Abstract
Pseudomonas luteola (P.luteola), formerly called Chryseomonas luteola, is a strict aerobic gram-negative bacillus, 0.8 to 1.0 µm wide and 1.5 to 2.5 µm long, considered an opportunistic pathogen found ubiquitously in humid environments, both in soil and water. It sporadically causes disease in animals and immunosuppressed humans or those subjected to invasive procedures such us peritoneal dialysis or catheterization. In ferrets, this infection was first described in Spain in 2012 and since then, cases have appeared occasionally in Finland, Austria, Australia, France, the United States and also in Spain. This pathogen is considered an emerging zoonotic disease in ferrets, causing respiratory disease, panniculitis, and abscesses due to pyogranulomatous or suppurative inflammation predominantly of the pleura, lung, mediastinum, panniculus or salivary glands, frequently with lethal consequences. The clinical case of a ferret, infected by Pseudomona luteola, presenting with ulcerative suppurative pododermatitis and ipsilateral popliteal purulent lymphadenitis, is described. Together with a complete resolution of the clinical case by means of a non-invasive medical management likely due to the rapid detection, identification, and treatment of the infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pseudomonas luteola, formerly known as Chryseomonas luteola, is a Gram-negative, aerobic, rod-shaped bacteria, 0,8–1 µm wide and 1,5–2,5 µm long with a non-staining capsule. The normal habitat of this bacterium remains unknown, although it is frequently found in damp environments, found in soil, and water (Rahav et al. 1995; Chihab et al. 2004). P. luteola is composed by a polysaccharide capsule with a multitrichous polar flagella that has been associated with cadmium and cobalt ion absorption. The bacteria forms yellow-orange pigmentated colonies (Ozdemir et al. 2005).
P. luteola is rarely a cause of disease in humans, but it has been found in medical implants, peritoneal dialysis catheters, in immunosuppressed patients and as a nosocomial infection in surgical procedures (Chihab et al. 2004; Barry 2021). P. luteola has also been described as a causative agent of endophthalmitis, endocarditis, peritonitis, septicemia and leg ulcers in patients with sickle cell disease (Tsakris et al. 2022; Casalta et al. 2005; Uy et al. 2007; Su et al. 2014; Bayhan et al. 2015).
In the veterinary literature, P. luteola infections that cause mortality have been reported in farmed rainbow trout (Altinok et al. 2007), none the less, it has also been found as a part of the normal gastrointestinal flora of healthy zebrafish (Danio rerio) (Cantas et al. 2012). This pathogen rarely causes disease in mammals, but it has been documented in cats (Milliron et al. 2021) causing pyogranulomatous panniculitis (Milliron et al. 2021) and ferrets (Schmidt et al. 2019).
This infection is considered an emergent disease in ferrets in Europe and presents with abscesses, panniculitis or respiratory disease (Baum et al. 2015; Martínez et al. 2012). This case report describes the isolation of P. luteola from a ferret with an ulcerative and pyogranulomatous pododermatitis, considered an atypical clinical presentation in this species; the role of this pathogen as an emerging zoonotic agent in ferrets is reviewed.
Material and methods
Case history
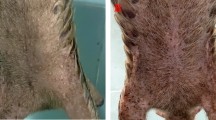
An 8-month-old intact male ferret (Mustela puturius furo) with a body weight of 1750 g was referred to the veterinary service for evaluation of an injury in the plantar aspect of the right hind limb, and the presence of lethargy and anorexia during the last 24 hours (Fig. 1a).
On clinical record, the ferret was fed a diet consisting of 70% raw food and 30% premium dry cat food. It was vaccinated against canine distemper receiving two doses of the vaccine with a one-month interval between them. The patient had an indoor lifestyle with outdoor access during weekends. The owners associated the wound with a field trip 6 days before. Physical examination was unremarkable other than pyrexia (40.5 ºC) and a painful, ulcerative and edematous lesion of 1 cm in diameter observed on the palmer aspect of the right paw, together with a firm and enlarged popliteal lymph node (1.5 cm × 1,75 cm) with no evidence of other lymph node enlargement. All other physiological parameters were within normal range, the ferret had a body condition score of 3/5, and cardiac and respiratory auscultation within normal limits. Two mls of blood were collected aseptically by jugular venipuncture for comprihensive blood tests including a blood count and a serum biochemical profile (Idexx, Westbrook, USA).
An incisional full-thickness biopsy of the ulcerative skin lesion was taken for histological examination. The ferret was premedicated with midazolam 0.2 mg/kg subcutaneously and butorphanol 0.2 mg/kg intramuscularly. Anesthesia was induced with alfaxalone 5 mg/kg administered intravenously, followed by tracheal intubation (2.0 mm) and maintenance with sevoflurane.
Additionally, various samples were taken by fine needle aspiration (FNA) of the affected right popliteal lymph node for cytological examination (Fig. 2). A Diff-Quick staining and bacterial culture was employed (isolation and antibiotic sensitivity testing was also undertaken). The aspirate was cultured on Blood agar (agar, 15 g/L, meat extract 10 g/L, peptone 10 g/L and sodium chloride 5 g/L, Merck), Chocolate Agar (146,093; Merck) and MacConkey agar (agar 12.0 g/L, bile salts 5.0 g/L, lactose 10.0 g/L, neutral red 0.075 g/L, peptone 20.0 g/L and sodium chloride 5.0 g/L, Merck). Two plates were incubated aerobically at 37 °C, and the other pairs of plates were incubated in anaerobic conditions at 37 °C. Plates were observed for bacterial growth after 16–20 h of cultivation. In cases of no or slow bacterial growth at first inspection, additional observations were performed at 24 h intervals. To isolate the types of growth colonies the MALDI-TOF mass spectrometry (VITEK MS, bioMérieux) and Knowledge Base database (version 3.0) was utilized. Finally, an antibiogram was performed. In addition, an incisional surgical biopsy of the dermal lesion was also obtained for histopathology tissue processing. This sample was fixed in 10% neutral-buffered formalin, embedded in paraffin, and 4 μm-thick sections were stained with hematoxylin and eosin (HE).
Results
Clinicopathological findings
Complete blood count, serum biochemistry, and serum protein electrophoresis revealed neutrophilia (7.56 (0.62–3.30 K/µL)), monocytosis (1,08 (0.18–0.90 K/µL)) and hyperglobulinemia (3.30 (1.80–3.10 g/dL)). While awaiting the results from cytology, bacterial culture and the biopsy, an empirical treatment was administered consisting of a combined antibiotic therapy of enrofloxacin (5 mg/kg/12 h) and amoxicillin-clavulanic acid (15 mg/kg/12 h), and meloxicam (0,1 mg/kg/24 h). With this treatment, the pododermatitis was partially resolved (Fig. 1b).
Cytological examination
Cytological evaluation of a sample taken by FNA and stained with Diff-Quick revealed purulent lymphadenitis with the presence of a high number of markedly degenerative neutrophils, a few macrophages and the presence of low number of intracellular bacteria.
Histopathological examination
Histopathological evaluation of the skin biopsy revealed an acute diffuse suppurative pododermatitis with ulceration and intralesional bacteria including coccobacilli and serpentine bacilli surrounded by a clear halo (Fig. 3).
Histopathological analysis from sampled pododermatitis. a). Complete ulceration and colonies of cocobacilli and serpentine bacilli with a clear halo infiltrating the superficial and deep dermis (arrows). HE, Bar: 1000 μm.; b). Colonies (arrows) surrounded by neutrophils and macrophages (arrowhead). HE, Bar: 100 μm
Bacterial culture and MALDI-TOF mass spectrometry
A single type of colony was isolated and identified as P. luteola using MALDI-TOF mass spectrometry. The antibiogram revealed that the P. luteola isolate was susceptible to ampicillin, amoxicillin-clavulanic acid, imipenem, meropenem, ciprofloxacin, enrofloxacin, marbofloxacin, pradofloxacin, gentamicin, tobramycin, doxycycline, cotrimoxazole, amikacin and tetracycline. P. luteola was resistant to cefalexin, cefuroxime, chloramphenicol and cefpodoxime. Finally, intermediate susceptibility was detected to trimethoprim-sulfamethoxazole and cefotaxime. Since the antibiogram revealed sensitivity to the antibiotics previously administered to the patient, a different antibiotic treatment was unnecessary. During the follow-up, the antibiotic therapy was continued for one month resulting in a complete resolution of pododermatitis and lymph node enlargement (Fig. 1c).
Discussion
P. luteola is considered an emerging zoonotic disease in ferrets causing respiratory signs, abscesses or pyogranulomatous panniculitis (Wyre 2020). Although there has been an increase of case descriptions, involving P. luteola, in ferrets in recent years, there are no publications to date describing pododermatitis. The first clinical descriptions of P. luteola infection in ferrets were reported in Spain (Martínez et al. 2012). Later, additional clinical cases were documented in Australia (Coignet 2012), France (Coignet 2012), Finland and Austria (Baum et al. 2015), and the United States (Schmidt et al. 2019). In general, the most common clinical presentations of this infection in ferrets include thoracic or peritoneal lesions, with respiratory clinical signs or peritonitis, followed by the presence of abscesses in different tissue locations (Wyre 2020). There was no description of pododermatitis caused by this pathogen in ferrets.
The route of infection and mode of transmission of P. luteola in ferrets have not been determined. In previous reports with respiratory clinical signs, abscesses and subcutaneous edema, there has been no evidence of nosocomial infections or wound contamination. In the ferret of this report, infection might have been associated to a field trip with the owners 6 days before presentation. Since this bacterium is ubiquitous in damp environments, found in both soil and water (Rahav et al. 1995), an abrasion or penetrating trauma to the footpad with secondary bacterial inoculation from an environmental source is possible but cannot be confirmed.
Cytology and histopathology are necessary to focus the diagnosis of this bacterial disease. Microscopic findings such a purulent or pyogranulomatous inflammation with intralesional pleomorphic bacteria including serpentine bacilli surrounded by a clear halo should suggest P. luteola infection as an aetiology. However, to confirm the diagnosis of P.luteola, bacterial isolation and identification are required. In these cases, pure cultures of yellow-pigmented colonies involving gram-negative rods are the common findings in Blood and Mac-Conkey agars (Martínez et al. 2012). In the present report, P. luteola was isolated in Blood Agar, Agar MacConkey and also in Chocolate Agar, and it was identified by mass spectrophotometry MALDI-TOF.
Pseudomonas spp. are one of the major pathogens causing healthcare-associated infections. Its capacity of adaptation, dissemination, intrinsic resistance to antimicrobials and ability to gain new mechanisms through mobile genetic elements, make the treatment of infections by this microorganism a challenge for the clinician. For example, P. aeruginosa, presents a reduced permeability in the external membrane, due to the expression of efflux pumps, and an inducible AmpC-type cephalosporinase or benzyl-penicillinase (Morita et al. 2014). In addition, P. aeruginosa is able to acquire new resistance determinants by horizontal transfer in the form of cassettes located in integrons, and in turn located in transposons or plasmids. P. aeruginosa produces beta-lactamases, but presents resistance to beta-lactam antibiotics (including extended spectrum) and carbapenem antibiotics (Oliver 2017). Resistance of P. luteola to first and second-generation cephalosporins, tetracycline, ampicillin and trimethoprim-sulfamethoxazole has been documented (Rahav et al. 1995; Yousefi et al. 2014). In other case reports in ferrets, treatment was based on antibiogram results and included amoxicillin, with clavulanic acid, amikacin or enrofloxacin (Schmidt et al. 2019). In this report, resistance to cephalexin and cefuroxime (first and second-generation of cephalosporins), cefovecin (third-generation cephalosporin) and chloramphenicol was found and P. luteola was sensitive to fluoroquinolones and other beta-lactam antibiotics, as previously described (Schmidt et al. 2019). Probably, P. luteola possesses different mechanisms of responses and resistance compared to P. aeruginosa.
This is the first case report of pododermatitis caused by P. luteola in a ferret. Complete resolution with medical treatment alone was possible. This success may be related to the promptness of the detection, identification and treatment. Consequently, P. luteola should be included in the dermatological differential diagnosis of pododermatitis in ferrets. In the majority of cases described in the literature, the diagnosis was made “post mortem” and in the few cases in which satisfactory treatment could be established, it was based on combined medical and surgical treatments. In the patient described in this case report, complete resolution was obtained with medical treatment alone, possibly due to the rapid detection, identification, and treatment of the infection. This case reinforces that P. luteola is more frequently sensitive to non-cephalosporin beta-lactams, and amoxicillin with clavulanic acid should be recommended as a first option in cases without an antibiogram.
Data availability
No datasets were generated or analysed during the current study.
References
Altinok I, Balta F, Capkin E, Kayis S (2007) Disease of rainbow trout caused by Pseudomonas luteola. Aquaculture 273:393–397
Barry M (2021) Pseudomonas luteola bacteremia in newly diagnosed systemic lupus erythematosus. Case Rep Infect Dis 14:4051378
Baum B, Richter B, Reifinger M, Klang A, Finnberg C, Loncaric I, Spergser J, Eisenberg T, Künzel F, Preis S, Pantchev N, Rütgen B, Guija de Arespacochaga A, Hewicker-Trautwein M (2015) Pyogranulomatous panniculitis in ferrets (Mustela putorius furo) with intralesional demonstration of Pseudomonas luteola. J Comp Pathol 152:114–118
Bayhan GI, Senel S, Tanir G, Ozkan S (2015) Bacteremia caused by Pseudomonas luteola in pediatric patients. Jpn J Infect Dis 68:50–54
Cantas L, Sørby JR, Aleström P, Sørum H (2012) Culturable gut microbiota diversity in zebrafish. Zebrafish 9:26–37
Casalta JP, Fournier PE, Habib G, Riberi A, Raoult D (2005) Prosthetic valve endocarditis caused by Pseudomonas luteola. BMC Infect Dis 5:82
Chihab W, Alaoui AS, Amar M (2004) Chryseomonas luteola identified as the source of serious infections in a Moroccan University Hospital. J Clin Microbiol 42:1837–1839
Coignet S (2012) Etude retrospective des infections a Pseudomonas luteola chez le furet. http://theses.vet-alfort.fr/telecharger.php?id=1893. Accessed 25 September 2023
Martínez J, Martorell J, Abarca ML, Olvera A, Ramis A, Woods L, Cheville N, Juan-Sallés C, Moya A, Riera A, Soto S (2012) Pyogranulomatous pleuropneumonia and mediastinitis in ferrets (Mustela putorius furo) associated with Pseudomonas luteola infection. J Comp Pathol 146:4–10
Milliron SM, Seyler ZG, Myers AN, Rodrigues Hoffmann A, Hnot M, Wiener DJ (2021) Pyogranulomatous panniculitis in a domestic cat associated with Pseudomonas luteola infection. Vet Dermatol 32:83-e15
Morita Y, Tomida J, Kawamura Y (2014) Responses of Pseudomonas aeruginosa to antimicrobials. Front Microbiol 4(Jan):1–8
Oliver A (2017) Epidemiology and carbapenem resistance mechanisms in Pseudomonas aeruginosa: Role of high-risk clones in multidrug resistance. Enferm Infecc Microbiol Clin 35(3):137–138
Ozdemir G, Ceyhan N, Manav E (2005) Utilization of an exopolysaccharide produced by Chryseomonas luteola TEM05 in alginate beads for adsorption of cadmium and cobalt ions. Bioresour Technol 96:1677–1682
Rahav G, Simhon A, Mattan Y, Moses AE, Sacks T (1995) Infections with Chryseomonas luteola (CDC group Ve-1) and flavimonas oryzihabitans (CDC group Ve-2). Medicine 74:83–88
Schmidt L, Doss G, Hawkins S, Blake C, Baumwart R, Kalish R, Rizzi T, Dreyfus J, Brandao J (2019) Cranial cervical abscessation and sialadenitis due to Pseudomonas luteola in two domestic ferrets (Mustela putorius furo) J Exot Pet Med 31:120–126
Su SY, Chao CM, Lai CC (2014) Peritoneal dialysis peritonitis caused by Pseudomonas luteola. Perit Dial Int 34:138–139
Tsakris A, Hassapopoulou H, Skoura L, Pournaras S, Douboyas J (2022) Leg ulcer due to Pseudomanas luteola in a patient with sickle cell disease. Diagn Microbiol Infect Dis 42:141–143
Uy HS, Leuenberger EU, de Guzman BB, Natividad FF (2007) Chronic, postoperative Pseudomonas luteola endophthalmitis. Ocul Immunol Inflamm 15:359–361
Wyre NR (2020) Emerging zoonotic diseases in ferrets. Vet Clin North Am Exot Anim Pract 23:299–308
Yousefi F, Shoja S, Honarvar N (2014) Empyema caused by Pseudomonas luteola: a case report. Jundishapur J Microbiol 7:e10923
Acknowledgements
The authors would like to thank (Janine E. Davies) for constructive criticism of the English revision.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Jacobo Giner and Sergio Villanueva-Saz conceived and designed the study; Jacobo Giner and Diego López-Sahuquillo performed the sample collection, María Eugenia Lebrero, Teresa Navarro and Carles Juan-Sallés wrote the manuscript. All authors reviewed the manuscript, Sergio Villanueva-Saz and Diana Marteles did the project management. Jacobo Giner, Álex Gómez and Carles Juan-Sallés corrected the manuscript. All authors approved the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
One ferret was sampled with the owner´s consent and due to spontaneous clinical disease. No additional ethical approval was required.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Giner, J., Lebrero, M.E., López-Sahuquillo, D. et al. Ulcerative and pyogranulomatous pododermatitis due to Pseudomonas luteola infection in a domestic ferret (Mustela putorius furo): a case report with literature review of this emerging zoonotic disease in ferrets. Vet Res Commun (2024). https://doi.org/10.1007/s11259-024-10464-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11259-024-10464-3