UV-C-induced impairment on cyanobacterial photosynthetic system, a one-off UV-C irradiation and a subsequent 6-day cultivation of Microcystis aeruginosa were conducted. The pulse amplitude modulation fluorometry was used to determine the variations of chlorophyll-a and light-induced curve (LIC); furthermore, the low temperature (77 K) fluorescence technique was used to analyze the emission spectra of photosynthetic apparatus. For 4.6 × 106 cells mL−1 M. aeruginosa suspension, 0–280 mJ cm−2 UV-C irradiation induced an accumulation of chlorophyll-a; however, higher dosage (>280 mJ cm−2) UV-C resulted in a decline of chlorophyll-a during the subsequent cultivation. The results of 77-K spectra demonstrated that UV-C induced intracellular damages of phycobilisome, photosystem I, photosystem II, and thylakoid. The LIC results showed that UV-C suppressed two important photosynthetic strategies: state transition and non-photochemical quenching. Consequently, the UV-C-induced impairment of cyanobacterial photosynthetic system can be summarized as a three-step process: (1) degradation of photosynthetic pigments, (2) decomposition of photosynthetic apparatus, and (3) inhibition of photoacclimation and photoprotection. These impairments resulted in a decline of cyanobacteria community. For 4.6 × 106 cells mL−1 cyanobacteria, 0–280 mJ cm−2 UV-C irradiation induced a decline-recovery procedure of M. aeruginosa cells, but 700–4200 mJ cm−2 UV-C irradiation caused a continuous decrease of cell density (from 4.5 × 106 cells mL−1 to 0) within 6-day cultivation. These results implied that, for a given biomass cyanobacteria community, there is an optimal UV-C dosage threshold to achieve complete inactivation.







Similar content being viewed by others
References
Azevedo, S. M. F. O., Carmichael, W. W., Jochimsen, E. M., Rinehart, K. L., Lau, S., Shaw, G. R., & Eaglesham, G. K. (2001). Human intoxication by microcystins during renal dialysis treatment in Caruaru-Brazil. Toxicology, 164, 32–32.
Bailey, S., & Grossman, A. (2008). Photoprotection in cyanobacteria: regulation of light harvesting. Photochemistry and Photobiology, 84, 1410–1420.
Boulay, C., Abasova, L., Six, C., Vass, I., & Kirilovsky, D. (2008). Occurrence and function of the orange carotenoid protein in photoprotective mechanisms in various cyanobacteria. BBA-Bioenergetics, 1777, 1344–1354.
Briand, J. F., Jacquet, S., Bernard, C., & Humbert, J. F. (2003). Health hazards for terrestrial vertebrates from toxic cyanobacteria in surface water ecosystems. Veterinary Research, 34, 361–377.
Campbell, D., Hurry, V., Clarke, A. K., Gustafsson, P., & Oquist, G. (1998). Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiology and Molecular Biology Reviews, 62, 667–683.
Chen, L. Z., Xie, M., Bi, Y. H., Wang, G. H., Deng, S. Q., & Liu, Y. D. (2012). The combined effects of UV-B radiation and herbicides on photosynthesis, antioxidant enzymes and DNA damage in two bloom-forming cyanobacteria. Ecotoxicology and Environmental Safety, 80, 224–230.
de Figueiredo, D. R., Azeiteiro, U. M., Esteves, S. M., Goncalves, F. J. M., & Pereira, M. J. (2004). Microcystin-producing blooms—a serious global public health issue. Ecotoxicology and Environmental Safety, 59, 151–163.
Gorbunov, M. Y., Kuzminov, F. I., Fadeev, V. V., Kim, J. D., & Falkowski, P. G. (2011). A kinetic model of non-photochemical quenching in cyanobacteria. BBA-Bioenergetics, 1807, 1591–1599.
He, Y. Y., & Hader, D. P. (2002). Reactive oxygen species and UV-B: effect on cyanobacteria. Photochemical and Photobiological Sciences, 1, 729–736.
Huang, J., Graham, N., Templeton, M. R., Zhang, Y., Collins, C., & Nieuwenhuijsen, M. (2009). A comparison of the role of two blue-green algae in THM and HAA formation. Water Research, 43, 3009–3018.
Karapetyan, N. V. (2007). Non-photochemical quenching of fluorescence in cyanobacteria. Biochemistry (Moscow), 72, 1127–1135.
Kirilovsky, D. (2007). Photoprotection in cyanobacteria: the orange carotenoid protein (OCP)-related non-photochemical-quenching mechanism. Photosynthesis Research, 93, 7–16.
Li, D. H., Xie, J., Zhao, J. Q., Xia, A. D., Li, D. H., & Gong, Y. D. (2004). Light-induced excitation energy redistribution in Spirulina platensis cells: “spillover” or “mobile PBSs”? BBA-Bioenergetics, 1608, 114–121.
Li, X., Zhao, Q., Zhou, W., Xu, L., & Wang, Y. (2015). Effects of chronic exposure to microcystin-LR on hepatocyte mitochondrial DNA replication in mice. Environmental Science & Technology, 49.
Lu, F., Wang, G. C., & Jin, H. C. (2011). Photosynthetic responses of thalli and isolated protoplasts of Bryopsis hypnoides (Bryopsidales, Chlorophyta) during dehydration. Chinese Journal of Oceanology and Limnology, 29, 334–342.
Ma, W. M., Ogawa, T., Shen, Y. G., & Mi, H. L. (2007). Changes in cyclic and respiratory electron transport by the movement of phycobilisomes in the cyanobacterium Synechocystis sp strain PCC 6803. BBA-Bioenergetics, 1767, 742–749.
MacColl, R. (1998). Cyanobacterial phycobilisomes. Journal of Structural Biology, 124, 311–334.
Mullineaux, C. W., & Emlyn-Jones, D. (2005). State transitions: an example of acclimation to low-light stress. Journal of Experimental Botany, 56, 389–393.
Nield, J., Morris, E. P., Bibby, T. S., & Barber, J. (2003). Structural analysis of the photosystem I supercomplex of cyanobacteria induced by iron deficiency. Biochemistry-Us, 42, 3180–3188.
Ou, H. S., Gao, N. Y., Deng, Y., Qiao, J. L., Zhang, K. J., Li, T., & Dong, L. (2011). Mechanistic studies of Microcystic aeruginosa inactivation and degradation by UV-C irradiation and chlorination with poly-synchronous analyses. Desalination, 272, 107–119.
Ou, H., Gao, N. Y., Deng, Y., Qiao, J. L., & Wang, H. (2012). Immediate and long-term impacts of UV-C irradiation on photosynthetic capacity, survival and microcystin-LR release risk of Microcystis aeruginosa. Water Research, 46, 1241–1250.
Parmar, A., Singh, N. K., Pandey, A., Gnansounou, E., & Madamwar, D. (2011). Cyanobacteria and microalgae: a positive prospect for biofuels. Bioresource Technology, 102, 10163–10172.
Rakhimberdieva, M. G., Stadnichuk, I. N., Elanskaya, T. V., & Karapetyan, N. V. (2004). Carotenoid-induced quenching of the phycobilisome fluorescence in Photosystem II-deficient mutant of Synechocystis sp. FEBS Letters, 574, 85–88.
Rakhimberdieva, M. G., Vavilin, D. V., Vermaas, W. F. J., Elanskaya, I. V., & Karapetyan, N. V. (2007). Phycobilin/chlorophyll excitation equilibration upon carotenoid-induced non-photochemical fluorescence quenching in phycobilisomes of the Synechoeystis sp PCC 6803. BBA-Bioenergetics, 1767, 757–765.
Ralph, P. J., & Gademann, R. (2005). Rapid light curves: a powerful tool to assess photosynthetic activity. Aquatic Botany, 82, 222–237.
Sakai, H., Katayama, H., Oguma, K., & Ohgaki, S. (2011). Effect of photoreactivation on ultraviolet inactivation of Microcystis aeruginosa. Water Science and Technology, 63, 1224–1229.
Scott, M., McCollum, C., Vasil’ev, S., Crozier, C., Espie, G. S., Krol, M., Huner, N. P. A., & Bruce, D. (2006). Mechanism of the down regulation of photosynthesis by blue light in the cyanobacterium Synechocystis sp PCC 6803. Biochemistry-Us, 45, 8952–8958.
Vaishampayan, A., Sinha, R. P., Hader, D. P., Dey, T., Gupta, A. K., Bhan, U., & Rao, A. L. (2001). Cyanobacterial biofertilizers in rice agriculture. Botanical Review, 67, 453–516.
Watson, S. B., Ridal, J., & Boyer, G. L. (2008). Taste and odour and cyanobacterial toxins: impairment, prediction, and management in the great lakes. Canadian Journal of Fisheries and Aquatic Sciences, 65, 1779–1796.
White, A. J., & Critchley, C. (1999). Rapid light curves: a new fluorescence method to assess the state of the photosynthetic apparatus. Photosynthesis Research, 59, 63–72.
Wilson, A., Ajlani, G., Verbavatz, J. M., Vass, I., Kerfeld, C. A., & Kirilovsky, D. (2006). A soluble carotenoid protein involved in phycobilisome-related energy dissipation in cyanobacteria. Plant Cell, 18, 992–1007.
Wilson, A., Boulay, C., Wilde, A., Kerfeld, C. A., & Kirilovsky, D. (2007). Light-induced energy dissipation in iron-starved cyanobacteria: roles of OCP and IsiA proteins. Plant Cell, 19, 656–672.
Yi, T., Xianzhong, M., Jiangyong, H., Mok, H. O. L., Lingyun, W., Au, D. W. T., Jia, Z., & Xihui, Z. (2013). Mechanisms of photosynthetic inactivation on growth suppression of Microcystis aeruginosa under UV-C stress. Chemosphere, 93, 637–644.
Zhou, H., & Smith, D. W. (2001). Advanced technologies in water and wastewater treatment. Canadian Journal of Civil Engineering, 28, 49–66.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (grant no. 51308224), the Science and Technology Planning Project of Guangdong Province, China (grant no. 2014A020216014), and the State Key Program of the National Natural Science Foundation of China (grant no. 21037001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, J., Ou, H., Chen, J. et al. Intrinsic Mechanism of UV-C-Induced Inactivation of Microcystis aeruginosa: Impairment on Photosynthetic System. Water Air Soil Pollut 227, 82 (2016). https://doi.org/10.1007/s11270-016-2770-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-2770-x