Abstract
Tendon calcification is a commonly associated with degenerative tendinopathy of the Achilles tendons in dogs. It is characterised by the formation of calcific deposits and is refractory to treatment, often re-forming after surgical removal. Little is known about its pathogenesis and therefore the aims of this study were to develop an in vitro model of canine tendon calcification and use this model to investigate mechanisms driving calcification. Cells from the canine Achilles tendon were cultured with different calcifying media to establish which conditions were best able to induce specific, cell-mediated calcification. Once optimum calcification conditions had been established, the effect of ATP treatment on calcification was assessed. Results revealed that 2 mM di-sodium phosphate combined with 2 mM calcium chloride provided the optimum calcifying conditions, increasing calcium deposition and expression of osteogenic-related genes similar to those observed in tendon calcification in vivo. ATP treatment inhibited calcification in a dose-dependent manner, reducing calcium deposition and increasing cell viability, while osteogenic-related genes were no longer upregulated. In conclusion, the in vitro model of canine tendon calcification developed in this study provides the ability to study mechanisms driving tendon calcification, demonstrating that ATP plays a role in modulating tendon calcification that should be explored further in future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tendon calcification is characterized by formation of calcific deposits within tendon, and is commonly associated with tendon pathology (Darrieutort-Laffite et al. 2018). Calcification has been reported in 30% of dogs presenting with shoulder pain, and has been detected in 13% and 20% of dogs presenting with supraspinatus and Achilles degeneration respectively (Maddox et al. 2013; Canapp et al. 2016; Gamble et al. 2017). Tendon calcification is also common in humans, likewise most often occurring in the supraspinatus and Achilles tendons in middle-aged individuals (Sansone et al. 2018; Giai Via et al. 2020). In humans, it is thought that calcium deposition within tendon is an active process, mediated by the resident cells (Uhthoff 1975; Darrieutort-Laffite et al. 2018). In canine tendons, hypoechoic areas surround regions of calcification, indicating the presence of inflammation (Mistieri et al. 2012). However, little is known regarding the pathogenesis of the disease in either species, and treatment options remain limited, often resulting in prolonged symptoms which do not resolve. Indeed, studies have shown that, while initial outcome after surgery for calcification of the supraspinatus tendons is good, long-term re-evaluation revealed reformation of calcification in all dogs assessed (Laitinen and Flo 2000, Lafuente et al. 2009).
A small number of studies have investigated the response of tendon-derived cells to calcifying media in vitro (Yue et al. 2016; Chen et al. 2019; Darrieutort-Laffite et al. 2019), however these studies have used levels of phosphate that far exceed those seen under physiological and pathological conditions in vivo (in excess of 5 mM). This results in dystrophic, non-specific deposition of mineral rather than specific cell-mediated calcification (Perpétuo et al. 2019). Thus, no in vitro models to induce specific tendon-derived cell calcification currently exist. Nevertheless, studies in other tissues have shown that with the appropriate conditions it is possible to model cell-mediated calcification in vitro. For example, in osteoblasts, the bone-forming cells, physiological mineralisation can be induced by culturing with 2–5 mM β-glycerophosphate (Taylor et al. 2014; Perpétuo et al. 2019). Whereas vascular calcification, another form of pathological calcification, can be modelled using vascular smooth muscle cells (VSMCs) cultured with sodium phosphate (≤ 5 mM) with or without calcium (usually as calcium chloride, ≤ 3 mM) (Patel et al. 2019).
Extracellular adenosine triphosphate (ATP) is an established signalling molecule that can act via P2 receptors to induce a range of functional effects in many different cell types (Burnstock 2007). ATP and related compound uridine triphosphate (UTP) have been shown to be potent inhibitors of physiological bone mineralisation, acting via both P2 receptor dependent and independent pathways (Hoebertz et al. 2000; Orriss et al. 2007, 2012). Similarly, ATP and UTP are also able to inhibit vascular calcification albeit via different mechanisms (Villa-Bellosta and Sorribas 2013, Patel et al. 2018). Whilst some of the actions of ATP and UTP are via P2 receptor mediated signalling, these compounds are also hydrolysed by ecto-nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1) to produce pyrophosphate (PPi). Since PPi is a ubiquitous and potent mineralisation inhibitor, some of the actions of ATP and UTP on mineralisation/calcification processes are likely to be indirect (Fleisch and Bisaz 1962, Orriss 2020).
The first aim of this study was to develop a novel, in vitro model of tendon calcification. The second aim was to use this model to investigate mechanisms driving calcification in cells derived from the canine Achilles tendon.
Methods
Isolation and culture of tendon-derived cells
Skeletally mature dogs (n = 3) euthanised for reasons unrelated to orthopaedic disease and donated to the cadaveric tissue donation programme with ethical (URN 2021 2072–2) and owner consent provided the samples (Donor details are displayed in Table 1). Within 3 h of euthanasia, the caudal hock region was aseptically prepared and through a linear skin incision, a 1.5 cm section of distal Common Calcaneal tendon (Gastrocnemius and conjoined tendon components) was isolated and resected from both hindlimbs. No tendons displayed macroscopic signs of injury or disease and samples were stored in Dulbecco’s Modified Eagle Media (DMEM) with primocin (100 µg/ml) prior to digestion at 5 °C for up to 24 h. The epitenon was then removed and tendons were finely minced and digested with 1 mg/mL pronase E (39052, VWR) per 1 g tissue for 2–4 h at 37 °C under constant agitation. Following pronase digestion, tissue was digested for a further 16 h with 0.5 mg/ml collagenase type IV (CLS-4, Lorne Laboratories) and 1 mg/mL dispase II (17105041, Invitrogen) at 37 °C with constant agitation. Cell suspensions were strained through a 70 µm filter and plated in T-75 cell culture plates. Tendon derived cells (TDCs) were cultured in low glucose DMEM supplemented with 10% FBS and antibiotic–antimycotic (complete mixture abbreviated to DMEM) until 80% confluence was reached, with media changes every 3–4 days.
Establishing optimum calcification conditions for tendon-derived cells
TDCs were cultured in phenol red-free DMEM supplemented with a range of phosphate sources that are commonly used to induce calcification. These included β-glycerophosphate and sodium phosphate dibasic (Na2HPO4, abbreviated to DIP) alone or in combination with calcium chloride (CaCl2). Based on pilot experiments (Fig. 1), it was determined that two types of calcifying media would be taken forward for detailed study; 5 mM DIP and 2 mM DIP & 2 mM CaCl2. Cells from each donor were seeded in 24-well plates at a density of 25,000 cells per well. Control cells were cultured under non-calcifying condition in DMEM supplemented with 50 μg/ml ascorbic acid. Calcifying cells were grown in DMEM supplemented with 50 μg/ml ascorbic acid and, either 5 mM DIP or 2 mM DIP & 2 mM CaCl2. To determine the effects of ATP on calcification in tendon-derived cells, both non-calcifying and calcifying media were supplemented with 10–100 µM ATP for the duration of the experiment. For all cultures, half media changes were performed every 3–4 days, and experiments were terminated after 8 days.
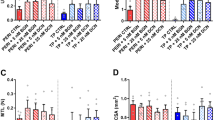
Developing a model of calcification in tendon-derived cells. (a) Phase contrast images of non-calcifying cells, dystrophic mineral deposition and cell mediated calcification. Non-specific dystrophic calcification occurs when phosphate and/or calcium is used to excess and is illustrated by the blue arrows. Cell-mediated calcification is less widespread and is shown by the red arrows. 2 mM DIP & 2 mM CaCl2 induced more cell-mediated calcification than 5 mM DIP. Scale bar: 100 µm. (b) Both calcifying conditions induced calcium deposition with a greater increase seen with DIP & CaCl2 (measured via colourimetric assay). Image analysis of fixed cell layers shows the effect of different calcifying conditions on (c) number of calcification nodules, (d) total nodule area and (e) average nodule size. Culture in calcifying conditions is associated with (f) increased cell death, (g) decreased tissue non-specific alkaline phosphatase (TNAP) activity and (h) higher ATP release. *p < 0.05; **p < 0.01; ***p < 0.001
Assessment of calcium deposition
The amount of calcium deposited by cells in each treatment group was quantified in two ways; image analysis and colorimetric assay. For image analysis, media was removed, wells were washed with PBS and fixed with 2.5% glutaraldehyde for 5 min, followed by 2 × PBS washes. Cells were rinsed 3 times with 70% ethanol and left to air dry at room temperature. Plates were scanned at 800 dpi (Epson Perfection 4990 Photo) and nodule number, total nodule area and average nodule size were measured using ImageJ as previously described (Perpétuo et al. 2019). In addition, plates were imaged using phase-contrast microscopy to visualise the calcification (Axiovert 35TV inverted microscope (Carl Zeiss Ltd, Cambridge, UK) with a 20 × LD ACHROPLAN objective.
To measure the calcium deposition by colourimetric assay, monolayers were washed twice with PBS and incubated overnight at room temperature with 0.6 M HCl. Calcium content was determined by measuring the stable interaction with o-cresolphthalein using a commercially available kit (Sigma-Aldrich, UK) and corrected for total protein concentration using the Bradford assay (Sigma-Aldrich, UK) (Patel et al. 2018).
Cell viability assay
Cells were cultured for 8 days in non-calcifying or calcifying media, with or without ATP. Cell viability was assessed using a CytoTox 96 Non-radioactive cytotoxicity assay (Promega) as described previously (Orriss et al. 2012). Briefly, media was removed from cultured cells and replaced with serum-free media. Cells were incubated at 37 °C in serum free media for 45 min to 1 h and media (25 µl) were collected to determine medium lactate dehydrogenase (LDH) levels (viability). To determine total cellular LDH levels, cells were lysed by addition of 1% Triton X-100 (v/v) to the media, and samples collected after 20 min. Assays were conducted according to the manufacturer’s instructions and samples were incubated at room temperature before reading at 495 nm. Cell viability (shown as percentage of dead cells) was calculated by expressing medium LDH as a percentage of the total cellular LDH.
Determination of tissue non-specific alkaline phosphatase (TNAP) activity
Cells were cultured as described above for 8 days. TNAP activity was assessed in cell lysates using a colorimetric assay (Sigma Aldrich), as previously described (Orriss et al. 2012). Enzyme activity was normalised to total protein content measured using the Bradford assay.
Measurement of ATP release
Release of ATP from non-calcifying and calcifying TDCs was measured after 8 days in culture. To avoid confounding effects of any treatments on the ATP measurements, culture medium was removed, cell layers washed and cells incubated with serum-free DMEM for 1 h. Following sample collection, ATP levels were measured immediately (Orriss et al. 2009). ATP release was normalised to cell number and viability monitored using the assay described above.
Gene arrays for tendon and osteogenic-related genes
To establish the effect of calcifying media and ATP treatment on gene expression, custom gene arrays for a panel of 36 tendon- and osteogenesis-related genes were performed (RT2 Profiler PCR Array, Qiagen). The list of genes measured is available in supplementary Table 1. Based on previous results, the following groups were compared: non-calcifying media; non-calcifying media plus 100 µM ATP; 2 mM DIP & 2 mM CaCl2; 2 mM DIP & 2 mM CaCl2 plus 100 µM ATP. After 8 days of culture, media was removed from cells and monolayers washed with PBS. Cells were lysed with Trizol and samples frozen prior to gene expression analysis. Total mRNA was extracted using Direct-zol RNA Microprep Kits (Zymo Research) with the inclusion of a DNase step and RNA quality assessed by measuring 260/280 nm absorbance ratio. 0.5 µg of mRNA was reverse transcribed to cDNA using an RT2 First Strand Kit (Qiagen) and arrays were performed according to the manufacturer’s instructions (RT2 Profiler PCR arrays, Qiagen). Gene expression was normalised using a panel of 6 housekeeping genes, and relative expression calculated according to the 2−ΔCT method (Livak and Schmittgen 2001). Statistical analysis was performed using the GeneGlobe data analysis tool (Qiagen).
Statistical analysis
Data were tested for normality using the Kolmogorov–Smirnov test. Data did not follow a normal distribution therefore Kruskal–Wallis tests followed by post-hoc analysis using the two-stage linear step-up procedure of Benjamini et al (2006) were used to determine significance between experimental groups. Significance was set at p < 0.05, with a trend towards significance at p < 0.1. Data analysis and visualisation was performed in GraphPad Prism (v10.0.2). Box plots show mean and interquartile range, with whiskers representing maximum and minimum data points.
Results
Developing a model of calcification in tendon-derived cells
Treatment of TDCs with both calcifying media resulted in specific, cell-mediated calcification rather than dystrophic, non-specific mineral deposition. There was significant increase in calcium deposition in calcifying cells compared to non-calcifying conditions, and a greater increase seen in response to DIP & CaCl2 treatment (Fig. 1a & b). Image analysis of fixed cell layers revealed that culturing cells in the presence of 5 mM DIP or 2 mM DIP & 2 mM CaCl2 resulted in a significant increase in the number of nodules formed (Fig. 1c). Similarly, total nodule area and average nodule size increased in response to calcifying media, with a greater response seen in cells treated with both DIP and CaCl2 (Fig. 1d & e). Cell viability was relatively high in cells cultured in non-calcifying media, with less than 10% cell death on average. Treatment with both calcifying media resulted in a significant increase (~ threefold) in the percentage of dead cells (Fig. 1f). TNAP activity was low in all groups with a significant reduction observed when cells were treated with calcifying media (Fig. 1g). Culturing cells with DIP only did not affect ATP release, but treatment with DIP and CaCl2 resulted in a significant increase in ATP release (Fig. 1h).
Effect of ATP treatment on calcification
Treatment of calcifying TDCs with ATP at a concentration of 100 µM caused a reduction in calcium deposition, particularly evident in cells cultured with 2 mM DIP & 2 mM CaCl2 (Fig. 2a). The amount of calcium deposited, as measured by colourimetric assay, was not affected by treatment with 10 µM ATP, whereas 100 µM ATP reduced calcium levels in calcifying conditions although these remained higher than in non-calcifying groups (Fig. 2b). Image analysis of fixed cell layers revealed that treatment with 10 µM ATP had no effect on the number of nodules formed, whereas 100 µM ATP reduced number of nodules to levels similar to those in cells cultured in non-calcifying media. Similar results were seen when measuring total and average nodule area, with a greater effect seen in cells cultured with 2 mM DIP & 2 mM CaCl2 compared with 5 mM DIP in the presence and absence of ATP (Fig. 2c-e). ATP treatment had no effect on calcification in non-calcifying TDCs (supplementary Fig. 1).
Effect of ATP treatment on calcium deposition under different calcifying conditions. (a) Representative phase contrast images demonstrate that ATP treatment reduces calcification in both calcium conditions tested. (b) the amount of calcium deposited decreased with increasing ATP concentration but remained significantly higher in cells treated with DIP and CaCl2. Image analysis of fixed cell layers showed that treatment with 100 µM ATP decreased nodule number (c), total nodule area (d), and average nodule size (e). Significant differences are shown relative to non-calcifying controls (# p < 0.1; *p < 0.05; **p < 0.01; ***p < 0.001) or to ATP concentration (ap < 0.1; bp < 0.05)
Effect of ATP treatment on cell viability and tissue non-specific alkaline phosphatase (TNAP) activity
The reduction in cell viability that occurred when cells were cultured in calcifying media was partially reversed with the addition of ATP at 100 µM, although the percentage of cell death remained significantly greater than in non-calcifying controls (Fig. 3a). Treatment with 10 µM ATP did not affect TNAP activity, whereas 100 µM ATP returned TNAP activity to levels similar to those seen in non-calcifying conditions (Fig. 3b). ATP treatment had no effect on cell viability or TNAP activity in non-calcifying TDCs (supplementary Fig. 1).
Effect of ATP treatment on cell viability and tissue non-specific alkaline phosphatase (TNAP) activity under different calcifying conditions. Percent cell death (a) tended to decrease with increasing ATP concentrations, but remained higher than under non-calcifying conditions. The decrease in TNAP activity (b) seen under calcifying conditions was reversed by treatment with ATP at a concentration of 100 µM only. Significant differences are shown relative to non-calcifying controls (# p < 0.1; *p < 0.05; **p < 0.01; ***p < 0.001) or to ATP concentration (bp < 0.05)
Effect of ATP treatment on ATP release
Treatment of cells under calcifying conditions with ATP significantly increased ATP release compared to non-calcifying controls. (Fig. 4). In cells treated with 5 mM DIP, this effect was more pronounced when 10 µM ATP was used, whereas in cells treated with 2 mM DIP & 2 mM CaCl2, treatment with 100 µM ATP had the largest effect. ATP treatment had no effect on ATP release in non-calcifying TDCs (supplementary Fig. 1).
Effect of ATP treatment on ATP release under different calcifying conditions. Treatment with 10 µM and 100 µM ATP caused an increase in ATP release under calcifying conditions. Significant differences are shown relative to non-calcifying controls (*p < 0.05; **p < 0.01; ***p < 0.001) or to ATP concentration (ap < 0.1; bp < 0.05; cp < 0.01)
Effect of calcifying media and ATP treatment on gene expression
Several genes were upregulated in calcifying TDCs compared to controls, including the osteogenic markers SPP1 (osteopontin) and BGLAP (osteocalcin) and those coding cell signalling proteins (FGFR1, BMP2, TGFBR1) (Fig. 5a). However, there were few changes in the expression of tendon-related genes. There were no differences in gene expression in non-calcifying cells with and without ATP treatment, indicating that ATP treatment alone had little effect on cell phenotype (Fig. 5b). In calcifying cells, addition of ATP caused an upregulation of the P2X7 receptor gene only (Fig. 5c). When comparing control and calcifying cells both treated with ATP, growth-factor related genes were upregulated (FGF1, FGF2, TGFBR2), and TFGBR2 and FMOD were downregulated in the calcifying group (Fig. 5d).
Effect of calcifying media and ATP treatment on expression of tenogenic and osteogenic related genes. Volcano plots showing gene expression in non-calcifying cells compared to calcifying cells (a) non-calcifying compared to non-calcifying, ATP-treated cells (b) calcifying compared to calcifying, ATP-treated cells (c) non-calcifying, ATP-treated cells compared to calcifying, ATP-treated cells (d). Significantly (p < 0.1, fold change > 2) regulated genes are labelled, red are upregulated, blue are down regulated
Discussion
We have developed an in vitro model in which specific, cell-mediated calcification can be induced in canine Achilles tendon-derived cells, without dystrophic mineral deposition or excessive cell death. We have used this model to demonstrate that tendon cell mineralisation can be inhibited by addition of ATP, supporting our hypothesis that dysregulation of pathways that regulate calcification could contribute to development of pathological tendon calcification. Further, we have identified key genes that are regulated both by calcification and ATP, providing targets for future studies to unpick the mechanisms governing tendon calcification, and ultimately for therapy.
There are several limitations associated with this study, including variations in donor breed, age and sex which may have contributed to the large variability in the data between individuals. Despite this, we were able to identify clear differences between calcifying and non-calcifying conditions and with ATP treatment. Another consideration is the use of cells from normal rather than diseased tendons, in which the pathways driving mineralisation may differ, and therefore this should be a focus of future studies. The sensitivity of the methods used to measure calcium deposition should also be considered. The image analysis methods used in this current study were developed to quantify physiological in vitro bone formation; however, the calcification nodules formed by TDCs are relatively small compared to bone nodules formed by osteoblasts (Perpétuo et al. 2019). Thus, it is possible that some of the smaller deposits could have been missed during the analysis. Therefore, we also utilised a colorimetric assay to measure deposited calcium. This approach, which is widely used in the study of vascular calcification, appeared to be more sensitive as it detected changes in the level of calcium deposition with 10 μM ATP as opposed to the 100 μM ATP with image analysis. Despite the differences in sensitivity, both methods showed the same overall trends.
A small number of studies have investigated calcification in tendon-derived cells, demonstrating that in rat tendon-derived stem cells, mineralisation can be inhibited by magnesium, with this inhibitory effect reversed by high concentrations of ATP (above 100 μM) (Yue et al. 2016). Similarly, stem/progenitor cells derived from mouse tail tendon show increased mineralisation when treated with interleukin-1β in the presence of osteogenic media (Chen et al. 2019). Cells derived from the rotator cuff tendon have also been reported to mineralise when treated with osteogenic media, which was accompanied by an increase in TNAP activity (Darrieutort-Laffite et al. 2019). However, these studies used high (10 mM) concentrations of β-glycerophosphate (BGP) to induce calcification, which can cause widespread, non-specific mineral deposition distinct from true bone formation or cell-mediated calcification, and also reduces cell viability and TNAP expression (Orriss et al. 2007; Hortells et al. 2015; Perpétuo et al. 2019). The results of these studies should be interpreted with caution, as they are unlikely to recapitulate the physiological environment that cells experience in vivo.
In the current study, we studied the effect of two types of calcifying media on tendon cell calcification. While both were able to induce specific, cell-mediated calcification, a greater degree of calcification occurred in cells treated with a combination of DIP and CaCl2. The reasons for this are unclear, but it could suggest that any phenotypic changes in TDCs that cause calcification require changes in extracellular calcium and phosphate (as opposed to just phosphate). Further work is clearly required to understand how changes in the extracellular environment drive pathological tendon calcification.
TNAP is a key enzyme involved in promoting physiological bone mineralisation. It hydrolyses a range of phosphate-containing molecules, including the inhibitor PPi, to produce the phosphate needed to drive mineralisation. Increased expression of TNAP in soft tissues has been associated with driving the development of pathological calcification including arterial medial calcification (Sheen et al. 2015). Therefore, this study investigated whether TNAP could play a role in calcification in tendon-derived cells. While TNAP activity was reduced in response to calcifying media, the absolute values of TNAP activity are approximately 1000-fold less than in mature osteoblasts that are actively mineralising (Patel et al. 2019). Therefore, TNAP is unlikely to play a significant role in driving mineral deposition in TDCs. However, TNAP activity was responsive to ATP treatment, returning to levels seen in non-calcifying controls.
ATP dose-dependently inhibited calcification, consistent with the inhibitory effect reported on bone mineralisation and vascular calcification (Hoebertz et al. 2000, Orriss et al. 2007, Orriss et al. 2012, Villa-Bellosta and Sorribas 2013, Patel et al. 2018). Since ATP is a universal P2 receptor agonist it is not possible to determine which P2 receptor subtype might be mediating this effect. Furthermore, it is likely that some of these inhibitory effects are mediated via purinergic independent mechanisms, as is seen in both osteoblasts and VSMCs (Patel et al. 2018).
It has also been shown that ATP, acting via the P2Y2 receptor, is capable of inducing its own release from osteocytes, osteoblasts and leukocytes (Kringelbach et al. 2014; De Ita et al. 2016; Orriss et al. 2017). Consistent with these earlier studies, calcifying tendon-derived cells cultured with ATP displayed an increased level of ATP release. Interestingly, this effect was not observed in the non-calcifying cells. This suggests that the changes that occur in these cells during calcification make them more responsive to extracellular ATP. The underlying reason for increased ATP release in these conditions is unclear, however, it could represent a feedback mechanism whereby the cells are responding to the calcification and trying to inhibit it.
When considering the response of tendon cells to calcifying media at the gene level, the upregulation of osteogenic markers SPP1 and BGLAP in calcifying cells indicate that these cells are taking on characteristics more typically associated with bone-forming osteoblasts. This suggests that the development of calcification is causing phenotypic changes in the tendon-derived cells, something that is also observed during the development of vascular calcification (Patel et al. 2019). Indeed, gene expression and protein-level analysis of biopsies from calcifying and non-calcifying regions of the human rotator cuff have shown an increase in SPP1/osteopontin within calcifying regions of the tendon (Takeuchi et al. 2001; Oliva et al. 2011; Grases et al. 2015; Cho et al. 2020). By contrast, induction of calcification with 10 mM BGP in rotator cuff derived cells did not regulate expression of SPP1 or BGLAP (Darrieutort-Laffite et al. 2019). These results indicate that the model of calcification developed in the current study may be more representative of in vivo calcification.
While exogenous ATP had no effect on gene expression under non-calcifying conditions, it ameliorated the changes in gene expression seen in calcifying cells compared to non-calcifying cells. This indicates that the ATP-mediated inhibition of calcification is preventing the phenotypic changes in TDCs that appear to occur during the development of calcification. Interestingly, an upregulation of the P2X7 receptor gene was observed in calcifying cells treated with ATP compared to non-treated calcifying cells. Previous work has shown that the P2X7 receptor plays a role in mediating ATP release from a range of cell types including bone cells, astrocytes and skin fibroblasts (Suadicani et al. 2006; Brandao-Burch et al. 2012; Flores-Muñoz et al. 2021). Therefore, this increase in P2X7 receptor expression could explain the increase in ATP release observed in tendon-derived cells. The P2X7 receptor has also been suggested to mediate some of the inhibitory effects of ATP on bone mineralisation (Orriss et al. 2012). Further work is clearly required to determine the role of the P2X7 receptor in the context of tendon calcification.
In conclusion, we have developed a novel in vitro model of tendon calcification, and used this model to demonstrate that extracellular ATP is able to inhibit the formation of calcification in tendon-derived cells. Future work should be conducted to establish the specific pathways driving tendon calcification and if these are similar in cells from normal and diseased tendons.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Benjamini Y, Krieger AM, Yekutieli D (2006) Adaptive linear step-up procedures that control the false discovery rate. Biometrika 933:491–507. https://doi.org/10.1093/biomet/93.3.491
Brandao-Burch A, Key ML, Patel JJ, Arnett TR, Orriss IR (2012) The P2X7 Receptor is an Important Regulator of Extracellular ATP Levels. Front Endocrinol 3:41. https://doi.org/10.3389/fendo.2012.00041
Burnstock G (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 872:659–797. https://doi.org/10.1152/physrev.00043.2006
Canapp SO, Canapp DA, Carr BJ, Cox C, Barrett JG (2016) Supraspinatus Tendinopathy in 327 Dogs: A Retrospective Study. Vet Evid 13. https://doi.org/10.18849/ve.v1i3.32
Chen Y, Xie Y, Liu M, Hu J, Tang C, Huang J, Qin T, Chen X, Chen W, Shen W, Yin Z (2019) Controlled-release curcumin attenuates progression of tendon ectopic calcification by regulating the differentiation of tendon stem/progenitor cells. Mater Sci Eng C 103:109711. https://doi.org/10.1016/j.msec.2019.04.090
Cho N, Lee S-G, Kim JO, Kim Y-A, Kim E-M, Park C, Ji J-H, Kim KK (2020) Identification of Differentially Expressed Genes Associated with Extracellular Matrix Degradation and Inflammatory Regulation in Calcific Tendinopathy Using RNA Sequencing. Calcif Tissue Int 1075:489–498. https://doi.org/10.1007/s00223-020-00743-x
Darrieutort-Laffite C, Blanchard F, Le Goff B (2018) Calcific tendonitis of the rotator cuff: From formation to resorption. Joint Bone Spine 856:687–692. https://doi.org/10.1016/j.jbspin.2017.10.004
Darrieutort-Laffite C, Arnolfo P, Garraud T, Adrait A, Couté Y, Louarn G, Trichet V, Layrolle P, Le Goff B, Blanchard F (2019) Rotator cuff tenocytes differentiate into hypertrophic chondrocyte-like cells to produce calcium deposits in an alkaline phosphatase-dependent manner. J Clin Med 810:1544. https://doi.org/10.3390/jcm8101544
De Ita M, Vargas MH, Carbajal V, Ortiz-Quintero B, López-López C, Miranda-Morales M, Barajas-López C, Montaño LM (2016) ATP releases ATP or other nucleotides from human peripheral blood leukocytes through purinergic P2 receptors. Life Sci 145:85–92. https://doi.org/10.1016/j.lfs.2015.12.013
Fleisch H, Bisaz S (1962) Mechanism of calcification: inhibitory role of pyrophosphate. Nature 195:911. https://doi.org/10.1038/195911a0
Flores-Muñoz C, Maripillán J, Vásquez-Navarrete J, Novoa-Molina J, Ceriani R, Sánchez HA, Abbott AC, Weinstein-Oppenheimer C, Brown DI, Cárdenas AM, García IE, Martínez AD (2021) Restraint of human skin fibroblast motility, migration, and cell surface actin dynamics, by Pannexin 1 and P2X7 receptor signaling. Int J Mol Sci 223:1069. https://doi.org/10.3390/ijms22031069
Gamble LJ, Canapp DA, Canapp OC (2017) Evaluation of Achilles Tendon Injuries with Findings from Diagnostic Musculoskeletal Ultrasound in Canines – 43 Cases. Vet Evid 23. https://doi.org/10.18849/ve.v2i3.92
Giai Via A, Oliva F, Padulo J, Oliva G, Maffulli N (2020) Insertional calcific tendinopathy of the achilles tendon and dysmetabolic diseases: An epidemiological survey. Clin J Sport Med 32:e68–e73. https://doi.org/10.1097/jsm.0000000000000881
Grases F, Muntaner-Gimbernat L, Vilchez-Mira M, Costa-Bauzá A, Tur F, Prieto RM, Torrens-Mas M, Vega FG (2015) Characterization of deposits in patients with calcific tendinopathy of the supraspinatus. Role of phytate and osteopontin. J Orthop Res 334:475–482. https://doi.org/10.1002/jor.22801
Hoebertz A, Townsend-Nicholson A, Glass R, Burnstock G, Arnett TR (2000) Expression of P2 receptors in bone and cultured bone cells. Bone 274:503–510. https://doi.org/10.1016/s8756-3282(00)00351-3
Hortells L, Sosa C, Millán Á, Sorribas V (2015) Critical parameters of the in vitro method of vascular smooth muscle cell calcification. PLoS One 1011:e0141751. https://doi.org/10.1371/journal.pone.0141751
Kringelbach TM, Aslan D, Novak I, Schwarz P, Jørgensen NR (2014) UTP-induced ATP release is a fine-tuned signalling pathway in osteocytes. Purinergic Signal 102:337–347. https://doi.org/10.1007/s11302-013-9404-1
Lafuente MP, Fransson BA, Lincoln JD, Martinez SA, Gavin PR, Lahmers KK, Gay JM (2009) Surgical treatment of mineralized and nonmineralized supraspinatus tendinopathy in twenty-four dogs. Vet Surg 383:380–387. https://doi.org/10.1111/j.1532-950X.2009.00512.x
Laitinen O, Flo G (2000) Mineralization of the supraspinatus tendon in dogs: a long-term follow-up. J Am Anim Hosp Assoc 363:262–267. https://doi.org/10.5326/15473317-36-3-262
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 254:402–408. https://doi.org/10.1006/meth.2001.1262
Maddox TW, May C, Keeley BJ, McConnell JF (2013) Comparison between shoulder computed tomography and clinical findings in 89 dogs presented for thoracic limb lameness. Vet Radiol Ultrasound 544:358–364. https://doi.org/10.1111/vru.12043
Mistieri MLA, Wigger A, Canola JC, Filho JGP, Kramer M (2012) Ultrasonographic evaluation of canine supraspinatus calcifying tendinosis. J Am Anim Hosp Assoc 486:405–410. https://doi.org/10.5326/jaaha-ms-5818
Oliva F, Barisani D, Grasso A, Maffulli N (2011) Gene expression analysis in calcific tendinopathy of the rotator cuff. Eur Cell Mater 21:548–557
Orriss IR (2020) Extracellular pyrophosphate: The body’s “water softener.” Bone 134:115243. https://doi.org/10.1016/j.bone.2020.115243
Orriss IR, Utting JC, Brandao-Burch A, Colston K, Grubb BR, Burnstock G, Arnett TR (2007) Extracellular nucleotides block bone mineralization in vitro: evidence for dual inhibitory mechanisms involving both P2Y2 receptors and pyrophosphate. Endocrinology 1489:4208–4216. https://doi.org/10.1210/en.2007-0066
Orriss IR, Knight GE, Utting JC, Taylor SE, Burnstock G, Arnett TR (2009) Hypoxia stimulates vesicular ATP release from rat osteoblasts. J Cell Physiol 2201:155–162. https://doi.org/10.1002/jcp.21745
Orriss IR, Key ML, Brandao-Burch A, Patel JJ, Burnstock G, Arnett TR (2012) The regulation of osteoblast function and bone mineralisation by extracellular nucleotides: The role of p2x receptors. Bone 513:389–400. https://doi.org/10.1016/j.bone.2012.06.013
Orriss IR, Guneri D, Hajjawi MOR, Shaw K, Patel JJ, Arnett TR (2017) Activation of the P2Y(2) receptor regulates bone cell function by enhancing ATP release. J Endocrinol 2333:341–356. https://doi.org/10.1530/joe-17-0042
Patel JJ, Zhu D, Opdebeeck B, D’Haese P, Millán JL, Bourne LE, Wheeler-Jones CPD, Arnett TR, MacRae VE, Orriss IR (2018) Inhibition of arterial medial calcification and bone mineralization by extracellular nucleotides: The same functional effect mediated by different cellular mechanisms. J Cell Physiol 2334:3230–3243. https://doi.org/10.1002/jcp.26166
Patel JJ, Bourne LE, Davies BK, Arnett TR, MacRae VE, Wheeler-Jones CPD, Orriss IR (2019) Differing calcification processes in cultured vascular smooth muscle cells and osteoblasts. Exp Cell Res 3801:100–113. https://doi.org/10.1016/j.yexcr.2019.04.020
Perpétuo IP, Bourne LE, Orriss IR (2019) Isolation and Generation of Osteoblasts. Methods Mol Biol 1914:21–38. https://doi.org/10.1007/978-1-4939-8997-3_2
Sansone V, Maiorano E, Galluzzo A, Pascale V (2018) Calcific tendinopathy of the shoulder: clinical perspectives into the mechanisms, pathogenesis, and treatment. Orthop Res Rev 10:63–72. https://doi.org/10.2147/ORR.S138225
Sheen CR, Kuss P, Narisawa S, Yadav MC, Nigro J, Wang W, Chhea TN, Sergienko EA, Kapoor K, Jackson MR, Hoylaerts MF, Pinkerton AB, O’Neill WC, Millán JL (2015) Pathophysiological role of vascular smooth muscle alkaline phosphatase in medial artery calcification. J Bone Miner Res 305:824–836. https://doi.org/10.1002/jbmr.2420
Suadicani SO, Brosnan CF, Scemes E (2006) P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci 265:1378–1385. https://doi.org/10.1523/jneurosci.3902-05.2006
Takeuchi E, Sugamoto K, Nakase T, Miyamoto T, Kaneko M, Tomita T, Myoui A, Ochi T, Yoshikawa H (2001) Localization and expression of osteopontin in the rotator cuff tendons in patients with calcifying tendinitis. Virchows Arch 4386:612–617. https://doi.org/10.1007/s004280000367
Taylor SE, Shah M, Orriss IR (2014) Generation of rodent and human osteoblasts. Bonekey Rep 3:585. https://doi.org/10.1038/bonekey.2014.80
Uhthoff HK (1975) Calcifying tendinitis, an active cell-mediated calcification. Virchows Arch A Pathol Anat Histol 3661:51–58. https://doi.org/10.1007/bf00438677
Villa-Bellosta R, Sorribas V (2013) Prevention of vascular calcification by polyphosphates and nucleotides- role of ATP. Circ J 778:2145–2151. https://doi.org/10.1253/circj.cj-13-0016
Yue J, Jin S, Li Y, Zhang L, Jiang W, Yang C, Du J (2016) Magnesium inhibits the calcification of the extracellular matrix in tendon-derived stem cells via the ATP-P2R and mitochondrial pathways. Biochem Biophys Res Commun 4781:314–322. https://doi.org/10.1016/j.bbrc.2016.06.108
Funding
Funded by the PetPlan Charitable Trust (S21-1018–1057).
Author information
Authors and Affiliations
Contributions
Conceptualization: CTT, IRO, RM; Formal analysis and investigation: DZ, NM, AM, CTT; Writing—original draft preparation: CTT, IRO; Writing—review and editing: CTT, IRO, DZ, NM, AM, RM; Funding acquisition: CTT, IRO, RM; Resources: RM; Supervision: CTT, IRO.
Corresponding author
Ethics declarations
Ethical approval
Tendon collection was performed post-mortem with owner consent and the study was approved by RVC’s Clinical Research Ethical Review Board (URN 2021 2072–2).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
IR Orriss and CT Thorpe joint senior authorship.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zamboulis, D.E., Marr, N., Moustafa, A. et al. Pathological calcification in canine tendon-derived cells is modulated by extracellular ATP. Vet Res Commun 48, 1533–1543 (2024). https://doi.org/10.1007/s11259-024-10331-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-024-10331-1