Abstract
This study proposes an ecological approach for preventing respiratory tract infections caused by Bordetella bronchiseptica in mammals using a mixture of carbohydrates. In an in vivo study, 51-day-old New Zealand rabbits were treated with a solution containing 1 × 107 CFUs of B. bronchiseptica and 250 μg of one of the following carbohydrates: N acetylglucosamine (GlcNAc), N acetylgalactosamine (GalNAc), alpha methyl mannose (AmeMan), alpha methyl glucose (AmeGlc) and sialic acid (Neu5AC). Positive (B. bronchiseptica) and negative (Physiological Saline Solution (PSS)) controls were included. Animals treated with GlcNAc or AmeGlc showed no clinical signs of infection and exhibited a significant reduction (p < 0.05) in the severity of microscopic lesions evaluated in the nasal cavity and lung compared with the positive controls. Additionally, the presence of bacteria was not detected through microbiological isolation or PCR in the lungs of animals treated with these sugars. Use of a mixture of GlcNAc and AmeGlc resulted in greater inhibition of microscopic lesions, with a significant reduction (p < 0.05) in the severity of these lesions compared to the results obtained using individual sugars. Furthermore, the bacterium was not detected through microbiological isolation, Polymerase Chain Reaction (PCR) or indirect immunoperoxidase (IIP) in this group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bordetella bronchiseptica is a motile Gram-negative coccobacillus and is part of the normal flora of the upper respiratory tracts of various host animals. B. bronchiseptica is currently known to contribute to various respiratory syndromes affecting a wide range of mammals, including dogs (Bemis et al. 1977; Molina et al. 2006; Fastres et al. 2020), cats (Welsh 1996), pigs (Pedersen and Barfod 1981; Saade et al. 2020), cattle (Liu et al. 2009), horses (Garcia-Cantu et al. 2000) and rabbits (Deeb et al. 1990; Gallego et al. 2013; Wang et al. 2020; Nielsen et al. 2021). There have also been cases of respiratory illnesses associated with B. bronchiseptica in immunocompromised humans (Woolfrey and Moody 1991; Llombart et al. 2006; Rath et al. 2008; Galeziok et al. 2009; Wang et al. 2020; Rybolt et al. 2022).
B. bronchiseptica adheres extensively to the epithelial cells of the upper respiratory tract of its hosts and it is mostly detected on the apical portion of the ciliated cells; it can also be found in the cytoplasm of neutrophils and macrophages. It adheres to the surface of the respiratory cells through structures and substances on its surface that act as adhesins, such as filamentous hemagglutinin (FHA), fimbriae, adenylate cyclase, pertactin and flagella (Savelkoul et al. 1996; Sisti et al. 2002; Hibrand-Saint Oyant et al. 2005; Edwards et al. 2005; Edwards 2006; Gallego et al. 2013; Saade et al. 2020), the first three of which are credited with mediating adhesion to host cells via carbohydrate-lectin interactions. Adhesion is the first step of infection and is therefore a central event in disease development. Once bacteria adhere to the surface of epithelial cells, they evade the cleaning mechanism of the mucociliary system and trigger infection via the expression of various virulence factors (Mattoo and Cherry 2005; Saade et al. 2020).
On the other side, the development of antibiotic resistance is a common problem in the treatment of bacterial infections (Davies and Davies 2010; D’Costa et al. 2011), as in the case of B. bronchiseptica, which exhibits high resistance to various antibacterial agents (Wettstein and Frey 2004; Zhao et al. 2011a; Mazumder et al. 2012). Additionally, vaccines aimed at preventing infection by bacteria of the Bordetella genus have shown contradictory results, for example, despite the wide application of vaccination programs, mainly against Bordetella pertussis in humans, the prevalence of diseases caused by these organisms has increased (Mooi et al. 2001; Gilberg et al. 2002; Ellis et al. 2002; Gopinathan et al. 2007; Zepp et al. 2011; Schulz et al. 2014). B. bronchiseptica infections also remain a common veterinary problem (Foley et al. 2002; Di Martino et al. 2007; Zhao et al. 2011a, b). Therefore, the development of new strategies and tools for prevention and control of diseases caused by B. bronchiseptica are required. One alternative for preventing and, perhaps, treating bacterial infections is anti-adhesive therapy based on the use of carbohydrates (CHOs) that block specific recognition sites between bacterial adhesins and receptors on host cell surfaces localized on molecules and structures that include carbohydrate-recognizing lectins residues (Ahmed et al. 2022).
The first test of CHO anti-adhesive therapy was developed in 1979 and demonstrated that the simultaneous injection of E. coli type 1 fimbria with alpha methyl mannopyranoside into mouse bladders reduced the rate of bacteriuria (Aronson et al. 1979). In vivo tests involving respiratory infections with Streptococcus pneumoniae in rabbits showed that simultaneously intratracheal administration of the bacteria with lacto-N-neotetraose and sialylated derivatives and intranasal administration of these oligosaccharides 24 h post-infection reduced the incidence of pneumonia and bacteremia (Idänpään-Heikkilä et al. 1997). An in vitro study using a mixture of fructose, mannose and galactose reported the inhibition of adhesion of Pseudomonas aeruginosa to bronchial epithelial cells and the development of pneumonia in mice intratracheally instilled with a mixture of the bacteria and sugars (Bucior et al. 2013).
The respiratory mucosal surface of rabbits, similar to that of other species, is rich in glycoproteins (Brandley and Schnaar 1986; Schulte et al. 1990; Muramatsu 2000), moreover, some adhesins, including B. bronchiseptica FHA, possess properties of lectins and the ability to bind to CHOs; on this basis, this study demonstrates that inhibiting the adhesion of B. bronchiseptica to the respiratory epithelium of rabbits using N-acetyl glucosamine and alpha methyl glucoside, alone or as a mixture, avoids the clinical signs of the disease and prevents the development of lesions in the respiratory epithelium of the nasal cavity and the lung caused by this microorganism.
Materials and methods
Bordetella bronchiseptica strain
B. bronchiseptica was isolated from the turbinates and tracheae of rabbits displaying signs of rhinitis and pneumonia from farms in Sabana de Bogotá, Colombia. The bacterium was grown in BHI agar for 24 h at 37 °C, and tests were conducted for differentiation (Gram staining, oxidase, catalase and urease tests). Molecular identification was performed via amplification of the gene encoding the ribosomal 16S rDNA subunit using the following primer pair: (27f) 5'-AGAGTTTGATCMTGGCTCAG- 3' and (1942R) 5'-TACGGYTACCTTGTTACGACTT-3', followed by sequencing, which showed 99.4% similarity with the reference strain Bordetella bronchiseptica RB50.
Sugars/Carbohydrates
Five CHOs were utilized in this study: N acetylglucosamine (GlcNAc), N acetylgalactosamine (GalNAc), alpha methyl mannose (AmeMan), alpha methyl glucose (AmeGlc) and sialic acid (Neu5AC) (Vector laboratories®, Burlingame, California, USA). The selection of these sugars was based on experiments using in vitro cultures of nasal cavities from rabbit fetuses, in which previous culture experiments showed that LCA (Lens culinaris), PNA (Arachis hypogaea), DBA (Dolichos biflorus), RCA120 lectin (Ricinus communis) and WGA (wheat germ agglutinin) significantly (p < 0.05) inhibited the adhesion of the bacteria to the apex of the ciliated epithelium (Carrillo et al. 2015). These lectins recognize one or more of these CHOs: GlcNAc, GalNAc, Neu5AC, galactose, mannose and glucose. Once the test results for the individual sugars were obtained, a mixture of the sugars that significantly inhibited microscopic injuries caused by B. bronchiseptica was used.
Animals
The experiments used a total of 42 clinically healthy New Zealand White rabbits that were 36 days old and were microbiologically negative for B. bronchiseptica and Pasteurella multocida. The animals underwent an adjustment period of 15 days in the laboratory, and at 51 days of age, they were randomized into 14 treatment groups of three rabbits per group (Table 1 and 2).
The administration procedure of the experimental solution as well as for euthanasia was similar. The animals were pre-anesthetized with acepromazine at 0.5 mg/kg SCT, xylazine at 5 mg/kg IM and ketamine at 35 mg/kg IM.
All procedures were approved and authorized by the Bioethics Committee of the Faculty of Veterinary Medicine and Animal Science, National University of Colombia (Act 006/2010).
Test solutions and experimental design
The test solutions included a mixture of 200 µL of B. bronchiseptica (at a concentration of 1 × 107 CFU/mL in PSS, resulting in a final concentration of 2 × 106 CFU of B. brochiseptica) combined with 200 µL of each sugar (at a concentration of 250 µg/200 µL in PSS, giving a final concentration of 250 µg of carbohydrate) as indicated in Table 1. Later a similar solution of B. bronchiseptica mixed with the two sugars (1:1) that obtained significant results was used at a concentration of 250 µg/200 µL in PSS, (resulting in a final concentration 250 ug of carbohydrate) as detailed in Table 2. These solutions were incubated for 15 min at room temperature.
Each animal received a total of 400 µL of each test solution: 200 µL administered intranasally (IN) and another 200 µL intratracheally (IT) simultaneously, as described in Table 1 and 2.
The animals were examined every 4 h beginning at the time of instillation, and the presentation of the following clinical signs was evaluated: hypothermia, cyanosis of the mucous membranes and ears, dyspnea (orthopnea, rapid shallow breathing at rest) and death. At 72 h post-instillation, the rabbits were euthanized with Euthanex® at a dose of 1 mL/5 kg intracardially.
Microbiological re-isolation and PCR
For microbiological re-isolation, samples from lungs were collected for standard microbiological and PCR techniques. Re-isolation was performed using swabs of the cranial lobe of the lung, which were cultured on BHI agar at 37 °C for 24 h for subsequent differentiation assays (Gram staining, oxidase test, catalase test).
DNA extraction was carried out in lung tissue using the commercially available ZR Genomic DNA™ Tissue Miniprep kit (Zymo Research Corp., California, U.S.A.). For the identification of B. bronchiseptica via PCR, the following primer sequences targeting the gene encoding filamentous hemagglutinin were used: (F) 5´-ATGACTGACGCAACGAACCGTTTCC-3´ and (R) 5´-GCGTTCTCGCCGGGCTCAGAAACTG-3´.
Tissue processing
After the animals were sacrificed, the heads were removed, and two transverse sections, each approximately 0.5 cm thick, were obtained from the nasal cavity, starting at the first premolar. The rib cage was removed, and the entire respiratory system was separated from the larynx and infused with 3.7% formaldehyde via the trachea, under pressure from a 20 cm column of water, to achieve complete fixation and further expansion of the lung tissue. Sagittal sections of all lung lobes were obtained. Turbinate samples and full lungs were fixed in 3.7% formalin buffer for 24 h at 4 °C. The sections of the nasal cavity were then subjected to a process of decalcification using a 10% solution of EDTA disodium salt, pH 7, at 4 °C for 8 days. All tissues were processed via routine techniques using hematoxylin–eosin (H&E).
Macroscopic evaluation of the lungs
All lungs were grossly evaluated and catalogued as apparently normal (AN) or according to the distribution of the lesions as having diffuse (D) or cranial lesions (CR).
Microscopic evaluation of tissues
The tissues stained with H&E were evaluated in a blinded manner by a researcher using a light microscope with a 40 × objective. For each nasal cavity, a total of 6 sections were evaluated: 2 from the dorsal region, 2 from the central region and 2 from the ventral region of the tissue. The evaluation criteria were migration of polymorphonuclear neutrophils into the respiratory epithelium, loss of cilia and presence of bacteria. Each of the changes was assigned a score according to its degree of severity, ranging from absent to severe (Table 3). For each nasal septum, scores were obtained for each lesion in each of the fields, and the average score for each lesion was calculated.
In the lung sections, each lobe was analyzed, and a score was assigned based on the degree of severity (Table 3) of the following lesions: septal thickening, the presence of detritus in the alveolar and/or bronchiolar lumen, the presence of bacteria and the presence of pneumonic foci. For each lung, the scores for each of the lobes and the average score for each lesion were calculated.
Indirect immunoperoxidase
The indirect immunoperoxidase (IIP) technique was developed to label B. bronchiseptica and determine its location in the tissues of the animals treated with the mixture of sugars. A primary polyclonal antiserum antibody against B. bronchiseptica was produced in sheep using a 1:1 dilution, and IgG secondary anti-sheep antibodies produced in donkey (Sigma, Aldrich®) at a 1:500 dilution were added. The commercial Liquid DAB Substrate Kit (Invitrogen™) was used to reveal the antibody binding. The nasal septum and lung tissues were observed with a light microscope, and a score was assigned to each tissue according to the labeling percentage (extent) of the bacteria adhered to the epithelium, ranging from absent to severe (Table 3).
Statistical analysis
The severity of lesions in the lung and nasal cavity, and the degree of labeling of B. bronchiseptica through IIP were rated from 0 to 6. The average scores for each group were evaluated via ANOVA to determine whether there were differences between treatments, which was followed by Dunnett's test to compare different treatments to the positive control (Zar 1998; Martínez et al. 2011). Differences were considered statistically significant at p < 0.05.
Results
Trials of individual sugars
Clinical signs
None of the animals treated with B. bronchiseptica and GlcNAc (group 1) or AmeGlc (group 2) showed clinical signs. Furthermore, at least two of the three rabbits treated with B. bronchiseptica and AmeMan (group 3), GalNAc (group 4) or Neu5AC (group 5) evidenced clinical signs, predominantly fever and cyanosis of the mucous membranes and ears. Additionally, one individual treated with B. bronchiseptica + AmeMan (group 3) died from infection. The animals in the positive control group (group 11) showed greater severity of clinical signs: two of the three animals died approximately 24 h post-inoculation. No animal in the control CHO groups (groups 6–10) or the negative control group (group 12) exhibited clinical signs. Figure 1 shows the number of rabbits with clinical signs in the experimental groups treated with B. bronchiseptica (group 11) or with B. bronchiseptica and sugar (groups 1–5).
Microbiological re-isolation and PCR
PCR and conventional microbiological isolation techniques detected B. bronchiseptica only in the lungs of the animals in groups 3, 4, 5 and 11, which developed clinical signs.
Gross evaluation of the lungs
The three rabbits treated with B. bronchiseptica + GlcNAc (group 1) presented apparently normal lungs, and only one animal in the group treated with B. bronchiseptica + AmeGlc (group 2) developed a pattern of diffuse pneumonia. In the positive control group (group 11) and the other groups inoculated with bacteria and sugars, at least two rabbits exhibited some pattern of lung injury. None of the negative control animals (group 12) or CHO controls (groups 6 to 10) showed lung injury (Fig. 2).
a Number of rabbits with the indicated patterns of pneumonia in each experimental group treated with B. bronchiseptica and sugar (groups 1–5) or with B. bronchiseptica (group 11 = positive control). b Lungs of a rabbit from the positive control group, note the cranioventral (darker regions) distribution of the bronchopneumonic lesions. c Lungs with a normal appearance from a rabbit treated with PSS (Group 12 = negative control). d Lungs with a normal appearance from a rabbit treated with B. bronchiseptica + GlcNAc (group 1). Bb: B. bronchiseptica
Microscopic evaluation of the nasal cavity and lungs
Nasal cavity
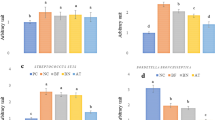
Only groups 1 (B. bronchiseptica + GlcNAc), 2 (B. bronchiseptica + AmeGlc) and 5 (B. bronchiseptica + Neu5AC) exhibited a lower severity (p < 0.05) of all lesions recorded in the nasal cavity compared with the positive control (group 11); in addition, the presence of bacteria was not observed in the first two groups, (Fig. 3a, b and c). Similarly, the negative control group (group 12) and the CHO controls (groups 6–10) were significantly different (p < 0.05) from the positive controls regarding all the microscopic lesions recorded in the nasal cavity. Figure 3d to 3f show histological sections of the rabbit nasal cavities with normal architecture and other with some lesions.
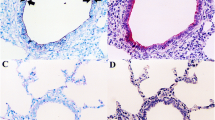
Severity of lesions based on microscopic evaluation of the respiratory epithelium in nasal cavities of rabbits in the groups treated with B. bronchiseptica + one CHO (groups 1–5) or B. bronchiseptica (group 11 = positive control). a Presence of PMNs. b Loss of cilia. c Severity of bacterial attachment to the apical surface of the respiratory epithelium. Histological section of the respiratory epithelium in nasal cavities of rabbits from (d) B. bronchiseptica + GlcNAc group (group 1) (H&E, 400X) and (e) Negative control (group 12) (H&E, 200X) show normal architecture. f Positive control (group 11) (H&E, 1000X) shows bacteria adhered between the cilia (arrows), loss of cilia (arrowheads) and PMNs infiltration between epithelial cells (asterisk); also, a dilatation of interepithelial spaces and disorganization of the epithelium is evident. Bb: B. bronchiseptica; *: significant difference (p < 0.05)
Lungs
Among the animals treated with B. bronchiseptica plus CHO, only groups 1 (B. bronchiseptica + GlcNAc) and 2 (B. bronchiseptica + AmeGlc), compared with the positive control group (group 11), exhibited a lower severity (p < 0.05) of all lung lesions (Fig. 4a to 4d). The negative controls (group 12) and the CHO controls (groups 6–10) were also significantly different (p < 0.05) from the positive control. Figure 4e to 4h show histological sections of a lung with normal architecture and others with some lesions.
Severity of lesions based on microscopic evaluation of the lungs of rabbits in the groups treated with B. bronchiseptica + one CHO (groups 1–5) or B. bronchiseptica (group 11 = positive control). a Lung septal thickening. b Pneumonic foci. c Accumulation of detritus in the alveolar and bronchiolar lumen. d Bacterial accumulation in the alveoli. Histological sections of rabbit lungs from e Negative control (group 12) with the normal architecture retained (H&E, 100X). f Positive control (group 11) (H&E, 100X) and (g) B. bronchiseptica + GalNAc (group 4) (H&E, 100X), presenting a noticeable loss of architecture, with septal thickening and the presence of abundant inflammatory cells in the space of the bronchioles and severe pneumonic foci. h B. bronchiseptica + AmeGlc (group 2) showed a slight degree of septal thickening (H&E, 100X). Bb: B. bronchiseptic, *: significant difference (p < 0.05)
Tests of sugar mixture: GlcNAc + AmeGlc
In each case in which clinical signs, gross lesions and the presence of microscopic lesions in both the nasal cavity and lungs of rabbits were significantly inhibited by the individual sugars GlcNAc and AmeGlc, a mixture of those sugars was used.
Clinical signs
None of the animals treated with B. bronchiseptica + the CHO mixture (group 13) or the mixture of CHOs (group 14) showed any clinical signs.
Microbiological re-isolation and PCR
B. bronchiseptica was not isolated from the lungs of any rabbits from groups 13 (B. bronchiseptica + CHO mixture) and 14 (CHO mixture), nor was identified by PCR technique.
Gross evaluation of the lungs
At necropsy, all the rabbits of group 13 (B. bronchiseptica + CHO mixture) and group 14 (mixed CHO) exhibited lungs that appeared normal.
Microscopic evaluation of the nasal cavity and lungs
Nasal cavity
The respiratory epithelium of the nasal cavity of rabbits treated with B. bronchiseptica + the CHO mixture (group 13) exhibited a normal appearance (Fig. 5a); this group showed significantly less loss of cilia (p < 0.05) compared with the rabbits treated with B. bronchiseptica + individual sugars (groups 1 and 2) (Fig. 5b).
a Comparison of the severity of microscopic lesions in the nasal cavity between animals treated with B. bronchiseptica + individual CHOs (groups 1 and 2), animals treated with B. bronchiseptica + the CHO mixture (group 13) and animals treated with CHO mixture (group 14 = negative control). Histological section of the respiratory epithelium in the nasal cavities of rabbits from (b) B. bronchiseptica + the CHO mixture (group 13), (H&E, 400X) and (c) Negative control (group 14) (H&E, 400X), show normal architecture. d Positive control (group 11) (H&E, 400X) shows a notable loss of most cilia (arrows), with the remaining cilia being shorter than normal; disorganization of the epithelial cells is evident. Bb: B. bronchiseptica; *: significant difference (p < 0.05)
Lungs
Significant differences (p < 0.05) in the severity of the thickening of the alveolar septa of the lungs were observed between group 13 (B. bronchiseptica + CHO mixture) and groups 1 (B. bronchiseptica + GlcNAc) and 2 (B. bronchiseptica + AmeGlc) and in the severity of the presence of pneumonic foci between groups 13 (B. bronchiseptica + CHO mixture) and 1 (B. bronchiseptica + GlcNAc) (Fig. 6a). The lungs of the rabbits in group 13 (B. bronchiseptica + CHO mixture) showed no microscopic changes indicative of a pneumonic process (Fig. 6b).
a Comparison of the severity of microscopic lesions in the lungs from animals treated with B. bronchiseptica + individual CHOs (groups 1 and 2) versus those treated with B. bronchiseptica + the mixture of GlcNAc and AmeGlc (group 13) and animals treated with CHO mixture (group 14 = negative control). Histological section of lung of a rabbit treated with B. bronchiseptica + the CHO mixture (group 13) showing (b) the normal architecture of the organ (H&E, 100X) and (c) staining indicative of the notable absence of B. bronchiseptica (IIP, 1000X). d Negative control (group 14) (IIP, 400X) where no specific labeling of the scant macrophages in the alveolar lumen is visible. e Positive control (group 11) (IIP, 400X) showing positive staining (brown color) indicating the presence of B. bronchiseptica both inside and outside of macrophages. Bb: B. bronchiseptica, *: significant difference (p < 0.05)
Indirect Immunoperoxidase
Moderate to severe positive IIP labeling of B. bronchiseptica was found in the nasal cavity and lungs of the rabbits in group 11 (positive control), while those of animals from group 13 (B. bronchiseptica + CHOs mixture) did not show positive staining (Fig. 6c to 6e) (p < 0.05).
Discussion
Despite the use of various vaccines for the prevention of B. bronchiseptica infection, respiratory disease caused by this microorganism remains a common cause of significant economic losses in the livestock sector (Wabacha and Mulei 2000; Zhao et al. 2011a, b; Mazumder et al. 2012; Wang et al. 2020). In addition, in canines, B. bronchiseptica causes severe respiratory disease mostly represented by the kennel cough syndrome (Bhardwaj et al. 2013; Corona et al. 2013; Fastres et al. 2020); furthermore, the increasing resistance of the microorganism to antibiotics is another major concern (Mazumder et al. 2012; Nielsen et al. 2021). Hence, the effort to develop new ways to prevent and/or control these diseases deserves increasing attention. The present study demonstrated that treatment with the sugars GlcNAc and AmeGlc individually prevented the expression of clinical signs and gross lung lesions (except in one rabbit) and significantly inhibited the microscopic damages caused by B. bronchiseptica in the nasal cavities and lungs. Furthermore, the mixture of these two sugars even more significantly inhibited the microscopic lesions compared with the individual sugars.
Apparently both carbohydrates function as inhibitors of bacterial adhesion to nasal and pulmonary epithelial cells in rabbits, in turn preventing infection and the onset of respiratory disease in these animals. This prevention strategy is proposed as an ecological alternative that does not destroy the pathogen, perhaps preventing the development of B. bronchiseptica resistance, as can occur when antibiotics and vaccines are employed. Another purpose of this strategy is to avoid damage to the animal, particularly to the architecture of the epithelium of the respiratory tract and, thus, its functionality. Degenerative changes, necrosis and inflammation, that occur during B. bronchiseptica infections, as in other types of bacterial infections, lead to a cascade of events that are often more harmful to the host than the infection by itself (Anderson et al. 1991; Tetley 1993; Mogensen 2009). Among these events, we highlight over-regulation of the inflammatory response, which induces the accumulation of PMNs and the subsequent release of reactive oxygen species and proteolytic enzymes that cause tissue damage (Wright et al. 2010; Segel et al. 2011; Saade et al. 2020). B. bronchiseptica can also damage the cilia (Dugal et al. 1990; Anderton et al. 2004; Saade et al. 2020); together with the excessive release of mucus by goblet cells, this phenomenon significantly alters the efficacy of the mucociliary system. By preventing epithelial damage, it should theoretically be possible to preserve the structure and function of the mucociliary clearance machinery, thus aiding the host in expelling the bacteria from the body.
AmeGlc and GlcNAc could block the adhesion of bacteria to the epithelium via two mechanisms: by binding to lectin-type adhesins on the surface of B. bronchiseptica and/or by binding to lectins on the surface of host epithelial cells. Different adhesins expressed on the surface of B. bronchiseptica can act individually, collectively or synergistically; these adhesins include pertactin, fimbriae and filamentous hemagglutinin (FHA). FHA is one of the most important adhesins found in B. bronchiseptica and can mediate the adhesion of the bacteria to both ciliated and non-ciliated cells (Cotter et al. 1998; Nicholson et al. 2009; Saade et al. 2020). FHA has at least three binding sites on host cells: one site composed of carbohydrates that mediates adhesion to respiratory ciliated epithelial cells and macrophages (Tuomanen et al. 1988; Prasad et al. 1993; Saade et al. 2020); another site composed of glycine-arginine-aspartic acid (RGD) sequences that mediates leukocyte adhesion (Relman et al. 1990; Saade et al. 2020); and a glycosaminoglycan for binding to heparin, heparan sulfate and other sulfated carbohydrates (Hannah et al. 1994; Saade et al. 2020). It has also been reported that the adherence of B. pertussis FHA is largely mediated by lectin-carbohydrate interactions. However, there are contradictions regarding which carbohydrates might mediate the adhesion of both B. pertussis and B. bronchiseptica. Tuomanen et al. (1988) demonstrated that the adhesion of the B. pertussis FHA to ciliated human tracheal epithelial cells can be inhibited by molecules that contain lactose, such as galactose, galactose β1-4-glucose, complex lactosamines and by antibodies directed against molecules of galactose-bound GlcNAc (Gal B1-3GlcNAc [4–1-a-Fuc]). However, GalNAc had no significant effect on the inhibition of adhesion, similar to the findings of the present study in B. bronchiseptica. It would therefore be appropriate to evaluate whether simpler molecules, such as lactose and galactose, have an increased ability to inhibit the adherence of B. bronchiseptica to the epithelium of the respiratory tract of rabbits. Plotkin and Bemis (1984) achieved 100% inhibition of the adherence of B. bronchiseptica to hamster fibroblasts (HLF) by using GlcNAc, which is consistent with the results of the present study in rabbits; these authors were also able to reverse the adherence of the bacteria to HLF by 96% after 45 min of incubation with this CHO at a dose of 0.25 M.
However, the FHA does not appear to be the only adhesin from B. bronchiseptica that can bind to sugar residues on the respiratory epithelia of different species. For example, the B. pertussis fimbrial subunit fim2, which is also expressed by B. bronchiseptica (Savelkoul et al. 1996), binds to sulfated sugars such as heparan sulfate, chondroitin sulfate and dextran sulfate (Geuijen et al. 1996). Nevertheless, the specific binding domains for other fimbrial subunits from both B. pertussis and B. bronchiseptica have not yet been identified. Furthermore, some studies claim that B. pertussis pertactin, which is also expressed by B. bronchiseptica, mediates binding to the cell surface via RGD sequences (Leininger et al. 1991) in proline-rich regions (Williamson 1994) or leucine protein interactions (Emsley et al. 1994). It has also been proposed that the adenylate cyclase toxin (Hibrand-Saint Oyant et al. 2005) and the flagella (Savelkoul et al. 1996) contribute to the adherence of B. bronchiseptica to eukaryotic cells, although the mechanism of the binding of these structures remains unclear.
Additionally, the possibility that GlcNAc and AmeGlc or even glucose (which is the carbohydrate basis of these glycoconjugates) could also block the adherence of B. bronchiseptica by recognizing other bacterial structures cannot be excluded. More recent studies report that even when the expression of the major adhesins of B. bronchiseptica is blocked, its adhesive power is retained (Mattoo 2003 in Edwards 2006), and those authors propose lipopolysaccharide (LPS) as a possible factor that may contribute to the adherence and colonization in absence of these structures. Although there are no studies that concretely support this hypothesis in the case of B. bronchiseptica, research in Salmonella enterica (Bravo et al. 2011), Pasteurella multocida (Gallego et al. 2017) and Helicobacter pylori (Chang et al. 2011), suggests that this molecule plays an important role in the early steps of adherence to host epithelial cells. GlcNAc is part of the structure of the LPS of B. bronchiseptica (Preston et al. 2006), and incubation with this carbohydrate, as carried out in the present study, would be one of the mechanisms by which this sugar interferes with the binding of the bacterium to surface lectins of the rabbit respiratory tract.
The expression of FHA and, to a lesser degree, of the fimbriae, has been reported to be necessary for maximum biofilm formation by B. bronchiseptica (Irie et al. 2004). Thus, it might be speculated that blocking the binding of these adhesins with AmeGlc, GlcNAc or their mixture, as performed in this work, would not only inhibit the bacterial adhesion to their respective cellular receptors but also interfere with the biofilm formation. Still another mechanism by which AmeGlc and GlcNAc might have exerted their anti-adhesive activity in this study is by promoting bacterial agglutination or aggregation. Bacterial aggregation has been induced in other Gram-positive and Gram-negative species by a mix of simple carbohydrates such as fucose, mannose and galactose, that unlike the clusters formed in biofilms, those formed by the addition of exogenous carbohydrates are not stable and rapidly disperse (Bucior et al. 2013). Therefore, it is possible that the sugars used in this study promote bacterial aggregation, forming clusters through a similar process to the one that occurs during biofilm formation, but which is not sufficiently strong to promote biofilm formation, whereas it is sufficient to inhibit the adherence of bacteria to the epithelium and facilitate the removal of the pathogen by the mucociliary clearance system of the host.
Results of our group demonstrated that the previous incubation of P. multocida with individual GlcNAc, AmeGlc and AmeMan and a mixture of them, significantly inhibited the adherence of the bacterium to the respiratory epithelium of rabbits in an in vivo model. Equally so, it prevented the expression of clinical signs, microscopic and macroscopic changes in the nasal septa and lungs of rabbits experimentally exposed to the microorganisms (Gallego et al. 2021).
This work demonstrates that the anti-adhesive activity of the mixture of GlcNAc and AmeGlc appears to have at least an additive effect, when compared with the results obtained using the two CHOs individually. In turn, the inhibitory effect of the CHO mixture would block a greater diversity of bacterial adhesion sites, which eventually may indirectly indicate the existence of different adhesion structures with different compositions on the surface of the microorganism. It should be noted that therapeutic substances such as the CHOs studied here, in addition to presenting obvious environmental and economic benefits, exhibit other properties, such as a low probability that microorganisms will eventually develop resistance against them (Sharon 2006; Gallego et al. 2021).
In conclusion, GlcNAc, AmeGlc and, to a greater extent, a mixture of the two sugars significantly inhibited the adherence of B. bronchiseptica to the apical surface of the respiratory epithelia of nasal cavity and inhibited the colonization of the bacteria in the rabbit lungs, through which the establishment of infection and clinical manifestations and macro- and microscopic lesions were prevented. Subsequent studies must aim to develop more stable glycoconjugates with more prolonged effects that can be directly applied to animals and to evaluate their therapeutic capacity for treating B. bronchiseptica infections.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahmed MN, Jahan R, Nissapatorn V, Wilairatana P, Rahmatullah M (2022) Plant lectins as prospective antiviral biomolecules in the search for COVID-19 eradication strategies. Biomed Pharmacother 146:112507. https://doi.org/10.1016/j.biopha.2021.112507
Anderson BO, Brown JM, Harken AH (1991) Mechanisms of neutrophil-mediated tissue injury. J Surg Res 51(2):170–179. https://doi.org/10.1016/0022-4804(91)90090-9
Anderton T, Maskell D, Preston A (2004) Ciliostasis is a key early event during colonization of canine tracheal tissue by Bordetella bronchiseptica. Microbiology (reading) 150(Pt 9):2843–2855. https://doi.org/10.1099/mic.0.27283-0
Aronson M, Medalia O, Schori L, Mirelman D, Sharon N, Ofek I (1979) Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl alpha-D-mannopyranoside. J Infect Dis 139(3):329–332. https://doi.org/10.1093/infdis/139.3.329
Bemis DA, Greisen HA, Appel MJ (1977) Pathogenesis of canine bordetellosis. J Infect Dis 135:753–762. https://doi.org/10.1093/infdis/135.5.753
Bhardwaj M, Singh B, Vadhana P (2013) Bordetella bronchiseptica infection and Kennel Cough in dogs. Adv Anim Vet Sci 1(3S):1–4
Brandley B, Schnaar R (1986) Cell-surface carbohydrates in cell recognition and response. J Leukoc Biol 40(1):97–111. https://doi.org/10.1002/jlb.40.1.97
Bravo D, Hoare A, Silipo A, Valenzuela C, Salinas C, Alvarez SA, Molinaro A, Valvano MA, Contreras I (2011) Different sugar residues of the lipopolysaccharide outer core are required for early interactions of Salmonella enterica serovars Typhi and Typhimurium with epithelial cells. Microb Pathog 50(2):70–80. https://doi.org/10.1016/j.micpath.2010.11.001
Bucior I, Abbott J, Song Y, Matthay MA, Engel JN (2013) Sugar administration is an effective adjunctive therapy in the treatment of Pseudomonas aeruginosa pneumonia. Am J Physiol Lung Cell Mol Physiol 305(5):L352–L363. https://doi.org/10.1152/ajplung.00387.2012
Carrillo M, Martínez N, Patiño P, Iregui C (2015) Inhibition of Pasteurella multocida adhesion to rabbit respiratory epithelium using lectins. Vet Med Int. https://doi.org/10.1155/2015/365428
Chang P, Wang C, You C, Kao M (2011) Effects of a HP0859 (rfaD) knockout mutation on lipopolysaccharide structure of Helicobacter pylori 26695 and the bacterial adhesion on AGS cells. Biochem Biophys Res Commun 405(3):497–502. https://doi.org/10.1016/j.bbrc.2011.01.060
Corona A, Vercelli A, Casassa A (2013) Molecular identification of Bordetella bronchiseptica by real-time PCR in dogs with respiratory disease. Veterinaria (cremona) 27(6):9–13
Cotter PA, Yuk MH, Mattoo S, Akerley BJ, Boschwitz J, Relman DA, Miller JF (1998) Filamentous hemagglutinin of Bordetella bronchiseptica is required for efficient establishment of tracheal colonization. Infect Immun 66:5921–5929. https://doi.org/10.1128/iai.66.12.5921-5929.1998
D’Costa V, King C, Kalan L, Morar M, Sung W, Schwarz C, Froese D, Zazula G, Calmels F, Debruyne R, Golding B, Poinar H, Wright G (2011) Antibiotic resistance is ancient. Nature 477:457–461. https://doi.org/10.1038/nature10388
Davies J, Davies D (2010) Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 74(3):417–433. https://doi.org/10.1128/MMBR.00016-10
Deeb B, DiGiacomo R, Bernard B, Silbernagel S (1990) Pasteurella multocida and Bordetella bronchiseptica infections in rabbits. J Clin Microbiol 28(1):70–75. https://doi.org/10.1128/jcm.28.1.70-75.1990
Di Martino B, Di Francesco C, Meridiani I, Marsilio F (2007) Etiological investigation of multiple respiratory infections in cats. New Microbiol 30:455–461
Dugal F, Girard C, Jacques M (1990) Adherence of Bordetella bronchiseptica 276 to porcine trachea maintained in organ culture. Appl Environ Microbiol 56(6):1523–1529. https://doi.org/10.1128/aem.56.6.1523-1529.1990
Edwards J, Groathouse N, Boitano S (2005) Bordetella bronchiseptica adherence to cilia is mediated by multiple adhesin factors and blocked by surfactant protein A. Infect Immun 73(6):3618–3626. https://doi.org/10.1128/IAI.73.6.3618-3626.2005
Edwards J (2006) Initial interactions between Bordetella bronchiseptica and tracheal epithelial cell cilia. PhD Dissertation, The University of Arizona. http://hdl.handle.net/10150/195708
Ellis J, Krakowka G, Dayton A, Konoby C (2002) Comparative efficacy of an injectable vaccine and an intranasal vaccine in stimulating Bordetella bronchiseptica-reactive antibody responses in seropositive dogs. J Am Vet Med Assoc 220(1):43–48. https://doi.org/10.2460/javma.2002.220.43
Emsley P, McDermott G, Charles IG, Fairweather NF, Isaacs NW (1994) Crystallographic characterization of pertactin, a membrane-associated protein from Bordetella pertussis. J Mol Biol 235:772–773. https://doi.org/10.1006/jmbi.1994.1029
Fastres A, Canonne MA, Taminiau B, Billen F, Garigliany MM, Daube G, Clercx C (2020) Analysis of the lung microbiota in dogs with Bordetella bronchiseptica infection and correlation with culture and quantitative polymerase chain reaction. Vet Res 51:46. https://doi.org/10.1186/s13567-020-00769-x
Foley J, Rand C, Bannasch M, Norris C, Milan J (2002) Molecular epidemiology of feline bordetellosis in two animal shelters in California, USA. Prev Vet Med 54:141–156. https://doi.org/10.1016/s0167-5877(02)00022-3
Galeziok M, Roberts I, Passalacqua J (2009) Bordetella bronchiseptica pneumonia in a man with acquired immunodeficiency syndrome: a case report. J Med Case Rep 3:76. https://doi.org/10.1186/1752-1947-3-76
Gallego C, Middleton A, Martínez N, Romero S, Iregui C (2013) Interaction of Bordetella bronchiseptica and its lipopolysaccharide with in vitro culture of respiratory nasal epithelium. Vet Med Int. https://doi.org/10.1155/2013/347086
Gallego C, Romero S, Esquinas P, Patiño P, Martínez N, Iregui C (2017) Assessment of Pasteurella multocida a lipopolysaccharide, as an adhesin in an in vitro model of rabbit respiratory epithelium. Vet Med Int. https://doi.org/10.1155/2017/8967618
Gallego C, Patiño P, Martínez N, Iregui C (2021) The effect of carbohydrates on the adherence of Pasteurella multocida to the nasal respiratory epithelium. An Acad Bras Ciênc. https://doi.org/10.1590/0001-3765202120190989
Garcia-Cantu M, Hartmann F, Brown C, Darien B (2000) Bordetella bronchiseptica and equine respiratory infections: a review of 30 cases. Equine Vet Educ 12:45–50
Geuijen CA, Willems R, Mooi F (1996) The major fimbrial subunit of Bordetella pertussis binds to sulfated sugars. Infect Immun 64:2657–2665. https://doi.org/10.1128/iai.64.7.2657-2665.1996
Gilberg S, Njamkepo E, du Chatelet IP, Partouche H, Gueirard P, Ghasarossian C, Schlumberger M, Guiso N (2002) Evidence of Bordetella pertussis infection in adults presenting with persistent cough in a french area with very high whole-cell vaccine coverage. J Infect Dis 186(3):415–418. https://doi.org/10.1086/341511
Gopinathan L, Kirimanjeswara G, Wolfe D, Kelley M, Harvill E (2007) Different mechanisms of vaccine-induced and infection-induced immunity to Bordetella bronchiseptica. Microbes Infect 9(4):442–448. https://doi.org/10.1016/j.micinf.2007.01.001
Hannah JH, Menozzi FD, Renauld G, Locht C, Brennan MJ (1994) Sulfated glycoconjugate receptors for the Bordetella pertussis adhesin filamentous hemagglutinin (FHA) and mapping of the heparin-binding domain on FHA. Infect Immun 62:5010–5019. https://doi.org/10.1128/iai.62.11.5010-5019
Hibrand-Saint Oyant L, Bourges D, Chevaleyre C, Raze D, Locht C, Salmon H (2005) Role of Bordetella bronchiseptica adenylate cyclase in nasal colonization and in development of local and systemic immune responses in piglets. Vet Res 36(1):63–77. https://doi.org/10.1051/vetres:2004056
Idänpään-Heikkilä I, Simon P, Zopf D, Vullo T, Cahill P, Sokol K, Tuomanen E (1997) Oligosaccharides interfere with the establishment and progression of experimental pneumococcal pneumonia. J Infect Dis 176:704–712
Irie Y, Mattoo S, Yuk MH (2004) The Bvg virulence control system regulates biofilm formation in Bordetella bronchiseptica. J Bacteriol 186(17):5692–5698. https://doi.org/10.1128/JB.186.17.5692-5698.2004
Leininger E, Roberts M, Kenimer JG, Charles IG, Fairweather N, Novotny P, Brennan MJ (1991) Pertactin, an Arg-Gly-Asp-containing Bordetella pertussis surface protein that promotes adherence of mammalian cells. Proc Natl Acad Sci U S A 88:345–349. https://doi.org/10.1073/pnas.88.2.345
Liu XM, Yang H, Zhang RX, Gao MC, Wang JW (2009) Isolation and identification of Bordetella bronchiseptica from calves. Chinese Veterinary Science 39(11):964–967
Llombart M, Chiner E, Senent C (2006) Necrotizing pneumonia due to Bordetella bronchiseptica in an immunocompetent woman. Arch Bronconeumol 42:255–256. https://doi.org/10.1016/s1579-2129(06)60457-6
Martínez M, Martínez N, Martínez R (2011) Diseño de experimentos en ciencias agropecuarias y biológicas con SAS, SPSS, R y STATISTIX. Fondo Nacional Universitario - Institución Auxiliar del Cooperativismo, Bogotá Colombia. https://isbn.cloud/9789589251577/diseno-de-experimentos-en-ciencias-agropecuarias-y-biologicas-con-sas-spss-r-y-statistix/
Mattoo S, Cherry J (2005) Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev 18(2):326–382. https://doi.org/10.1128/CMR.18.2.326-382.2005
Mazumder Y, Das A, Kar D, Shome B, Dutta B, Rahman H (2012) Isolation of Bordetella bronchiseptica from pigs in north east India. J Anim Sci Adv 2(4):396–406
Mogensen T (2009) Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 22(2):240–273. https://doi.org/10.1128/CMR.00046-08
Molina G, Rosales M, Bárcenas G, Montaraz J (2006) Isolation and characterization of Bordetella bronchiseptica strains from canine origin. Vet Mex 37:313–325
Mooi F, van Loo I, King A (2001) Adaptation of Bordetella pertussis to vaccination: a cause for its reemergence? Emerg Infect Dis 7(3 Suppl):526–528. https://doi.org/10.3201/eid0707.017708
Muramatsu T (2000) Protein-bound carbohydrates on cell-surface as targets of recognition: an odyssey in understanding them. Glycoconj J 17(7–9):577–595. https://doi.org/10.1023/a:1011078627247
Nicholson TL, Brockmeier SL, Loving CL (2009) Contribution of Bordetella bronchiseptica filamentous hemagglutinin and pertactin to respiratory disease in swine. Infect Immun 77(5):2136–2146. https://doi.org/10.1128/IAI.01379-08
Nielsen SS, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin-Bastuji B, Gonzales Rojas JL, Gortazar C, Herskin M, Michel V, Miranda MA, Padalino B, Pasquali P, Roberts HC, Spoolder H, Stahl K, Velarde A, Viltrop A, Winckler C, Dewulf J, Guardabassi L, Hilbert F, Mader R, Baldinelli F, Alvarez J (2021) Assessment of animal diseases caused by bacteria resistant to antimicrobials: rabbits. EFSA J 19(12):6999. https://doi.org/10.2903/j.efsa.2021.6999
Pedersen KB, Barfod K (1981) The aetiological significance of Bordetella bronchiseptica and Pasteurella multocida in atrophic rhinitis of swine. Nord Vet Med 33(12):513–522
Plotkin BJ, Bemis DA (1984) Adherence of Bordetella bronchiseptica to hamster lung fibroblasts. Infect Immun 46(3):697–702. https://doi.org/10.1128/iai.46.3.697-702.1984
Prasad SM, Yin Y, Rodzinski E, Tuomanen EI, Masure HR (1993) Identification of a carbohydrate recognition domain in filamentous hemagglutinin from Bordetella pertussis. Infect Immun 61:2780–2785. https://doi.org/10.1128/iai.61.7.2780-2785.1993
Preston A, Petersen BO, Duus JØ, Kubler-Kielb J, Ben-Menachem G, Li J, Vinogradov E (2006) Complete structures of Bordetella bronchiseptica and Bordetella parapertussis lipopolysaccharides. J Biol Chem 281(26):18135–18144. https://doi.org/10.1074/jbc.M513904200
Rath B, Register K, Wall J, Sokol D, Van Dyke R (2008) Persistent Bordetella bronchiseptica pneumonia in an immunocompetent infant and genetic comparison of clinical isolates with Kennel Cough vaccine strains. Clin Infect Dis 46:905–908. https://doi.org/10.1086/528858
Relman D, Tuomanen E, Falkow S, Golenbock DT, Saukkonen K, Wright SD (1990) Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell 61:1375–1382. https://doi.org/10.1016/0092-8674(90)90701-f
Rybolt LE, Sabunwala S, Greene JN (2022) Zoonotic bacterial respiratory infections associated with cats and dogs: A case series and literature review. Cureus 14(4):e24414. https://doi.org/10.7759/cureus.24414
Saade G, Deblanc C, Bougon J, Marois-Crehan C, Fablet C, Auray G, Belloc C, Leblanc-Maridor M, Gagnon C, Zhu J, Gottschalk M, Summerfield A, Simon G, Bertho N, Meurens F (2020) Coinfections and their molecular consequences in the porcine respiratory tract. Vet Res 51:80. https://doi.org/10.1186/s13567-020-00807-8
Savelkoul P, de Kerf D, Willems R, Mooi F, van der Zeijst B, Gaastra W (1996) Characterization of the fim2 and fim3 fimbrial subunit genes of Bordetella bronchiseptica: roles of Fim2 and Fim3 fimbriae and flagella in adhesion. Infect Immun 64(12):5098–5105. https://doi.org/10.1128/iai.64.12.5098-5105.1996
Schulte B, Harley R, Spicer S (1990) Carbohydrate histochemistry. In: Gil J (ed) Models of Lung Disease: Microscopy and structural methods, 1st edn. CRC Press, Boca Raton, USA, p 52. https://doi.org/10.1201/9781003066248
Schulz B, Kurz S, Weber K, Balzer H, Hartmann K (2014) Detection of respiratory viruses and Bordetella bronchiseptica in dogs with acute respiratory tract infections. Vet J 201(3):365–369. https://doi.org/10.1016/j.tvjl.2014.04.019
Segel G, Halterman M, Lichtman M (2011) The paradox of the neutrophil’s role in tissue injury. J Leukoc Biol 89:359–372. https://doi.org/10.1189/jlb.0910538
Sharon N (2006) Carbohydrates as future anti-adhesion drugs for infectious diseases. Biochim Biophys Acta 1760(4):527–537. https://doi.org/10.1016/j.bbagen.2005.12.008
Sisti F, Fernández J, Rodríguez M, Lagares A, Guiso N, Hozbor D (2002) In vitro and in vivo characterization of a Bordetella bronchiseptica mutant strain with a deep rough lipopolysaccharide structure. Infect Immun 70(4):1791–1798. https://doi.org/10.1128/IAI.70.4.1791-1798.2002
Tetley T (1993) New perspectives on basic mechanisms in lung disease. Proteinase imbalance: its role in lung disease. Thorax 48(5):560–565. https://doi.org/10.1136/thx.48.5.560
Tuomanen E, Towbin H, Rosenfelder G, Braun D, Larson G, Hansson GC, Hill R (1988) Receptor analogs and monoclonal antibodies that inhibit adherence of Bordetella pertussis to human ciliated respiratory epithelial cells. J Exp Med 168:267–277. https://doi.org/10.1084/jem.168.1.267
Wabacha J, Mulei CM (2000) The economic impact of progressive atrophic rhinitis in grower-finisher pigs in a medium-scale piggery in Kenya. Bull Anim Health Prod Afr 48(3):189–191
Wang J, Sun S, Chen Y, Chen D, Sang L, Xie X (2020) Characterisation of Bordetella bronchiseptica isolated from rabbits in Fujian, China. Epidemiol Infect 148:E237. https://doi.org/10.1017/S0950268820001879
Welsh R (1996) Bordetella bronchiseptica infections in cats. J Am Anim Hosp Assoc 32(2):153–158. https://doi.org/10.5326/15473317-32-2-153
Wettstein K, Frey J (2004) Comparison of antimicrobial resistance pattern of selected respiratory tract pathogens isolated from different animal species. Schweiz Arch Teerheilkd 146:417–422. https://doi.org/10.1024/0036-7281.146.9.417
Williamson MP (1994) The structure and function of proline-rich regions in proteins. Biochem J 297(Pt 2):249–260. https://doi.org/10.1042/bj2970249
Woolfrey BF, Moody JA (1991) Human infections associated with Bordetella bronchiseptica. Clin Microbiol Rev 4:243–255. https://doi.org/10.1128/cmr.4.3.243
Wright H, Moots R, Bucknall R, Edwards S (2010) Neutrophil function in inflammation and inflammatory diseases. Rheumatology (oxford) 49(9):1618–1631. https://doi.org/10.1093/rheumatology/keq045
Zar J (1998) Biostatistical analysis. Prentice Hall, London
Zepp F, Heininger U, Mertsola J, Bernatowska E, Guiso N, Roord J, Tozzi A, Van Damme P (2011) Rationale for pertussis booster vaccination throughout life in Europe. Lancet Infect Dis 11(7):557–570. https://doi.org/10.1016/S1473-3099(11)70007-X
Zhao Z, Wang C, Xue Y, Tang X, Wu B, Cheng X, He Q, Chen H (2011a) The occurrence of Bordetella bronchiseptica in pigs with clinical respiratory disease. Vet J 188(3):337–340. https://doi.org/10.1016/j.tvjl.2010.05.022
Zhao Z, Xue Y, Wang C, Ding K, Wu B, He Q, Cheng X, Chen H (2011b) Antimicrobial susceptibility of Bordetella bronchiseptica isolates from pigs with respiratory diseases on farms in China. J Vet Med Sci 73(1):103–106. https://doi.org/10.1292/jvms.10-0184
Funding
Open access funding provided by Colombia Consortium. This study was financed by Ministry of Science, Technology and Innovation of Colombia and the National University of Colombia.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. The preparation of the material and the compilation were carried out by Pilar Patiño, Carolina Gallego and Carlos Iregui. Data analysis was carried out by Pilar Patiño, Nhora Martinez and Alba Rey. The first draft of the manuscript was written by Pilar Patiño and Carlos Iregui. All authors commented on previous versions of the manuscript and made their contributions and suggestions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Statement of animal ethics
The in vivo experimental protocols in this study were submitted to evaluation by the Bioethics and Animal Welfare Committee of the Veterinary Faculty of the National University of Colombia (Act 006/2010). This committee is based on international and national guidelines that govern the use, care and welfare of animals used for scientific purposes, in accordance with Law 84 of 1989 of the Congress of the Republic of Colombia: National Statute for the Protection of Animals, Chapter V: On the sacrifice of animals, article 17, literal j.: For experimental, investigative or scientific purposes and Chapter VI: On the use of live animals in experiments and research, Articles 23 and 24 and Resolution 8430 of 1993 of the Ministry of Health, which establishes the scientific, technical and administrative standards for health research: Title V: Biomedical research with animals, articles 87 and 88.
Competing of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Patiño, P., Gallego, C., Martínez, N. et al. Effect of carbohydrates on the adhesion of Bordetella bronchiseptica to the respiratory epithelium in rabbits. Vet Res Commun 48, 1481–1495 (2024). https://doi.org/10.1007/s11259-024-10307-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-024-10307-1