Abstract
The classic development of vaccines is lengthy, tedious, and may not necessarily be successful as demonstrated by the case of HIV. This is especially a problem for emerging pathogens that are newly introduced into the human population and carry the inherent risk of pandemic spread in a naïve population. For such situations, a considerable number of different platform technologies are under development. These are also under development for pathogens, where directly derived vaccines are regarded as too complicated or even dangerous due to the induction of inefficient or unwanted immune responses causing considerable side-effects as for dengue virus. Among platform technologies are plasmid-based DNA vaccines, RNA replicons, single-round infectious vector particles, or replicating vaccine-based vectors encoding (a) critical antigen(s) of the target pathogens. Among the latter, recombinant measles viruses derived from vaccine strains have been tested. Measles vaccines are among the most effective and safest life-attenuated vaccines known. Therefore, the development of Schwarz-, Moraten-, or AIK-C-strain derived recombinant vaccines against a wide range of mostly viral, but also bacterial pathogens was quite straightforward. These vaccines generally induce powerful humoral and cellular immune responses in appropriate animal models, i.e., transgenic mice or non-human primates. Also in the recent first clinical phase I trial, the results have been quite encouraging. The trial indicated the expected safety and efficacy also in human patients, interestingly independent from the level of prevalent anti-measles immunity before the trial. Thereby, recombinant measles vaccines expressing additional antigens are a promising platform for future vaccines.
Similar content being viewed by others
History of measles vaccine
The measles are a scourge of mankind since about 7000 to 8000 years [1] and accounted for millions of deaths each year, in historic times [2]. Due to its extremely high transmission rate with an R0 of 12-18 [3], the disease spreads like wildfire among naïve populations and usually is a childhood disease. Pathogenic wild-type measles virus (MV) induces a transient, albeit pronounced immune suppression in infected patients rendering those susceptible to secondary infections especially under low hygiene conditions. This is one cause for the considerable mortality rate of the measles in low-developed countries [4]. Nevertheless, even in industrial countries, a mortality rate of about 1 in 1000 infections is prevalent due to direct effects of the MV infection, such as neurologic complications, i.e., primary measles encephalitis, acute disseminated encephalomyelitis (ADEM), MV inclusion-body encephalitis (MIBE), or subacute sclerotizing pan-encephalitis (SSPE), which concur with increasing fatality rates from 5% to 100% [5].
Therefore, the availability of first effective and safe measles vaccine authorized in 1963 by the FDA has been a blessing for mankind. The first vaccines have been derived of a primary pathogenic wild-type virus isolate from a boy named David Edmonston in 1954 [6]. This virus isolate was passaged many times on different primary cells and cell lines. A total number of 52 passages on human and 28 passages on chicken cells [7] yielded an attenuated strain named Edmonston seed B, which proofed attenuated in macaques but protected those against infection with pathogenic strains [8]. Also for human children, who have to be vaccinated as early as possible after maternal antibodies have gone, this vaccine showed efficacy in protecting against the measles, albeit a significant number of children became febrile after immunization [9]. Therefore, the vaccine strain was passaged another 48 times on chicken embryonal fibroblast including 40 passages at reduced temperature to give rise to even more attenuated vaccine strains Moraten [10]. Other vaccine strains derived from the Edmonston lineage by comparable passaging history are Schwarz, Edmonston-Zagreb, or AIK-C [7, 10, 11]. Edmonston-lineage vaccine strains are or have been licensed vaccines in the US, the EU, or Japan, and confer high efficacy accompanied by a remarkable safety profile [12, 13]. Indeed, the measles vaccine is even recommended for otherwise compromised collectives, such as HIV-infected patients, as long as immune suppression is not severe, i.e., CD4+ T lymphocyte counts are > 200/mm3 [12]. In parallel, other life-attenuated measles vaccine strains, e.g., Leningrad 16 or CAM-70, have been developed independently and used [13, 14].
Therefore, MV vaccine strains can be regarded both in terms of safety and efficacy as an ideal vector platform to build recombinant vaccines on, which present antigens of choice to the immune system in an extremely immune-stimulatory context.
Reverse genetics of mononegavirales
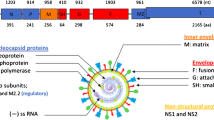
To use a chosen vaccine as a vector platform, technologies have to be available that allow genetic modification of the vector platform such that an antigen of choice can additionally be expressed or presented by the recombinant vaccine. MV belongs to the order of mononegavirales, the family of paramyxoviridae, and is the prototype of morbilliviruses. As such, the MV genome is a non-segmented, single-stranded linear RNA with a total length of 15,894 nucleotides in negative sense orientation (-ssRNA), with the different genes arranged in single cassettes, so called transcription units [15]. The genome structure and especially the negative-sense orientation of the RNA of mononegavirales genomes renders the transcription of viral mRNAs and replication of the virus genomes critically dependent on the virus-encoded ribonucleoprotein complexes (RNPs) consisting of the RNA genome complexed by the nucleocapsid protein N, the phosphoprotein P, and the RNA-dependent RNA-polymerase (RdRP) so called large protein L [16].
Therefore, the generation of recombinant mononegavirales from (biotechnologically modified) plasmids becomes complex, since not only the RNA genome, but also all protein components of the viral RNP have to be simultaneously present in a single cell to yield replicative centers that release recombinant, infectious virus. This process is even more complicated by the so-called “rule-of-six” [17]. The viral nucleocapsid protein N tightly covers viral RNAs, and the N-RNA complex is the template for viral genome transcription and replication [18]. However, only genomes whose nucleotide number is a multiply of six can be observed in naturally occurring Paramyxoviridae or give rise to respective recombinant viruses [17]. This implies on the one hand that each modification of these viruses’ genomes has to take this basic rule into account, but also that transcriptional start and stop of the recombinant, plasmid-encoded RNA genomes has to be on the spot. For this purpose, a T7 polymerase-driven expression system for all viral RNP-complex proteins, i.e., N, P, and L, as well as for the viral RNA antigenome has been set up, the latter additionally flanked 3′ by a Hepatitis delta virus ribozyme, respectively, to ensure its correct length. Such a system was first established for rabies virus and allowed generation, a so-called rescue, of replicating rabies virus from plasmid DNA [19].
Generation of recombinant measles virus
To generate recombinant MV, an Edmonston B vaccine-derived laboratory strain was chosen, that had been passaged multiple times on Vero cells, before. The complete virus genome engineered to encode an additional tag sequence was cloned into a pBluescript plasmid backbone under the control of the T7 promoter and flanked 3′ by the hepatitis virus δ-ribozyme. This plasmid could be used after co-transfection together with an also T7-driven expression plasmid encoding the MV polymerase into the 293-3-46 helper cell line, which expresses the T7 polymerase as well as MV N and P proteins (Fig. 1a), to rescue infectious and replicating recombinant MV [20]. As an alternative to the use of a stably transfected cell line, a system using 4 plasmids encoding the MV genome, as well as expression of MV N, P, and L proteins can be used together with a replication-deficient vaccine-T7 vector, MVA-T7 (Fig. 1b), to rescue infectious MV [21]. Finally, it has been shown that recombinant MV can be generated very efficiently using expression plasmids for MV genome as well as RNP proteins driven by the PolII-dependent CMVie-Promoter (Fig. 1c) to rescue MV with correct genome length [22]. Due to the availability of the reverse genetics system for MV, the respective genome was subsequently modified by essentially duplication of the critical stretches of the intergenic region between the L and H [23] or P and M gene cassettes [24]. These intergenic regions are highly conserved and contain Stop-Start signal sequences, which are recognized by the viral polymerase complex during mRNA transcription [25]. Thereby, transcription of singular viral mRNAs is realized. By duplication of this region, an additional Stop-Start signal is introduced into the genome, giving rise to a Stop-Start-Stop-Start signal sequence pattern. Thereby, an additional transcription unit (ATU) is introduced, which can be used to express additional cargo genes by introducing the respective ORF after the first Start-signal. This renders the possibility to mark recombinant MV by genes encoding marker proteins such as GFP [26] or luciferase [27] that allow tracking of the virus, but in principle any genes may be encoded given that they do not interfere with viral replication. In terms of payload, recombinant MV have been generated that encoded up to three additional genes [28] or a total extra size of 6 kb, thereby expanding the genome size of nearly a factor 1.5. Usually, recombinant MVs with extra gene cassettes reveal replication properties very similar to the parental empty MV, both in kinetics and titers. Sometimes, replication may be impaired, but this is related not only to length, but also to the position and the biology of the encoded protein. In general, extra genes next to the 3′ end may have a larger impact on replication, while extra cytoplasmic proteins are less problematic than those proteins targeted via the endoplasmic reticulum to the cell membrane or for secretion [29]. The variability in genome size has been attributed to the helical structure of the RNP complex that can easily be expanded by the addition of extra N protein residues in tandem with the pleomorphic particle structure allowing the packaging of larger RNP complexes. The latter was impressively visualized by the demonstration of co-packaging of at least two genomes in MV particles [30]. Thus, recombinant MV allows the insertion of quite large additional coding sequences generally without losing efficient replicative capacity.
Schematic depiction of different MV rescue systems available. To generate infectious MV particles, the exact full-length RNA genome or anti-genome has simultaneously to be available in a given cell together with the viral ribonucleoprotein complex proteins N, P, and L. For this purpose, the genomes were originally driven by T7 polymerase and ended with a delta ribozyme, yielding the need for co-expression of the T7 polymerase, in addition. a For this purpose, 293-3-46 rescue cells stably expressing MV-N, MV-P, and the T7 polymerase are co-transfected with the MV genome plasmid of interest, e.g., p(+)MV, and a T7 polymerase-driven expression plasmid for MV-L, e.g., pEMC.La. b As an alternative, T7-driven expression plasmids for the MV genome as well as for the other components of the RNP can be co-transfected e.g., into 293T cells, which are superinfected by a replication-deficient vaccinia vector, e.g., MVA-T7. c Finally, successful rescue has been demonstrated after co-transfection of DNA polymerase II driven expression plasmids for the MV genome as well as for the other components of the RNP complex. Hatched boxes promoter sequences; dark gray boxes termination/polyadenylation signals; white boxes individual genes
Measles virus-derived recombinant vaccines
While IL-12 encoding genes had been first to become introduced into MV genomes [23], it was realized soon that such an MV can be used as a vector to express and present other pathogens’ critical antigens to the immune system in the context of a highly immunogenic MV replication. First, the Hepatitis B Virus surface and core antigens were chosen to be expressed by a recombinant MV via an ATU following the P gene cassette [24]. These recombinant viruses were shown to express the desired additional antigens, and the HBsAg-encoding MV provoked significant humoral antigen-specific immune responses [24] in the transgenic, MV-susceptible IFNAR−/−-CD46Ge mouse model [31].
To take optimal advantage of the vector platform also with respect to regulatory issues, further MV genomes were cloned that were identical to authorized MV vaccine strains. Today, genomes encompassing the exact sequences of the vaccine strains Schwarz [32], Moraten [33], Edmonston-Zagreb [34], MVbv [35], or AIK-C [36] are available to generate recombinant platform-based vaccines. On this basis, a variety of recombinant MV has been generated that mostly target viral pathogens, but also bacterial antigens, namely such of Helicobacter pylori [37], induce significant immunity when expressed from the MV platform (Table 1).
Pre-clinical and clinical data
As depicted in Table 1, 11 out of 17 recombinant MV-derived vaccines have been tested in the available mouse model IFNAR-/--CD46Ge, transgenic mice with a type I interferon receptor deficiency, but expressing the human homologue of the MV vaccine strain receptor CD46 as transgene with human tissue specificity [31]. For each of the analyzed recombinant vaccines, the induction of significant humoral immune responses has been found (Table 1). The induction of these responses includes the isotype switch from IgM to IgG, and the induced antibodies can have neutralizing activity, as demonstrated e.g., for the vaccines expressing soluble E protein of WNV [66], CHIKV virus-like particles [38], or the unmodified or the soluble version of the MERS-CoV Spike glycoprotein [59]. Reyes del Valle et al. have further shown that the amount of expressed foreign antigen determines the strength of the induced antibody response [46]. However, the most effective vaccines may not necessarily be those with the additional antigen encoded most up-front in the MV genome, but may be determined empirically by testing different positions for the ATU and the respective antigen. For example, MERS-S expression is higher when encoded after the H rather than the P cassette [59].
Furthermore, also powerful cellular immune responses can be triggered by MV-derived vaccines against additionally encoded antigens. The induction of antigen-specific T cell responses has been demonstrated against a couple of MV-encoded antigens, including HIV-1 Env [53], WNV-E [67], and, again, MERS-S [59].
The protective capacity of these responses has been demonstrated first for the WNV-vaccine, when immunized mice survived an otherwise lethal challenge with WNV [66]. Meanwhile, protection by the respective recombinant vaccines has been demonstrated for a number of other MV-based vaccines (Table 1). While the mouse data may be challenged due to the knock-out of the IFNAR, which is necessary to allow basic replication of MV in receptor-transgenic mice [31], 7 MV-based vaccines have progressed to the non-human primate (NHP) model, the only other alternative to transgenic mice besides cotton rats, which have been used to characterize 3 vaccines, due to lack of other species for MV susceptibility [68]. Also in NHPs or cotton rats, recombinant MV encoding foreign antigens remained immunogenic and induced immune responses against the latter (Table 1).
Based on these data, the MV-CHIKV and MV-F4 vaccines, which are Schwarz-strain MVs encoding CHIKV VLPs and HIV-envelope glycoprotein, respectively, have progressed into phase I clinical trials [39, 69]. While the HIV-vaccine trial data are not available, yet [69], the CHIKV-vaccine trial gave some interesting insights into efficacy of MV-derived vaccines in man [39]: In a cohort of 36 patients that were vaccinated with doses between 1.5 × 104 and 3 × 105 infectious particles with a booster immunization at the same dose level 28 or 90 days after the first shot, the vaccine induced immune responses in all volunteers after two shots, in the high dose group even without a booster immunization. Even more important, the study revealed the expected excellent safety profile with no serious and just 4 severe solicited adverse events (headache, local erythema, local induration, local pain, and pyrexia) recorded among all participants. Interestingly, the strength of antibody responses hosted by individual patients was independent from pre-formed anti-measles immunity (due to previous measles infection or vaccination) as demonstrated by stratifying patient cohorts for prevalent MV-neutralizing Ab-responses before the trial and analyzing the induced CHIKV-immunity. Thus, clinical trial data are with respect to the impact of pre-formed anti-measles immunity in accordance with the results of the preclinical animal models. The available pre-clinical data also suggest an if at all minor impact of anti-measles immunity on the efficacy of recombinant vaccines using the MV platform technology [34, 38, 51, 56]. Thereby, recombinant MV vaccine platform-derived vaccines should also be suited for patients that already have acquired anti-measles immunity.
Such pre-formed anti-measles immunity has to be expected and therefore considerations of efficacy despite immunity have to be regarded as quite important, since anti-vector immunity has been considered to be associated with unwanted effects on the efficacy of adenovirus-derived vectors [70]. While gutless adenovirus vector systems, but especially poxvirus vectors such as MVA, could harbor larger genetic inserts than recombinant MV [71]. Nevertheless, the 4–6 kb extra cargo allowed even in separate expression cassettes in MV should be sufficient for most antigens. Moreover, recombinant adenovirus-based vaccine candidates have already progressed up to phase III clinical trials [70], but there is in total much more clinical experience with the MV vaccine backbone itself considering billions of doses of measles vaccine, which have been used, yet. Looking at the MV vaccine platform and these other currently available vaccine platforms such as MVA, other poxviruses or adenoviruses in general, a direct side-by-side comparison especially considering efficacy is yet to be done, but most likely the effectiveness of the compared vectors will in the end also depend on the nature of the antigen to be expressed as well as on the immune responses required for protection.
Summary and outlook
Due to the advent of a reverse genetic system, the generation of recombinant, vaccine-strain-derived measles viruses became possible that can be used as vectors to stimulate effective humoral and cellular immune responses against other pathogens including emerging infections. These vectored vaccines proved to be effective against a whole range of viral and also bacterial pathogens in a number of animal models as well as in first clinical trials. Most importantly, the extraordinary advantageous safety profile of measles vaccines also applied to the recombinant platform-based vaccines, which have been analyzed, yet.
Therefore, recombinant vaccine-strain-derived measles viruses belong to the set of potential vaccine platforms that are well suited to develop new, urgently needed vaccines, be it against emerging infections or other diseases, where classic vaccine development has not delivered effective vaccines, yet. Future clinical trials will provide further insight into efficacy and characteristics of the MV vector system, i.e., if the immunity triggered in human patients will be protective to the same extent as shown in the animal models, or if the longevity of immune responses against the measles vaccines will also be found in the responses triggered against the additional antigen. In any case, the promising data available yet suggest that these vaccines should be an excellent home-base for modern vaccine development to provide sharp weapons against current and future challenges.
References
R.A. Weiss, The Leeuwenhoek Lecture 2001, Animal origins of human infectious disease. Philos. Trans. R. Soc. Lond. B. 356(1410), 957–977 (2001). doi:10.1098/rstb.2001.0838
D. Naniche, Human immunology of measles virus infection. Curr. Top. Microbiol. Immunol. 330, 151–171 (2009)
D.N. Durrheim, N.S. Crowcroft, P.M. Strebel, Measles - The epidemiology of elimination. Vaccine 32(51), 6880–6883 (2014). doi:10.1016/j.vaccine.2014.10.061
P.A. Rota, W.J. Moss, M. Takeda et al., Measles. Nat. Rev. Dis. Primers. 2, 16049 (2016). doi:10.1038/nrdp.2016.49
D.L. Fisher, S. Defres, T. Solomon, Measles-induced encephalitis. QJM 108(3), 177–182 (2015). doi:10.1093/qjmed/hcu113
J.F. Enders, T.C. Peebles, Propagation in tissue cultures of cytopathogenic agents from patients with measles. Proc. Soc. Exp. Biol. Med. 86(2), 277–286 (1954)
Flint SJ, Racaniello VR, Rall GF (2015) Principles of virology, 4th edition
J.F. Enders, Development of attenuated measles virus vaccines. Am. J. Dis. Child. 103(3), 335 (1962). doi:10.1001/archpedi.1962.02080020347030
S.L. Katz, C.H. Kempe, F.L. Black et al., Studies on an attenuated measles-virus vaccine. VIII. General summary and evaluation of the results of vaccine. N. Engl. J. Med. 263, 180–184 (1960). doi:10.1056/NEJM196007282630408
J.S. Rota, Z.D. Wang, P.A. Rota et al., Comparison of sequences of the H, F, and N coding genes of measles virus vaccine strains. Virus Res. 31(3), 317–330 (1994)
A.J.F. Schwarz, Preliminary tests of a highly attenuated measles vaccine. Arch. Pediatr. Adolesc. Med. 103(3), 386 (1962). doi:10.1001/archpedi.1962.02080020398042
H.Q. McLean, A.P. Fiebelkorn, J.L. Temte et al., Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep (2013) 62(RR-04): 1–34
S. Ueda, Development of measles vaccines in Japan. Vaccine 27(24), 3230–3231 (2009). doi:10.1016/j.vaccine.2009.02.066
P.N. Burgasov, O.G. Andzaparidze, V.F. Popov, The status of measles after five years of mass vaccination in the USSR. Bull. World Health Organ. 49(6), 571–576 (1973)
J.K. Rose, Complete intergenic and flanking gene sequences from the genome of vesicular stomatitis virus. Cell 19(2), 415–421 (1980). doi:10.1016/0092-8674(80)90515-2
A.K. Pattnaik, G.W. Wertz, Replication and amplification of defective interfering particle RNAs of vesicular stomatitis virus in cells expressing viral proteins from vectors containing cloned cDNAs. J. Virol. 64(6), 2948–2957 (1990)
P. Calain, L. Roux, The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J. Virol. 67(8), 4822–4830 (1993)
J. Curran, D. Kolakofsky, Replication of paramyxoviruses. Adv. Virus Res. 54, 403–422 (1999)
M.J. Schnell, T. Mebatsion, K.K. Conzelmann, Infectious rabies viruses from cloned cDNA. EMBO J. 13(18), 4195–4203 (1994)
F. Radecke, P. Spielhofer, H. Schneider et al., Rescue of measles viruses from cloned DNA. EMBO J. 14(23), 5773–5784 (1995)
H. Schneider, P. Spielhofer, K. Kaelin et al., Rescue of measles virus using a replication-deficient vaccinia-T7 vector. J. Virol. Methods 64(1), 57–64 (1997)
A. Martin, P. Staeheli, U. Schneider, RNA polymerase II-controlled expression of antigenomic RNA enhances the rescue efficacies of two different members of the Mononegavirales independently of the site of viral genome replication. J. Virol. 80(12), 5708–5715 (2006). doi:10.1128/JVI.02389-05
M. Singh, M.A. Billeter, A recombinant measles virus expressing biologically active human interleukin-12. J. Gen. Virol. 80(Pt 1), 101–106 (1999). doi:10.1099/0022-1317-80-1-101
M. Singh, R. Cattaneo, M.A. Billeter, A recombinant measles virus expressing hepatitis B virus surface antigen induces humoral immune responses in genetically modified mice. J. Virol. 73(6), 4823–4828 (1999)
R. Cattaneo, G. Rebmann, A. Schmid et al., Altered transcription of a defective measles virus genome derived from a diseased human brain. EMBO J. 6(3), 681–688 (1987)
W.P. Duprex, S. McQuaid, L. Hangartner et al., Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. J. Virol. 73(11), 9568–9575 (1999)
H.T. Ong, K. Hasegawa, A.B. Dietz et al., Evaluation of T cells as carriers for systemic measles virotherapy in the presence of antiviral antibodies. Gene Ther. 14(4), 324–333 (2007). doi:10.1038/sj.gt.3302880
A. Zuniga, Z. Wang, M. Liniger et al., Attenuated measles virus as a vaccine vector. Vaccine 25(16), 2974–2983 (2007). doi:10.1016/j.vaccine.2007.01.064
M.A. Billeter, H.Y. Naim, S.A. Udem, Reverse genetics of measles virus and resulting multivalent recombinant vaccines: applications of recombinant measles viruses. Curr. Top. Microbiol. Immunol. 329, 129–162 (2009)
M. Rager, S. Vongpunsawad, W.P. Duprex et al., Polyploid measles virus with hexameric genome length. EMBO J. 21(10), 2364–2372 (2002). doi:10.1093/emboj/21.10.2364
B. Mrkic, J. Pavlovic, T. Rulicke et al., Measles virus spread and pathogenesis in genetically modified mice. J. Virol. 72(9), 7420–7427 (1998)
C. Combredet, V. Labrousse, L. Mollet et al., A molecularly cloned Schwarz strain of measles virus vaccine induces strong immune responses in macaques and transgenic mice. J. Virol. 77(21), 11546–11554 (2003)
P. Devaux, V. von Messling, W. Songsungthong et al., Tyrosine 110 in the measles virus phosphoprotein is required to block STAT1 phosphorylation. Virology 360(1), 72–83 (2007). doi:10.1016/j.virol.2006.09.049
H. Mok, X. Cheng, Q. Xu et al., Evaluation of measles vaccine virus as a vector to deliver respiratory syncytial virus fusion protein or Epstein-Barr virus glycoprotein gp350. Open Virol. J. 6, 12–22 (2012). doi:10.2174/1874357901206010012
A. Zuniga, M. Liniger, T.N.A. Morin et al., Sequence and immunogenicity of a clinically approved novel measles virus vaccine vector. Hum. Vaccin. Immunother. 9(3), 607–613 (2013)
A. Sawada, K. Komase, T. Nakayama, AIK-C measles vaccine expressing fusion protein of respiratory syncytial virus induces protective antibodies in cotton rats. Vaccine 29(7), 1481–1490 (2011). doi:10.1016/j.vaccine.2010.12.028
I.D. Iankov, I.H. Haralambieva, E. Galanis, Immunogenicity of attenuated measles virus engineered to express Helicobacter pylori neutrophil-activating protein. Vaccine 29(8), 1710–1720 (2011). doi:10.1016/j.vaccine.2010.12.020
S. Brandler, C. Ruffie, C. Combredet et al., A recombinant measles vaccine expressing chikungunya virus-like particles is strongly immunogenic and protects mice from lethal challenge with chikungunya virus. Vaccine 31(36), 3718–3725 (2013). doi:10.1016/j.vaccine.2013.05.086
K. Ramsauer, M. Schwameis, C. Firbas et al., Immunogenicity, safety, and tolerability of a recombinant measles-virus-based chikungunya vaccine: a randomised, double-blind, placebo-controlled, active-comparator, first-in-man trial. Lancet Infect. Dis. 15(5), 519–527 (2015). doi:10.1016/S1473-3099(15)70043-5
S. Brandler, M. Lucas-Hourani, A. Moris et al., Pediatric measles vaccine expressing a dengue antigen induces durable serotype-specific neutralizing antibodies to dengue virus. PLoS Negl. Trop. Dis. 1(3), e96 (2007). doi:10.1371/journal.pntd.0000096
S. Brandler, C. Ruffie, V. Najburg et al., Pediatric measles vaccine expressing a dengue tetravalent antigen elicits neutralizing antibodies against all four dengue viruses. Vaccine 28(41), 6730–6739 (2010). doi:10.1016/j.vaccine.2010.07.073
H.-M. Hu, H.-W. Chen, Y.-J. Hsiao et al., The successful induction of T-cell and antibody responses by a recombinant measles virus-vectored tetravalent dengue vaccine provides partial protection against dengue-2 infection. Hum Vaccin Immunother 12(7), 1678–1689 (2016). doi:10.1080/21645515.2016.1143576
I.S. Harahap-Carrillo, I. Ceballos-Olvera, J.R.-D. Valle, Immunogenic subviral particles displaying domain III of Dengue 2 envelope protein vectored by measles virus. Vaccines 3(3), 503–518 (2015). doi:10.3390/vaccines3030503. (Basel)
C. Swett-Tapia, L. Bogaert, P. de Jong et al., Recombinant measles virus incorporating heterologous viral membrane proteins for use as vaccines. J. Gen. Virol. (2016). doi:10.1099/jgv.0.000523
J. Reyes-del Valle, G. Hodge, M.B. McChesney et al., Protective anti-hepatitis B virus responses in rhesus monkeys primed with a vectored measles virus and boosted with a single dose of hepatitis B surface antigen. J. Virol. 83(17), 9013–9017 (2009). doi:10.1128/JVI.00906-09
J.R. del Valle, P. Devaux, G. Hodge et al., A vectored measles virus induces hepatitis B surface antigen antibodies while protecting macaques against measles virus challenge. J. Virol. 81(19), 10597–10605 (2007). doi:10.1128/JVI.00923-07
J. Reyes-del Valle, C. La Fuente, M.A. de Turner et al., Broadly neutralizing immune responses against hepatitis C virus induced by vectored measles viruses and a recombinant envelope protein booster. J. Virol. 86(21), 11558–11566 (2012). doi:10.1128/JVI.01776-12
M. Satoh, M. Saito, K. Tanaka et al., Evaluation of a recombinant measles virus expressing hepatitis C virus envelope proteins by infection of human PBL-NOD/Scid/Jak3null mouse. Comp. Immunol. Microbiol. Infect. Dis. 33(6), e81–e88 (2010). doi:10.1016/j.cimid.2010.02.006
M. Guerbois, A. Moris, C. Combredet et al., Live attenuated measles vaccine expressing HIV-1 Gag virus like particles covered with gp160DeltaV1V2 is strongly immunogenic. Virology 388(1), 191–203 (2009). doi:10.1016/j.virol.2009.02.047
M. Liniger, A. Zuniga, T.N.A. Morin et al., Recombinant measles viruses expressing single or multiple antigens of human immunodeficiency virus (HIV-1) induce cellular and humoral immune responses. Vaccine 27(25–26), 3299–3305 (2009). doi:10.1016/j.vaccine.2009.01.057
C. Lorin, L. Mollet, F. Delebecque et al., A single injection of recombinant measles virus vaccines expressing human immunodeficiency virus (HIV) type 1 clade B envelope glycoproteins induces neutralizing antibodies and cellular immune responses to HIV. J. Virol. 78(1), 146–157 (2004)
C. Lorin, L. Segal, J. Mols et al., Toxicology, biodistribution and shedding profile of a recombinant measles vaccine vector expressing HIV-1 antigens, in cynomolgus macaques. Naunyn Schmiedebergs Arch Pharmacol 385(12), 1211–1225 (2012). doi:10.1007/s00210-012-0793-4
C. Lorin, F. Delebecque, V. Labrousse et al., A recombinant live attenuated measles vaccine vector primes effective HLA-A0201-restricted cytotoxic T lymphocytes and broadly neutralizing antibodies against HIV-1 conserved epitopes. Vaccine 23(36), 4463–4472 (2005). doi:10.1016/j.vaccine.2005.04.024
R. Stebbings, M. Fevrier, B. Li et al., Immunogenicity of a recombinant measles-HIV-1 clade B candidate vaccine. PLoS ONE 7(11), e50397 (2012). doi:10.1371/journal.pone.0050397
R. Stebbings, B. Li, C. Lorin et al., Immunogenicity of a recombinant measles HIV-1 subtype C vaccine. Vaccine 31(51), 6079–6086 (2013). doi:10.1016/j.vaccine.2013.09.072
G. Gupta, V. Giannino, N. Rishi et al., Immunogenicity of next-generation HPV vaccines in non-human primates: measles-vectored HPV vaccine versus Pichia pastoris recombinant protein vaccine. Vaccine (2016). doi:10.1016/j.vaccine.2016.07.051
G. Cantarella, M. Liniger, A. Zuniga et al., Recombinant measles virus-HPV vaccine candidates for prevention of cervical carcinoma. Vaccine 27(25–26), 3385–3390 (2009). doi:10.1016/j.vaccine.2009.01.061
A. Higuchi, H. Toriniwa, T. Komiya et al., Recombinant measles AIK-C vaccine strain expressing the prM-E antigen of Japanese encephalitis virus. PLoS ONE 11(3), e0150213 (2016). doi:10.1371/journal.pone.0150213
A.H. Malczyk, A. Kupke, S. Prufer et al., A highly immunogenic and protective middle east respiratory syndrome coronavirus vaccine based on a recombinant measles virus vaccine platform. J. Virol. 89(22), 11654–11667 (2015). doi:10.1128/JVI.01815-15
Z. Wang, L. Hangartner, T.I. Cornu et al., Recombinant measles viruses expressing heterologous antigens of mumps and simian immunodeficiency viruses. Vaccine 19(17–19), 2329–2336 (2001)
M. Yoneda, Study of pathogenicity of Nipah virus and its vaccine development. Uirusu 64(1), 105–112 (2014). doi:10.2222/jsv.64.105
Y. Yamaji, T. Nakayama, Recombinant measles viruses expressing respiratory syncytial virus proteins induced virus-specific CTL responses in cotton rats. Vaccine 32(35), 4529–4536 (2014). doi:10.1016/j.vaccine.2014.06.024
N. Escriou, B. Callendret, V. Lorin et al., Protection from SARS coronavirus conferred by live measles vaccine expressing the spike glycoprotein. Virology 452–453, 32–41 (2014). doi:10.1016/j.virol.2014.01.002
M. Liniger, A. Zuniga, A. Tamin et al., Induction of neutralising antibodies and cellular immune responses against SARS coronavirus by recombinant measles viruses. Vaccine 26(17), 2164–2174 (2008). doi:10.1016/j.vaccine.2008.01.057
D.L. Bolton, S. Santra, C. Swett-Tapia et al., Priming T-cell responses with recombinant measles vaccine vector in a heterologous prime-boost setting in non-human primates. Vaccine 30(41), 5991–5998 (2012). doi:10.1016/j.vaccine.2012.06.029
P. Despres, C. Combredet, M.-P. Frenkiel et al., Live measles vaccine expressing the secreted form of the West Nile virus envelope glycoprotein protects against West Nile virus encephalitis. J. Infect. Dis. 191(2), 207–214 (2005). doi:10.1086/426824
S. Brandler, P. Marianneau, P. Loth et al., Measles vaccine expressing the secreted form of West Nile virus envelope glycoprotein induces protective immunity in squirrel monkeys, a new model of West Nile virus infection. J. Infect. Dis. 206(2), 212–219 (2012). doi:10.1093/infdis/jis328
B. Mrkic, B. Odermatt, M.A. Klein et al., Lymphatic dissemination and comparative pathology of recombinant measles viruses in genetically modified mice. J. Virol. 74(3), 1364–1372 (2000)
ClinicalTrials.gov, An Open-label, Phase I, Dose-escalation and Safety Study of Two Intramuscular Injections of a Dose of 2.9 Log or 4 Log CCID50 of the Recombinant HIV I Clade B Measles Vaccine Vector in Healthy Adults.: NCT01320176. https://clinicaltrials.gov/, 2012. Accessed 15 Nov 2016
S.P. Buchbinder, D.V. Mehrotra, A. Duerr et al., Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372(9653), 1881–1893 (2008). doi:10.1016/S0140-6736(08)61591-3
H. Meyer, G. Sutter, A. Mayr, Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J. Gen. Virol. 72(Pt 5), 1031–1038 (1991). doi:10.1099/0022-1317-72-5-1031
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no potential conflict of interest. The author is supported by the German Center for Infection Research (DZIF; TTU 01.802).
Additional information
Edited by Anja Ehrhardt and Florian Kreppel.
Rights and permissions
About this article
Cite this article
Mühlebach, M.D. Vaccine platform recombinant measles virus. Virus Genes 53, 733–740 (2017). https://doi.org/10.1007/s11262-017-1486-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-017-1486-3