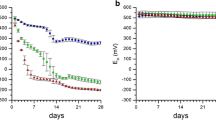
Abstract
Redox conditions play an outstanding role in controlling the behaviour of trace elements in soil environments. They are not only sensitive to water saturation but also to soil temperature because many redox reactions are mediated by microorganisms. In this study, we investigated the influence of oxidising (oxygen predominant), weakly reducing (MnIII,IV reduction) and moderately reducing (FeIII reduction) conditions at three temperature regimes (7, 15 and 25 °C) on the solubility of ten trace elements. Multimetal-contaminated topsoil (pH 5.8) from a floodplain in Germany was investigated with the following aqua regia-soluble concentrations: Zn 903, Cu 551, Cr 488, Pb 354, Ni 93.5, As 35.7, Co 22.4, Sb 20.5, Cd 8.3 and Mo 6.5 mg kg−1. Soil suspensions were held at fixed redox potential in microcosm experiments, sampled at every third day and analysed for trace elements. Time to achieve weakly and particularly moderately reducing conditions was temperature dependent and increased in the order 25 °C < 15 °C < 7 °C. Under oxidising conditions, the solubility of the trace elements was low. Reductive dissolution of Mn oxides under weakly reducing conditions was accompanied by a release of Co and Mo. Reductive dissolution of Fe oxides (and of remaining Mn oxides) under moderately reducing conditions additionally led to a release of As, Cd, Cr, Ni and Pb, whereas Cu and Zn were hardly affected. Antimony revealed a different behaviour because, after a first increase, a continuous decrease in its concentration was observed soon after the onset of weakly reducing conditions. We conclude that soil temperature should be considered as a master variable, to distinguish between weakly and moderately reducing soil conditions, and that it is necessary to keep element-specific behaviour in mind when dealing with the effects of redox conditions in soils on trace element solubility.






Similar content being viewed by others
References
Abdel-Samad, H., & Watson, P. R. (1997). An XPS study of the adsorption of chromate on goethite (α-FeOOH). Applied Surface Science, 108(3), 371–377.
Ackermann, J., Vetterlein, D., Kuehn, T., Kaiser, K., & Jahn, R. (2010). Minerals controlling arsenic distribution in floodplain soils. European Journal of Soil Science, 61(4), 588–598.
Adriano, D. C. (2001). Trace elements in terrestrial environments: biogeochemistry, bioavailability, and risks of metals (2nd ed.). New York: Springer.
Antić-Mladenović, S., Rinklebe, J., Frohne, T., Stärk, H.-J., Wennrich, R., Tomić, Z., & Liĉina, V. (2011). Impact of controlled redox conditions on nickel in a serpentine soil. Journal of Soils and Sediments, 11(3), 406–415.
Bartlett, R. J., & James, B. R. (1993). Redox chemistry of soils. Advanced Agronomy, 50, 151–208.
Bauer, M., & Blodau, C. (2006). Mobilization of arsenic by dissolved organic matter from iron oxides, soils and sediments. Science of the Total Environment, 354(2–3), 179–190.
Bennett, B., & Dudas, M. J. (2003). Release of arsenic and molybdenum by reductive dissolution of iron oxides in a soil with enriched levels of native arsenic. Journal of Environmental Engineering Science, 2(4), 265–272.
BGR (2008). Flächenrepräsentative Hintergrundwerte für As, Sb, Be, Mo, Co, Se, Tl, U und V in Böden Deutschlands aus länderübergreifender Sicht. http://www.bgr.bund.de/DE/Themen/Boden/Produkte/Schriften/Downloads/Hintergrundwerte.pdf?__blob=publicationFile&v=2. Accessed 10 May 2013.
Bohn, H. L., Myer, R. A., & O’Connor, G. A. (2001). Soil chemistry (3rd ed.). New York: Wiley.
Burns, R. G. (1976). The uptake of cobalt into ferromanganese nodules, soils, and synthetic manganese (IV) oxides. Geochimica et Cosmochimica Acta, 40(1), 95–102.
Carbonell, A., Porthouse, J. D., Mulbah, C. K., Delaune, R. D., & Patrick, W. H. (1999). Metal solubility in phosphogypsum-amended sediment under controlled pH and redox conditions. Journal of Environmental Quality, 28(1), 232–242.
Charlatchka, R., & Cambier, P. (2000). Influence of reducing conditions on solubility of trace metals in contaminated soils. Water, Air, and Soil Pollution, 118(1), 143–168.
Chuan, M. C., Shu, G. Y., & Liu, J. C. (1996). Solubility of heavy metals in a contaminated soil: effects of redox potential and pH. Water, Air, and Soil Pollution, 90(3), 543–556.
Cogger, C. G., Kennedy, P. E., & Carlson, D. (1992). Seasonally saturated soils in the Puget Lowland: 2. Measuring and interpreting redox potentials. Soil Science, 154(1), 50–58.
Collins, R. N., & Kinsela, A. S. (2010). The aqueous phase speciation and chemistry of cobalt in terrestrial environments. Chemosphere, 79(8), 763–771.
Connell, W. E., & Patrick, W. H. (1968). Sulfate reduction in soil—effects of redox potential and pH. Science, 159(3810), 86–87.
Cornelis, G., Van Gerven, T., & Vandecasteele, C. (2012). Antimony leaching from MSWI bottom ash: modelling of the effect of pH and carbonation. Waste Management, 32(2), 278–286.
Cornu, S., Cattle, J. A., Samouelian, A., Laveuf, C., Guilherme, L. R. G., & Alberic, P. (2009). Impact of redox cycles on manganese, iron, cobalt, and lead in nodules. Soil Science Society of America Journal, 73(4), 1231–1241.
Deschamps, E., Ciminelli, V. S. T., Weidler, P. G., & Ramos, A. Y. (2003). Arsenic sorption onto soils enriched in Mn and Fe minerals. Clays and Clay Minerals, 51(2), 197–204.
Deuel, L. E., & Swoboda, A. R. (1972). Arsenic solubility in a reduced environment. Soil Science Society of America Proceedings, 36(2), 276–278.
Devai, I., Patrick, W. H., Neue, H. U., DeLaune, R. D., Kongchum, M., & Rinklebe, J. (2005). Methyl mercury and heavy metal content in soils of rivers Saale and Elbe (Germany). Analytical Letters, 38(6), 1037–1048.
Du Laing, G., Rinklebe, J., Vandecasteele, B., Meers, E., & Tack, F. M. G. (2009). Trace metal behaviour in estuarine and riverine floodplain soils and sediments: a review. Science of the Total Environment, 407(13), 3972–3985.
Farmer, J. G., Paterson, E., Bewley, R. J. F., Geelhoed, J. S., Hillier, S., Meeussen, J. C. L., Lumsdon, D. G., Thomas, R. P., & Graham, M. C. (2006). The implications of integrated assessment and modelling studies for the future remediation of chromite ore processing residue disposal sites. Science of the Total Environment, 360(1–3), 90–97.
Favre, F., Boivin, P., & Wopereis, M. C. S. (1997). Water movement and soil swelling in a dry, cracked Vertisol. Geoderma, 78(1–2), 113–123.
Fendorf, S. E. (1995). Surface-reactions of chromium in soils and waters. Geoderma, 67(1–2), 55–71.
Fendorf, S., & Kocar, B. D. (2009). Biogeochemical processes controlling the fate and transport of arsenic: implications for South and Southeast Asia. Advances in Agronomy, 104, 137–164.
Fendorf, S., Eick, M. J., Grossl, P., & Sparks, D. L. (1997). Arsenate and chromate retention mechanisms on goethite. 1. Surface structure. Environmental Science and Technology, 31(2), 315–320.
Filella, M., & Williams, P. A. (2012). Antimony interactions with heterogeneous complexants in waters, sediments and soils: a review of binding data for homologous compounds. Chemie der Erde - Geochemistry, 72, 49–65.
Goldberg, S., Forster, H. S., & Godfrey, C. L. (1996). Molybdenum adsorption on oxides, clay minerals, and soils. Soil Science Society of America Journal, 60(2), 425–432.
Gotoh, S., & Patrick, W. H. (1972). Transformation of manganese in a waterlogged soil as affected by redox potential and pH. Soil Science Society of America Proceedings, 36(5), 738–742.
Grybos, M., Davranche, M., Gruau, G., & Petitjean, P. (2007). Is trace metal release in wetland soils controlled by organic matter mobility or Fe-oxyhydroxides reduction? Journal of Colloid and Interface Science, 314(2), 490–501.
Grybos, M., Davranche, M., Gruau, G., Petitjean, P., & Pedrot, M. (2009). Increasing pH drives organic matter solubilization from wetland soils under reducing conditions. Geoderma, 154(1–2), 13–19.
Gustafsson, J. P. (2001). Modelling the acid-base properties and metal complexation of humic substances with the Stockholm Humic Model. Journal of Colloid and Interface Science, 244(1), 102–112.
Hem, J. D., Roberson, C. E., & Lind, C. J. (1985). Thermodynamic stability of CoOOH and its co-precipitation with manganese. Geochimica et Cosmochimica Acta, 49(3), 801–810.
Hess, R. E., & Blanchar, R. W. (1977). Dissolution of arsenic from waterlogged and aerated soil. Soil Science Society of America Journal, 41(5), 861–865.
Hooda, P. S. (2010). Trace elements in soils. New York: Wiley.
James, B. R., & Bartlett, R. J. (1983). Behavior of chromium in soils. 5. Fate of organically complexed Cr (III) added to soil. Journal of Environmental Quality, 12(2), 169–172.
Jiang, J., Xu, R., Wang, Y., & Zhao, A. (2008). The mechanism of chromate sorption by three variable charge soils. Chemosphere, 71(8), 1469–1475.
Kay, J. T., Conklin, M. H., Fuller, C. C., & O’Day, P. A. (2001). Processes of nickel and cobalt uptake by a manganese oxide forming sediment in Pinal Creek, Globe Mining District, Arizona. Environmental Science and Technology, 35(24), 4719–4725.
Klemm, W., Greif, A., Broekaert, J. A. C., Siemens, V., Junge, F. W., van der Veen, A., Schultze, M., & Duffek, A. (2005). A study on arsenic and the heavy metals in the Mulde river system. Acta Hydrochimica et Hydrobiologica, 33(5), 475–491.
Knechtenhofer, L. A., Xifra, I. O., Scheinost, A. C., Fluhler, H., & Kretzschmar, R. (2003). Fate of heavy metals in a strongly acidic shooting-range soil: small-scale metal distribution and its relation to preferential water flow. Journal of Plant Nutrition and Soil Science, 166(1), 84–92.
LANUV (2003). Hintergrundwerte für anorganische und organische Stoffe in Oberböden Nordrhein-Westfalens. Landesumweltamt Nordrhein-Westfalens, Düsseldorf. http://www.lanuv.nrw.de/boden/bodenschutz/HGW_Internet_2003-3.pdf. Accessed 10 May 2013.
Li, Y. C., Ge, Y., Zhang, C. H., & Zhou, Q. S. (2010). Mechanisms for high Cd activity in a red soil from southern China undergoing gradual reduction. Australian Journal of Soil Research, 48(4), 371–384.
Manceau, A., Drits, V. A., Silvester, E., Bartoli, C., & Lanson, B. (1997). Structural mechanism of Co2+ oxidation by the phyllomanganate buserite. American Mineralogist, 82(11–12), 1150–1175.
Mansfeldt, T., & Overesch, M. (2013). Arsenic mobility and speciation in a Gleysol with petrogleyic properties: a field and laboratory approach. Journal of Environmental Quality, 42(4), 1130–1141.
Martell, A., Smith, R., & Motekaitis, R. (2004). NIST critically selected stability constants of metal complexes. Gaithersburg: National Institute of Standards and Technology.
Masscheleyn, P. H., Delaune, R. D., & Patrick, W. H. (1991). Effect of redox potential and pH on arsenic speciation and solubility in a contaminated soil. Environmental Science and Technology, 25(8), 1414–1419.
McLaren, R. G., Lawson, D. M., & Swift, R. S. (1986). Sorption and desorption of cobalt by soils and soil components. Journal of Soil Science, 37(3), 413–426.
Meers, E., Unamuno, V. R., Du Laing, G., Vangronsveld, J., Vanbroekhoven, K., Samson, R., Diels, L., Geebelen, W., Ruttens, A., Vandegehuchte, M., & Tack, F. M. G. (2006). Zn in the soil solution of unpolluted and polluted soils as affected by soil characteristics. Geoderma, 136(1–2), 107–119.
Mehra, O. P., & Jackson, M. L. (1960). Iron oxide removal from soils and clays by a dithionite-citrate system buffered with sodium bicarbonate. Clays and Clay Minerals, 7, 317–327.
Mirlean, N., Roisenberg, A., & Chies, J. O. (2007). Metal contamination of vineyard soils in wet subtropics (southern Brazil). Environmental Pollution, 149(1), 10–17.
Mitsunobu, S., Harada, T., & Takahashi, Y. (2006). Comparison of antimony behaviour with that of arsenic under various soil redox conditions. Environmental Science and Technology, 40(23), 7270–7276.
Mitsunobu, S., Takahashi, Y., & Terada, Y. (2010). u-XANES evidence for the reduction of Sb(V) to Sb(III) in soil from Sb mine tailing. Environmental Science and Technology, 44(4), 1281–1287.
Murray, J. W., & Dillard, J. G. (1979). The oxidation of cobalt(II) adsorbed on manganese dioxide. Geochimica et Cosmochimica Acta, 43(5), 781–787.
Olivie-Lauquet, G., Gruau, G., Dia, A., Riou, C., Jaffrezic, A., & Henin, O. (2001). Release of trace elements in wetlands: role of seasonal variability. Water Research, 35(4), 943–952.
Onstott, T. C., Chan, E., Polizzotto, M. L., Lanzon, J., & DeFlaun, M. F. (2011). Precipitation of arsenic under sulfate reducing conditions and subsequent leaching under aerobic conditions. Applied Geochemistry, 26(3), 269–285.
Oremland, R. S., & Stolz, J. F. (2005). Arsenic, microbes and contaminated aquifers. Trends in Microbiology, 13(2), 45–49.
Oscarson, D. W., Huang, P. M., Hammer, U. T., & Liaw, W. K. (1983). Oxidation and sorption of arsenite by manganese-dioxide as influenced by surface-coatings of iron and aluminium-oxides and calcium-carbonate. Water, Air, and Soil Pollution, 20(2), 233–244.
Overesch, M., Rinklebe, J., Broll, G., & Neue, H. U. (2007). Metals and arsenic in soils and corresponding vegetation at Central Elbe river floodplains (Germany). Environmental Pollution, 145(3), 800–812.
Patrick, W. H. (1966). Apparatus for controlling the oxidation-reduction potential of waterlogged soils. Nature, 212(5067), 1278–1279.
Patrick, W. H., & Jugsujinda, A. (1992). Sequential reduction and oxidation of inorganic nitrogen, manganese, and iron in flooded soil. Soil Science Society of America Journal, 56(4), 1071–1073.
Patrick, W. H., Williams, B. G., & Moraghan, J. T. (1973). Simple system for controlling redox potential and pH in soil suspension. Soil Science Society of America Journal, 37(2), 331–332.
Paul, E. A., & Claark, F. E. (1996). Soil microbiology and biochemistry (2nd ed.). San Diego: Academic.
Pelfrêne, A., Gassama, N., & Grimaud, D. (2009). Mobility of major-, minor- and trace elements in solutions of a planosolic soil: distribution and controlling factors. Applied Geochemistry, 24(1), 96–105.
Rabenhorst, M. C. (2005). Biologic zero: a soil temperature concept. Wetlands, 25(3), 616–621.
Rabenhorst, M. C., & Castenson, K. L. (2005). Temperature effects on iron reduction in a hydric soil. Soil Science, 170(9), 734–742.
Rai, D., Sass, B. M., & Moore, D. A. (1987). Chromium(III) hydrolysis constants and solubility of chromium(III) hydroxide. Inorganic Chemistry, 26(3), 345–349.
Ravenscroft, P., Brammer, H., & Richards, K. S. (2009). Arsenic pollution: a global synthesis. Chichester: Wiley-Blackwell.
Schenk, R. (1994). Verteilung und Dynamik von Schwermetallen in Sedimenten der Wupper. Düsseldorf: Universität Düsseldorf.
Schulz-Zunkel, C., & Krueger, F. (2009). Trace metal dynamics in floodplain soils of the river Elbe: a review. Journal of Environmental Quality, 38(4), 1349–1362.
Schwab, A. P., & Lindsay, W. L. (1983). The effect of redox on the solubility and availability of manganese in a calcareous soil. Soil Science Society of America Journal, 47(2), 217–220.
Schwertmann, U. (1964). Differenzierung der Eisenoxide des Bodens durch photochemische Extraction mit saurer Ammoniumoxalatlösung. Zeitschrift für Pflanzenernährung, Düngung und Bodenkunde, 105, 194–202.
Shams, K. M., Tichy, G., Sager, M., Peer, T., Bashar, A., & Jozic, M. (2009). Soil contamination from tannery wastes with emphasis on the fate and distribution of tri- and hexavalent chromium. Water, Air, and Soil Pollution, 199(1–4), 123–137.
Simpson, S. L., Rosner, J., & Ellis, J. (2000). Competitive displacement reactions of cadmium, copper, and zinc added to a polluted, sulfidic estuarine sediment. Environmental Toxicology and Chemistry, 19(8), 1992–1999.
Smedley, P. L., & Kinniburgh, D. G. (2002). A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry, 17(5), 517–568.
Smith, R. M., & Martell, A. E. (1976). Critical stability constants: inorganic complexes (4th ed.). New York: Plenum.
Takahashi, Y., Ohtaku, N., Mitsunobu, S., Yuita, K., & Nomura, M. (2003). Determination of the As(III)/As(V) ratio in soil by X-ray absorption near-edge structure (XANES) and its application to the arsenic distribution between soil and water. Analytical Science, 19(6), 891–896.
van der Geest, H. G., & León Paumen, M. (2008). Dynamics of metal availability and toxicity in historically polluted floodplain sediments. Science of the Total Environment, 406(3), 419–425.
Vaughan, K. L., Rabenhorst, M. C., & Needelman, B. A. (2009). Saturation and temperature effects on the development of reducing conditions in soils. Soil Science Society of America Journal, 73(2), 663–667.
Viollier, E., Inglett, P. W., Hunter, K., Roychoudhury, A. N., & van Cappellen, P. (2000). The ferrozine method revisited: Fe(II)/Fe(III) determination in natural waters. Applied Geochemistry, 15(6), 785–790.
Wan, X. M., Tandy, S., Hockmann, K., & Schulin, R. (2013). Changes in Sb speciation with waterlogging of shooting range soils and impacts on plant uptake. Environmental Pollution, 172, 53–60.
Wang, G., & Staunton, S. (2006). Evolution of water-extractable copper in soil with time as a function of organic matter amendments and aeration. European Journal of Soil Science, 57(3), 372–380.
Weber, F. A., Voegelin, A., & Kretzschmar, R. (2009). Multi-metal contaminant dynamics in temporarily flooded soil under sulfate limitation. Geochimica et Cosmochimica Acta, 73(19), 5513–5527.
Weber, F.-A., Hofacker, A. F., Voegelin, A., & Kretzschmar, R. (2010). Temperature dependence and coupling of iron and arsenic reduction and release during flooding of a contaminated soil. Environmental Science and Technology, 44(1), 116–122.
Weigand, H., Mansfeldt, T., Baumler, R., Schneckenburger, D., Wessel-Bothe, S., & Marb, C. (2010). Arsenic release and speciation in a degraded fen as affected by soil redox potential at varied moisture regime. Geoderma, 159(3–4), 371–378.
Wichard, T., Mishra, B., Myneni, S. C. B., Bellenger, J. P., & Kraepiel, A. M. L. (2009). Storage and bioavailability of molybdenum in soils increased by organic matter complexation. Nature Geoscience, 2(9), 625–629.
Wightwick, A. M., Mollah, M. R., Partington, D. L., & Allinsono, G. (2008). Copper fungicide residues in Australian vineyard soils. Journal of Agricultural and Food Chemistry, 56(7), 2457–2464.
Wilson, S. C., Lockwood, P. V., Ashley, P. M., & Tighe, M. (2010). The chemistry and behaviour of antimony in the soil environment with comparisons to arsenic: a critical review. Environmental Pollution, 158(5), 1169–1181.
WRB and IUSS Working Group (2006). World reference base for soil resources. A framework for international classification, correlation and communication. World soil resources reports 103. FAO, Rome
Xu, N., Christodoulatos, C., & Braida, W. (2006). Adsorption of molybdate and tetrathiomolybdate onto pyrite and goethite: effect of pH and competitive anions. Chemosphere, 62(10), 1726–1735.
Zachara, J. M., Fredrickson, J. K., Smith, S. C., & Gassman, P. L. (2001). Solubilization of Fe(III) oxide-bound trace metals by a dissimilatory Fe(III) reducing bacterium. Geochimica et Cosmochimica Acta, 65(1), 75–93.
Zeien, H., & Brümmer, G. W. (1989). Chemische Extraktion zur Bestimmung von Schwermetallbindungsformen in Böden. Mitteilungen der Deutschen Bodenkundlichen Gesellschaft, 59, 505–510.
Zhi-Guang, L. (1985). Oxidation-reduction potential. In Y. Tian-ren (Ed.), Physical chemistry of paddy soils (pp. 1–26). Berlin: Springer.
Acknowledgments
This research was supported by the German Research Foundation (DFG) under contract no. Ma 2143/9-1 and the RheinEnergieStiftung Jugend/Beruf, Wissenschaft under contract no. W-09-2-018.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hindersmann, I., Mansfeldt, T. Trace Element Solubility in a Multimetal-Contaminated Soil as Affected by Redox Conditions. Water Air Soil Pollut 225, 2158 (2014). https://doi.org/10.1007/s11270-014-2158-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-2158-8