Abstract
Virus monitoring in small mammals is central to the design of epidemiological control strategies for rodent-borne zoonotic viruses. Synanthropic small mammals are versatile and may be potential carriers of several microbial agents. In the present work, a total of 330 fecal samples of small mammals were collected at two sites in the North of Portugal and screened for zoonotic hepatitis E virus (HEV, species Paslahepevirus balayani). Synanthropic small mammal samples (n = 40) were collected in a city park of Porto and belonged to the species Algerian mouse (Mus spretus) (n = 26) and to the greater white-toothed shrew (Crocidura russula) (n = 14). Furthermore, additional samples were collected in the Northeast region of Portugal and included Algerian mouse (n = 48), greater white-toothed shrew (n = 47), wood mouse (Apodemus sylvaticus) (n = 43), southwestern water vole (Arvicola sapidus) (n = 52), Cabrera’s vole (Microtus cabrerae) (n = 49) and Lusitanian pine vole (Microtus lusitanicus) (n = 51). A nested RT-PCR targeting a part of open reading frame (ORF) 2 region of the HEV genome was used followed by sequencing and phylogenetic analysis. HEV RNA was detected in one fecal sample (0.3%; 95% confidence interval, CI: 0.01–1.68) from a synanthropic Algerian mouse that was genotyped as HEV-3, subgenotype 3e. This is the first study reporting the detection of HEV-3 in a synanthropic rodent, the Algerian mouse. The identified HEV isolate is probably the outcome of either a spill-over infection from domestic pigs or wild boars, or the result of passive viral transit through the intestinal tract. This finding reinforces the importance in the surveillance of novel potential hosts for HEV with a particular emphasis on synanthropic animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatitis E virus (HEV) is a small nonenveloped single-stranded positive sense RNA virus that belongs to the Hepeviridae family, subfamily Orthohepevirinae, genus Paslahepevirus. species balayani (Purdy et al. 2022). In both developing and industrialized countries, HEV is the main cause of acute hepatitis, reported to infect around 20 million individuals and resulting in 3.3 million symptomatic cases and 44,000 fatalities annually worldwide (WHO 2022). Hepatitis E was identified as a zoonotic illness in the late 1990s and pigs were discovered to be the primary natural host of HEV genotypes 3 and 4 (Dalton et al. 2008). The intake of undercooked pork meat was soon linked to a significant number of autochthonous cases in several European countries (Pavio et al. 2015).
HEV (Paslahepevirus balayani) includes variants detected in humans, pigs, wild boar, deer, mongoose, rabbits, camels and other animals, with 8 genotypes recognized until today (HEV-1 - HEV-8), whereby HEV-3, HEV-4 and HEV-7 with zoonotic transmission (Smith et al. 2020). Genotypes HEV-3 and HEV-4 are transmitted via contact with infected animals, especially pigs, or mainly by consumption of contaminated pork meat products (Velavan et al. 2021). These two genotypes have been detected in Sus scrofa domesticus (domestic pigs) and Sus scrofa scrofa (wild boars), as well as in humans (Berto et al. 2012; de la Villalba et al. 2013; Santos-Silva et al. 2023). Despite numerous studies on HEV-3 and HEV-4, particularly in high-income countries where transmission of these two genotypes via the fecal-oral route and infections acquired through environmental contamination with animal feces is still mostly unknown, other potential transmission routes are being investigated, such as milk (King et al. 2018; Treagus et al. 2021; Santos-Silva et al. 2022).
In rats, the first description of HEV was in 1993 in the former Soviet Union, in a region that experienced a viral hepatitis outbreak in human in July-October 1989 having been pointed out to rodents a certain role in the process of the spread of the virus (Karetnyĭ et al. 1993). However, molecular detection and characterization of HEV RNA in Norway rats (Rattus norvegicus) only occurred in 2010 (Johne et al. 2010b). Moreover, currently a growing body of data shows evidence of the circulation of HEV-3 in small mammals, like rats and rabbits (Lack et al. 2012; Ryll et al. 2017).
Besides the initial reports of HEV-3 in small mammals, additional Hepeviridae members have also been found, namely Rocahepevirus ratti. Putative genotypes of rat Hepatitis E virus (species Rocahepevirus ratti; ratHEV) HEV-C3 and HEV-C4 were identified in A. chevrieri and E. melanogaster, respectively (Wang et al. 2018), however, there is a high likelihood of numerous additional orthohepeviruses being present in different rodent species and geographic locations (Wang et al. 2018). Genotypes of Paslahepevirus balayani species xhibit a greater divergence than other hepeviruses species (Reuter et al. 2020) and are distinctly different from genotypes HEV-C1 and HEV-C2 of Rocahepevirus ratti (Wang et al. 2020).
The zoonotic potential of rocahepeviruses is now recognized. Interestingly, ratHEV replication in a human-derived cell line was demonstrated (Jirintai et al. 2014; Li et al. 2015). Notwithstanding, experimental infection in monkeys and domestic pigs failed (Purcell et al. 2011; Cossaboom et al. 2012), however a recent study successfully inoculated ratHEV in Rhesus and Cynomolgus monkeys (Yang et al. 2022). Nevertheless, ratHEV infection has been linked to chronic hepatitis and acute hepatitis (Sridhar et al. 2018; Andonov et al. 2019). Additionally, ratHEV human infections have also recently been reported in Europe (Rivero-Juarez et al. 2022; Rodriguez et al. 2023), and IgG anti-ratHEV-reactive IgG antibodies have been detected in forestry workers in Germany and Japan (Dremsek et al. 2012; Li et al. 2013; de Cock et al. 2022).
Small mammals include animals of the order Eulipotyphla (formerly known as Insectivora) and the order Rodentia. They have extremely versatile habits, inhabiting various locations with the ability to settle between wild and urban environments (Bencatel et al. 2017). The full spectrum of small mammals, including synanthropic animals that have adapted to human environments, representing additional HEV reservoirs and playing a role in the epidemiology of zoonotic HEV is still far from being clarified. Synanthropic animals thrive in close proximity to human populations and may frequently come into contact with humans and their habitats, thereby increasing the potential for zoonotic transmission. Detecting spill-over infections in small mammals is valuable as it helps map the occurrence of HEV strains in different regions. Understanding the crucial role of synanthropic animals in the epidemiology of HEV is essential for developing effective prevention and control strategies, as they can serve as additional reservoirs and vectors for the virus. Consequently, comprehensive research that encompasses both wildlife and synanthropic animals is necessary to gain a complete understanding of the complex dynamics of HEV transmission.
In Portugal, HEV infection in humans has been widely reported (Berto et al. 2012; Mesquita et al. 2016; Moraes et al. 2022) and HEV RNA has been detected in domestic pigs and in wild mammals, such as wild boar and red deer (Moraes et al. 2022; Santos-Silva et al. 2023), however no studies have been ever carried out in small mammals. Hence the aim of this study was to perform a screening and to provide genetic characterization of HEV detected in synanthropic and wild small mammals from Portugal.
Materials and methods
Sampling and collection
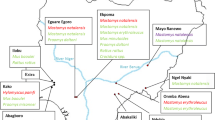
A total of 330 fecal samples from synanthropic and wild small mammals were collected. Samples from synanthropic animals, represented by the wild species Algerian mouse (Mus spretus) (n = 26) and by the greater white-toothed shrew (Crocidura russula) (n = 14), were collected in the spring of 2014 from a public city park in Porto, Portugal (Fig. 1). The wild small mammal fecal samples (n = 290) were collected during ecological studies (Barão et al. 2022; Lux et al. 2023) from six different species in the spring of 2020 in the Northeast region of Portugal (Trás-os-Montes). Wild small mammal droppings were collected from 24 different sampling units, representing six different habitat types and were from one insectivore (greater white-toothed shrew, Crocidura russula, n = 47) and from five species of rodents, including two species of the family Muridae (wood mouse, Apodemus sylvaticus, n = 43; Algerian mouse, Mus spretus, n = 48) and three species of the family Cricetidae (southwestern water vole, Arvicola sapidus, n = 52; Cabrera’s vole, Microtus cabrerae, n = 49; Lusitanian pine vole, Microtus lusitanicus, n = 51).
Wild small mammal species of the samples were previously determined by molecular analysis of a short 12 S rRNA gene fragment to genetically identify the host species (Barão et al. 2022). Fresh samples were immediately transported to the laboratory and kept frozen at -20 °C until further analysis.
(A) Map of Portugal. Red and green squares highlight the sampling site of synanthropic and wild small mammals, respectively. (B) Location of the 24 sampling units centered in olive grove patches. Common names of small mammal species are as follows: wood mouse (Apodemus sylvaticus), southwestern water vole (Arvicola sapidus), greater white-toothed shrew (Crocidura russula), Cabrera’s vole (Microtus cabrerae), Lusitanian pine vole (Microtus lusitanicus) and Algerian mouse (Mus spretus)
Sample preparation and RNA extraction
Individual fecal suspensions (10% in phosphate-buffered saline, pH 7.2) and 0.2 g sterile silicone microbeads (Precellys Lysing kits, Bertin Technologies SAS, Montigny-le-Bretonneux, France) were prepared. Fecal samples were shredded with plastic pestles, vortexed for 5 min using the cell disruptor Disruption Genie (Scientific industries, Inc., Bohemia, NY, USA) and centrifugated at 8,000 × g for 5 min according to previously described methods (Mesquita et al. 2010). Nucleic acid extraction was carried out from 140 µL of clarified supernatants using the automatic nucleic acid extraction machine QIAcube® (Qiagen, Hilden, Germany) and QIAamp® viral RNA mini kit (Qiagen, Hilden, Germany). The RNA was eluted in 50 µL of RNase-free ultrapure water and kept at -80 °C until further use.
Amplification of HEV RNA
All RNA extracts were tested for HEV RNA by a pangenotypic nested RT-PCR assay that targets the open reading frame (ORF) 2 region that encodes the viral capsid protein of the HEV genome (exclusively for HEV genotypes of species Paslahepevirus balayani, amplifying a 467 nucleotides (nt)-long genome fragment in the second round spanning between nt 5930–6334 (Frías et al. 2021).
RNA extracts were also tested by a broad-spectrum nested RT-PCR assay targeting the RNA-dependent RNA-polymerase (RdRp) gene of the ORF1 region of the HEV genome (nt 331–334 ) spanning nt 4285–4616 (numbering according to genotype 3 strain Meng accession number AF082843), that was developed for detection of novel hepeviruses (Johne et al. 2010b). For the first round, Qiagen One-Step™ RT-PCR kit (Qiagen®, Hilden, Germany) was used for both assays and for the second round, 5 µL of the first-round products were used as templates with GoTaq® (Promega™, WI, U.S.A.), all according to the manufacturer’s instructions. The WHO PEI 6329/10 subgenotype 3a standard (accession number AB630970, provided by the Paul Ehrlich-Institute, Langen, Germany) was used as a positive control and RNase-free water as negative control. Amplification reactions, with the corresponding positive and negative controls (nuclease – free water), were conducted in Bio-Rad T100TM Thermal Cycler. The conditions for first rounds were an initial reverse transcription (RT) step for 15 min at 45ºC followed by 3 min at 95ºC (enzyme activation, denaturation of template DNA). For the pangenotypic nested RT-PCR assay the thermal profile includes 40 cycles of 95 ºC for 15 s, 52 ºC for 15 s, and 72 ºC for 2 s, with a final elongation at 72 ºC for 10 min. For the broad-spectrum nested RT-PCR assay the same conditions were followed except the annealing temperature was set as 50 ºC. Both second rounds followed the same conditions, excluding the RT step.
If samples were positive for any of the above molecular approaches, HEV RNA quantification was also attempted using a broad-spectrum real-time RT-PCR (RT-qPCR) assay targeting the open reading frame ORF3 region with primers/probe (TaqMan) as previously described (Jothikumar et al. 2006). The RT-qPCR was performed using iTaq Universal Probes One-Step Kit (Bio-Rad Laboratories, USA) at a final total of 20 µL reaction mixture volume in a CFX Connect Real-Time thermocycling System (Bio-Rad Laboratories, USA). The thermal cycling regimen for the RT-qPCR reaction included initial reverse transcription (RT) at 50 °C for 10 min, followed by a simultaneous step for reverse transcriptase inactivation and the initial denaturation of cDNA at 95 °C for 3 min. Subsequently, 45 cycles of amplification were carried out, involving denaturation at 95 °C for 15 s and annealing/extension at 55 °C for 15 s.
Sequencing and phylogenetic analysis
RT-PCR products were separated by electrophoresis at 100 V for 40 min on a 1.5 % agarose gel stained with Xpert Green Safe DNA gel dye (GriSP®) and documented using the ChemiDoc XRS system with ImageLab software (Bio-Rad, Hercules, CA, USA). Bands of the expected size were excised and treated enzymatically to remove unincorporated primers and nt using Illustra™ ExoProStar™ (Sigma Aldrich® Darmstadt, Germany). Amplicons were further sequenced in both directions with the dideoxy-chain termination method using the BigDye Terminator v1.1 Cycle Sequencing kit (PE Applied Biosystems, Foster City, CA, USA). Sequence editing and multiple alignments were performed with the BioEdit software package, version 2.1 (Ibis Biosciences, Carlsbad, CA, USA). Aligned sequences were compared to sequences found in the NCBI (GenBank) nucleotide database, retrieved on 1 February 2023 (http://blast.ncbi.nlm.nih.gov/Blast). Phylogenetic analysis was performed using MEGA version X software (Kumar et al. 2018). The maximum-likelihood (ML) approach was used to infer this analysis (Tamura 1992; Kumar et al. 2018), and Tamura-Nei model was used to estimate the maximum likelihood (ML) bootstrap values using 1000 replicates. The Tamura-Nei model was determined by MEGA version X (Kumar et al. 2018) as the best replacement. Further typing was performed with the HEVnet genotyping tool (Mulder et al. 2019) to identify genotypes/subgenotypes of HEV.
Results
From the 330 fecal samples tested, one was positive for HEV RNA by the pangenotypic nested RT-PCR assay that targets the ORF2 (0.3%; 95% confidence interval, CI: 0.01–1.68) (Table 1). No sample was positive using the broad-spectrum nested ORF1-targeting RT-PCR assay and the in-house broad-spectrum RT-qPCR assay targeting the ORF3 region. The HEV RNA positive stool sample originates from a synanthropic Algerian mouse, which represented an occurrence of 1.4% (95% CI: 0.03–7.3) for this species when considering both synanthropic and wild Algerian mouse and 2.5% (95% CI: 0.06–13.16) for the total of the synanthropic samples collected. The respective generated consensus sequence was identified as HEV subgenotype 3e by Blast search. Phylogenetic analysis based on the 467 nt-long partial region of the ORF2 confirmed clustering with HEV-3 subgenotype e (Fig. 2), being closely related to variants of human (MK167982, from United Kingdom) and animal (MT840367, wild boar from Italy) origin. Furthermore, pairwise nt sequence similarity of the positive sample and the positive control was 81.2%. The sequence of HEV detected in this study is available in GenBank database under accession number OK545865. The mean pairwise distances analysis between each reference HEV sequences, as recommended for HEV subtyping studies (Smith et al. 2020), and the positive (51 M) sample is described in Table 2. No sample was positive for ratHEV RNA.
Phylogenetic analysis of HEV sequence found in a synanthropic Algerian mouse from Portugal. HEV-3 found in this study (OK545865) and the closest related variants of human (MK167982, from United Kingdom) and animal (MT840367, wild boar from Italy) origin and their respective accession numbers are highlighted in bold in the tree inferred using the MEGA X software and the Interactive Tree of Life (iTOL) based on 50 nucleotide HEV sequences as well as 49 strains of various genotypes obtained from GenBank.
Discussion
The potential role and impact of small mammals as a HEV reservoir is still largely unknown. The present study offers the first molecular-based proof of the presence and identification of the zoonotic HEV-3 in a synanthropic Algerian mouse in Portugal.
The first molecular identification of a hepevirus (ratHEV, species Rocahepevirus ratti) in rodents was in Norway rat feces and liver samples in Germany in 2010 (Johne et al. 2010a, b). Since then ratHEV strains have been detected in rats across at least three continents (Asia, America, and Europe), indicating that rats are infected with the virus throughout a large geographic range (Reuter et al. 2020). In addition, ratHEV has been found in a variety of wild rat species, including Norway rats (Rattus norvegicus) and Black rats (Rattus rattus) in many European countries (Johne et al. 2010a; Ryll et al. 2017; Simanavicius et al. 2018; Murphy et al. 2019), United States of America (Purcell et al. 2011), Hong Kong (Sridhar et al. 2021), Indonesia (Mulyanto et al. 2014; Primadharsini et al. 2018). Furthermore, ratHEV has also been detected in synanthropic rats (Porea et al. 2023), and in humans (Rivero-Juarez et al. 2022) from Europe. In the present study, ratHEV was not detected in any of the 290 wild small mammal fecal samples that included one insectivore and five rodent species. Further studies assessing urban rats/rodents (Rattus sp.) should be conducted in Portugal in order to estimate the zoonotic risk of infection, considering the findings in neighboring countries, such as France and Spain.
Nevertheless, in this study, HEV-3 subgenotype 3e was detected in the fecal sample of a synanthropic Algerian mouse, that goes in accordance to results from previous studies that also report this human pathogenic genotype in small mammals (Lack et al. 2012). However, no accurate assessment of the variety of hepevirus species/genotypes circulating in wild small mammals in Portugal can be made due to the extremely low number of positive samples. Furthermore, the positive sample was identified using the pangenotypic nested RT-PCR assay that targets the ORF2 region that encodes the viral capsid protein of the HEV genome and specific to the species Paslahepevirus balayani and not with the broad-spectrum nested RT-PCR assay targeting the RdRp gene of the ORF1 region of the HEV genome and the broad-spectrum RT-qPCR assay targeting the ORF3 region. These differences may be attributed to the genetic variability of the virus, which may have hindered detection with the other sets of primers.
According to the HEVTool (Mulder et al. 2019) and phylogenetic analysis, the HEV strain detected in the Algerian mouse was associated with strains that cause more severe illness in humans (Subissi et al. 2019). Since synanthropic small mammals live in close association with people and benefit from their surroundings and activities and although the uncertainty in how HEV behaves in synanthropic and wild small mammals, there is some concern that it could spread and infect humans and other species. However, as previously mentioned, the HEV positive sample found in this study could potentially be result of a spill-over infection or of passive viral transit through the intestinal tract, which is highly unlikely to be transmitted to humans or animals. In a study from Italy, rats inhabiting a pig farm were found to have HEV-3e (the same subgenotype found in pigs), highlighting the possible involvement of rats in the spread of this virus (De Sabato et al. 2020) or at least a spill-over effect that enables the tracking of this particular virus strain within a specific geographic area. Interestingly, in previous studies, HEV-3 RNA was detected in intestinal contents, but not in the liver of mice from a pig farm house and was considered to be a virus that had entered the intestine from ingested feces of pigs rather than infection of mice (Grierson et al. 2019). In addition, studies show the absence of genotype HEV-3 in liver tissue samples from wild rats, supporting the argument that rats, like mice, are only accidental hosts of HEV (Takahashi et al. 2022). Nevertheless, further investigations may be required before discarding the possibility of HEV replication in rats. A study reported wild-type and immunodeficient mice being resistant to HEV-3 infection, yet HEV RNA and anti-HEV antibodies were detected in rats inoculated with HEV, indicating a successful infection (Schlosser et al. 2019).
In our study a low HEV detection rate was seen in synanthropic and wild small mammals (0.3%), with HEV-3 being identified. A similar detection rate (0.2%) has been reported in a study that detected HEV-3 in one Norway rat in a survey conducted in 11 European countries (1/508, from Belgium) (Ryll et al. 2017), while in USA 7.85% (35/446) of the Rattus spp. surveyed positive for HEV-3 having some been classified as subgenotype 3a (Lack et al. 2012). Besides rats, HEV-3a has also been detected in another small mammal (namely yellow-necked field mouse, Apodemus flavicollis) in Europe (Prpić et al. 2019). Furthermore, this HEV genotype has been identified in other mammal species, such as wild boar, from the same region of Portugal as our study (Mesquita et al. 2016; Santos-Silva et al. 2023). Therefore, the question whether rodents are truly natural reservoirs of human HEV or act as only intermediate hosts or bioindicators is not yet conclusively answered (Kenney 2019; Wang et al. 2020).
While the detection rate in this study is relatively low, the results suggest a potential susceptibility of these small rodents to HEV infection. Furthermore, strikingly HEV was only detected in a synanthropic rodent that is closely associated with people and that benefits from people surroundings and activities, whereas no non-synanthropic wild small mammal have shown evidence of HEV RNA. As such, the present study on HEV in small mammals of Portugal as a potential source for hepevirus transmission highlights the necessity for further research to improve risk assessment for human health.
In conclusion, this is the first time that HEV subgenotype 3e was identified in an Algerian mouse. However, the HEV isolate identified is most likely the result of a spillover infection from domestic pigs, or possibly the result of passive viral transit through the intestinal tract. Moreover, our study is the first hepevirus report in Portuguese small mammals, raising questions about their susceptibility to HEV and their role in HEV epidemiology.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Andonov A, Robbins M, Borlang J et al (2019) Rat Hepatitis E Virus linked to severe Acute Hepatitis in an Immunocompetent patient. J Infect Dis 220:951–955. https://doi.org/10.1093/infdis/jiz025
Barão I, Queirós J, Vale-Gonçalves H et al (2022) Landscape characteristics affecting small Mammal occurrence in heterogeneous Olive Grove Agro-ecosystems. Conservation 2:51–67. https://doi.org/10.3390/conservation2010005
Bencatel J, Álvares F, Moura A, Barbosa AM (2017) Atlas de mamíferos de Portugal
Berto A, Mesquita JR, Hakze-van der Honing R et al (2012) Detection and characterization of Hepatitis E Virus in Domestic pigs of different ages in Portugal. Zoonoses Public Health 59:477–481. https://doi.org/10.1111/j.1863-2378.2012.01488.x
Cossaboom CM, Córdoba L, Sanford BJ et al (2012) Cross-species infection of pigs with a novel rabbit, but not rat, strain of hepatitis E virus isolated in the United States. J Gen Virol 93:1687–1695. https://doi.org/10.1099/vir.0.041509-0
Dalton HR, Bendall R, Ijaz S, Banks M (2008) Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis 8:698–709. https://doi.org/10.1016/S1473-3099(08)70255-X
de Cock M, Fonville M, de Vries A et al (2022) Screen the unforeseen: microbiome-profiling for detection of zoonotic pathogens in wild rats. Transbound Emerg Dis 69:3881–3895. https://doi.org/10.1111/tbed.14759
de la Villalba CM, Owot M, Benedito JC E-C, et al (2013) Hepatitis E virus genotype 3 in humans and swine, Cuba. Infect Genet Evol J Mol Epidemiol Evol Genet Infect Dis 14:335–339. https://doi.org/10.1016/j.meegid.2012.12.022
De Sabato L, Di Bartolo I, Lapa D et al (2020) Molecular characterization of HEV Genotype 3 in Italy at Human/Animal Interface. Front Microbiol 11:1–9. https://doi.org/10.3389/fmicb.2020.00137
Dremsek P, Wenzel JJ, Johne R et al (2012) Seroprevalence study in forestry workers from eastern Germany using novel genotype 3- and rat hepatitis E virus-specific immunoglobulin G ELISAs. Med Microbiol Immunol 201:189–200. https://doi.org/10.1007/s00430-011-0221-2
Frías M, López-López P, Zafra I et al (2021) Development and clinical validation of a pangenotypic PCR-based assay for the detection and quantification of hepatitis E virus (Orthohepevirus A Genus). J Clin Microbiol 59:1–7. https://doi.org/10.1128/JCM.02075-20
Grierson SS, McGowan S, Cook C et al (2019) Molecular and in vitro characterisation of hepatitis E virus from UK pigs. Virology 527:116–121. https://doi.org/10.1016/j.virol.2018.10.018
Jirintai S, Tanggis, Mulyanto et al (2014) Rat hepatitis E virus derived from wild rats (Rattus rattus) propagates efficiently in human hepatoma cell lines. Virus Res 185:92–102. https://doi.org/10.1016/j.virusres.2014.03.002
Johne R, Heckel G, Plenge-Bönig A et al (2010a) Novel hepatitis E virus genotype in Norway rats, Germany. Emerg Infect Dis 16:1452–1455. https://doi.org/10.3201/eid1609.100444
Johne R, Plenge-Bönig A, Hess M et al (2010b) Detection of a novel hepatitis E-like virus in faeces of wild rats using a nested broad-spectrum RT-PCR. J Gen Virol 91:750–758. https://doi.org/10.1099/vir.0.016584-0
Jothikumar N, Cromeans TL, Robertson BH et al (2006) A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J Virol Methods 131:65–71. https://doi.org/10.1016/j.jviromet.2005.07.004
Karetnyĭ IV, Dzhumalieva DI, Usmanov RK et al (1993) [The possible involvement of rodents in the spread of viral hepatitis E]. Zhurnal Mikrobiol Epidemiol i Immunobiol 52–56
Kenney SP (2019) The current host range of hepatitis E viruses. Viruses 11:https://doi.org/10.3390/v11050452
King NJ, Hewitt J, Perchec-Merien AM (2018) Hiding in Plain Sight? It’s time to investigate other possible transmission routes for Hepatitis E Virus (HEV) in developed countries. Food Environ Virol 10:225–252. https://doi.org/10.1007/s12560-018-9342-8
Kumar S, Stecher G, Li M et al (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Lack JB, Volk K, Van Den Bussche RA (2012) Hepatitis E virus genotype 3 in wild rats, United States. Emerg Infect Dis 18:1268–1273. https://doi.org/10.3201/eid1808.120070
Li TC, Yoshizaki S, Ami Y et al (2013) Susceptibility of laboratory rats against genotypes 1, 3, 4, and rat hepatitis E viruses. Vet Microbiol 163:54–61. https://doi.org/10.1016/j.vetmic.2012.12.014
Li TC, Yang T, Yoshizaki S et al (2015) Construction and characterization of an infectious cDNA clone of rat hepatitis E virus. J Gen Virol 96:1320–1327. https://doi.org/10.1099/vir.0.000072
Lux L, Ulrich RG, Santos-Silva S et al (2023) Detection and molecular characterization of Giardia and Cryptosporidium Spp. Circulating in Wild Small mammals from Portugal. Animals 13. https://doi.org/10.3390/ani13030515
Mesquita JR, Barclay L, Nascimento MSJ, Vinjé J (2010) Novel norovirus in dogs with diarrhea. Emerg Infect Dis 16:980–982. https://doi.org/10.3201/eid1606.091861
Mesquita JR, Oliveira RMS, Coelho C et al (2016) Hepatitis E Virus in Sylvatic and Captive Wild Boar from Portugal. Transbound Emerg Dis 63:574–578. https://doi.org/10.1111/tbed.12297
Moraes Df, Lopez-Lopez P, Palmeira JD et al (2022) Screening for hepatitis E virus genotype 3 in red deer (Cervus elaphus) and fallow deer (Dama dama), Portugal, 2018–2020. Transbound Emerg Dis 69:2764–2768. https://doi.org/10.1111/tbed.14427
Mulder AC, Kroneman A, Franz E et al (2019) HEVnet: a one health, collaborative, interdisciplinary network and sequence data repository for enhanced hepatitis e virus molecular typing, characterisation and epidemiological investigations. Eurosurveillance 24:1–6. https://doi.org/10.2807/1560-7917.ES.2019.24.10.1800407
Mulyanto, Suparyatmo JB, Andayani IGAS et al (2014) Marked genomic heterogeneity of rat hepatitis E virus strains in Indonesia demonstrated on a full-length genome analysis. Virus Res 179:102–112. https://doi.org/10.1016/j.virusres.2013.10.029
Murphy EG, Williams NJ, Jennings D et al (2019) First detection of Hepatitis E virus (Orthohepevirus C) in wild brown rats (Rattus norvegicus) from Great Britain. Zoonoses Public Health 66:686–694. https://doi.org/10.1111/zph.12581
Pavio N, Meng XJ, Doceul V (2015) Zoonotic origin of hepatitis e. Curr Opin Virol 10:34–41. https://doi.org/10.1016/j.coviro.2014.12.006
Porea D, Raileanu C, Crivei LA et al (2023) First detection of Hepatitis E Virus (Rocahepevirus ratti genotype C1) in Synanthropic Norway rats (Rattus norvegicus) in Romania. Viruses 15. https://doi.org/10.3390/v15061337
Primadharsini PP, Mulyanto, Wibawa IDN et al (2018) The identification and characterization of novel rat hepatitis E virus strains in Bali and Sumbawa, Indonesia. Arch Virol 163:1345–1349. https://doi.org/10.1007/s00705-018-3736-7
Prpić J, Keros T, Vucelja M et al (2019) First evidence of hepatitis E virus infection in a small mammal (yellow-necked mouse) from Croatia. PLoS ONE 14:1–9. https://doi.org/10.1371/journal.pone.0225583
Purcell RH, Engle RE, Rood MP et al (2011) Hepatitis E virus in rats, Los Angeles, California, USA. Emerg Infect Dis 17:2216–2222. https://doi.org/10.3201/eid1712.110482
Purdy MA, Drexler JF, Meng X-J et al (2022) ICTV Virus Taxonomy Profile: Hepeviridae 2022. J Gen Virol 103:1–2. https://doi.org/10.1099/jgv.0.001778
Reuter G, Boros Á, Pankovics P (2020) Review of Hepatitis E virus in rats: evident risk of Species Orthohepevirus C to Human zoonotic infection and disease. https://doi.org/10.3390/v12101148. Viruses 12:
Rivero-Juarez A, Frias M, Perez AB et al (2022) Orthohepevirus C infection as an emerging cause of acute hepatitis in Spain: first report in Europe. J Hepatol 77:326–331. https://doi.org/10.1016/j.jhep.2022.01.028
Rodriguez C, Marchand S, Sessa A et al (2023) Orthohepevirus C Hepatitis, an underdiagnosed disease? J Hepatol 79:e39–e41
Ryll R, Bernstein S, Heuser E et al (2017) Detection of rat hepatitis E virus in wild Norway rats (Rattus norvegicus) and black rats (Rattus rattus) from 11 European countries. Vet Microbiol 208:58–68. https://doi.org/10.1016/j.vetmic.2017.07.001
Santos-Silva S, Gonçalves HMR, Rivero-Juarez A et al (2022) Detection of hepatitis E virus in milk: current evidence for viral excretion in a wide range of mammalian hosts. Transbound Emerg Dis 69:3173–3180. https://doi.org/10.1111/tbed.14683
Santos-Silva S, Moraes DF, da SD, López-López P et al (2023) Survey of Zoonotic Diarrheagenic Protist and Hepatitis E Virus in Wild Boar (Sus scrofa) of Portugal. Anim an open Access J from MDPI 13. https://doi.org/10.3390/ani13020256
Schlosser J, Dähnert L, Dremsek P et al (2019) Different outcomes of experimental hepatitis E virus infection in diverse mouse strains, Wistar rats, and rabbits. Viruses 11:1–11. https://doi.org/10.3390/v11010001
Simanavicius M, Tamosiunas PL, Petraityte-Burneikiene R et al (2018) Generation in yeast and antigenic characterization of hepatitis E virus capsid protein virus-like particles. Appl Microbiol Biotechnol 102:185–198. https://doi.org/10.1007/s00253-017-8622-9
Smith DB, Izopet J, Nicot F et al (2020) Update: proposed reference sequences for subtypes of hepatitis E virus (species Orthohepevirus A). J Gen Virol 101:692–698. https://doi.org/10.1099/jgv.0.001435
Sridhar S, Yip CCY, Wu S et al (2018) Rat hepatitis E virus as cause of persistent hepatitis after liver transplant. Emerg Infect Dis 24:2241–2250. https://doi.org/10.3201/eid2412.180937
Sridhar S, Yip CC-Y, Wu S et al (2021) Transmission of Rat Hepatitis E virus infection to humans in Hong Kong: a clinical and epidemiological analysis. Hepatology 73:10–22. https://doi.org/10.1002/hep.31138
Subissi L, Peeters M, Lamoral S, et al (2019) Subtype-specific differences in the risk of hospitalisation among patients infected with hepatitis E virus genotype 3 in Belgium, 2010–2018. Epidemiol Infect 147:5–8. https://doi.org/10.1017/S0950268819001122
Takahashi M, Kunita S, Kawakami M et al (2022) First detection and characterization of rat hepatitis E virus (HEV-C1) in Japan. Virus Res 314:198766. https://doi.org/10.1016/j.virusres.2022.198766
Tamura K (1992) Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G + C-content biases. Mol Biol Evol 9:678–687. https://doi.org/10.1093/OXFORDJOURNALS.MOLBEV.A040752
Treagus S, Wright C, Baker-Austin C et al (2021) The Foodborne transmission of Hepatitis E Virus to humans. Food Environ Virol 13:127–145. https://doi.org/10.1007/s12560-021-09461-5
Velavan TP, Pallerla SR, Johne R et al (2021) Hepatitis E: an update on one health and clinical medicine. Liver Int off J Int Assoc Study Liver 41:1462–1473. https://doi.org/10.1111/liv.14912
Wang B, Li W, Zhou J-H et al (2018) Chevrier’s Field Mouse (Apodemus chevrieri) and Père David’s vole (Eothenomys melanogaster) in China carry orthohepeviruses that form two putative novel genotypes within the species Orthohepevirus C. Virol Sin 33:44–58. https://doi.org/10.1007/s12250-018-0011-8
Wang B, Harms D, Yang X, Lou, Bock CT (2020) Orthohepevirus C: an expanding species of emerging hepatitis e virus variants. Pathogens 9:1–23. https://doi.org/10.3390/pathogens9030154
WHO (2022) Hepatitis E. https://www.who.int/news-room/fact-sheets/detail/hepatitis-e
Yang F, Li Y, Li Y et al (2022) Experimental cross-species transmission of Rat Hepatitis E Virus to Rhesus and Cynomolgus monkeys. Viruses 14. https://doi.org/10.3390/v14020293
Acknowledgements
Sérgio Santos-Silva thanks Fundação para a Ciência e a Tecnologia (FCT) for the financial support of his Ph.D work under the scholarship 2021.09461.BD contract through the Maria de Sousa-2021 program. H. M. R. Gonçalves work was supported through the project PTDC/BTM-MAT/30858/2017. Antonio Rivero-Juarez is the recipient of a Miguel Servet Research Contract by the Ministerio de Ciencia, Promoción y Universidades of Spain (CP18/00111). This work is financed by national funds through FCT - Fundação para a Ciência e a Tecnologia, I.P., under the projects UIDB/04750/2020, UIDB/50006/2020 and LA/P/0064/2020. Additional support was provided by FEDER funds through the Programa Operacional Factores de Competitividade (COMPETE), and by national funds through the FCT, within the scope of the project AGRIVOLE—PTDC/BIA-ECO/31728/2017.
Funding
This research was funded by Fundação para Ciência e Tecnologia (FCT), grant number 2021.09461.BD.
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection and analysis were performed by Sérgio Santos-Silva, Danny Franciele da Silva Dias Moraes, Pedro López-López, Joana Paupério, João Queirós, Laura Lux. The first draft of the manuscript was written by Sérgio Santos-Silva, and João R. Mesquita and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Animal ethical statement
No animals were sacrificed for the purpose of this study and all applicable institutional and/or national/international guidelines for the care and use of animals have been followed.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Santos-Silva, S., Moraes, D.F.d.D., López-López, P. et al. Detection of hepatitis E virus genotype 3 in an Algerian mouse (Mus spretus) in Portugal. Vet Res Commun 48, 1803–1812 (2024). https://doi.org/10.1007/s11259-024-10293-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-024-10293-4