Abstract
The increasing frequency of methicillin-resistant (MR) staphylococci in humans and animals need special attention for their difficult treatment and zoonotic character, therefore novel antimicrobial compounds on a natural base against antibiotic-resistant bacteria are requested. Currently, bacteriocins/enterocins present a new promising way to overcome this problem, both in prevention and treatment. Therefore, the preventive and medicinal effect of dipeptide enterocin EntA/P was evaluated against MR Staphylococcus epidermidis SEP3/Tr2a strain in a rabbit model, testing their influence on growth performance, glutathione-peroxidase (GPx) enzyme activity, phagocytic activity (PA), secretory (s)IgA, and jejunal morphometry (JM). Eighty-eight rabbits (aged 35 days, meat line M91, both sexes) were divided into experimental groups S (SEP3/Tr2a strain; 1.0 × 105 CFU/mL; dose 500µL/animal/day for 7 days, between days 14 and 21 to simulate the pathogen attack), E (EntA/P; 50 µL/animal/day, 25,600 AU/mL in two intervals, for preventive effect between days 0 and 14; for medicinal effect between days 28 and 42), E + S (EntA/P + SEP3/Tr2a; preventive effect; SEP3/Tr2a + EntA/P; medicinal effect) and control group (C; without additives). Higher body weight was recorded in all experimental groups (p < 0.001) compared to control data. The negative influence/attack of the SEP3Tra2 strain on the intestinal immunity and environment was reflected as decreased GPx activity, worse JM parameters and higher sIgA concentration in infected rabbits. These results suggest the promising preventive use of EntA/P to improve the immunity and growth of rabbits, as well as its therapeutic potential and protective role against staphylococcal infections in rabbit breeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The routine use of antibiotics in agriculture leads to the alarming rise of antibiotic-resistant bacteria in food animals, presenting a potential public health concern and critical economic issue (increased morbidity/mortality rate; Vidovic and Vidovic 2020). Staphylococci are frequently found as commensals of the skin and mucous membranes, but many of them are also opportunistic pathogens, causing local pyogenic and systemic infections - toxemia and septicemia in livestock, mostly in cattle, pigs, poultry and rabbits (Bonvegna et al. 2021; Silva et al. 2022). From coagulase-positive staphylococci (CPS), Staphylococcus aureus has special human and veterinary importance, because of its methicillin-resistance (MR) and multidrug-resistance (MDR). Nevertheless, in recent years, the rate of methicillin-resistant coagulase-negative staphylococci (MR-CNS), such as S. epidermidis, S. haemolyticus and S. chromogenes, has become more numerous and are involved in nosocomial infections and various animal diseases, due to their invasiveness, biofilm-forming ability, toxin production, hemolysins (Igbinosa et al. 2016). The increasing frequency of methicillin-resistant and multidrug-resistant staphylococci (MRS, MDRS) in animals, containing resistance and pathogenicity genes, needs special attention for their difficult treatment and zoonotic character (Vidovic and Vidovic 2020; Silva et al. 2022; Kasela et al. 2023). Therefore, new antimicrobial compounds directed against antibiotic-resistant bacteria are requested. Current research is focusing on alternative approaches: pro-, pre-, syn-, parapro- and postbiotics – including antimicrobial proteins, organic and fatty acids, herbal extracts and essential oils, to overcome this problem, substitute synthetic drugs in therapy and use preventively as natural feed additives in animal production and veterinary medicine (Zamojska et al. 2021). Among different bioactive compounds, bacteriocins may be considered as promising alternative. These ribosomally synthesized antimicrobial peptides with a broad antimicrobial spectrum produced by Gram-negative and Gram-positive bacteria as well, mostly by lactic acid bacteria (LAB), including enterococci (producing bacteriocins named mostly enterocins (Ents); Franz et al. 2007) are commonly used in the food industry as biopreservatives. However, many bacteriocins have been investigated, only nisin and pediocin PA-1 are considered safe by the Food and Drug Administration (FDA) and approved in the food industry (EFSA 2017), with antimicrobial effect against pathogenic/spoilage bacteria, including Staphylococcus aureus (Silva et al. 2018; Vera-Santander et al. 2023). Bacteriocins/enterocins application is also increasingly applied in livestock farms to improve the health and productivity of food animals, regarding their biosafety, antimicrobial, antioxidant, and immunomodulatory activities, and low tendency to develop resistance compared to conventional antibiotics (Bemena et al. 2014; Ben Lagha et al. 2017; Vieco-Saiz et al. 2019; Hernández-González et al. 2021; Pogány Simonová et al. 2020). In vitro antimicrobial and antibiofilm effect of bacteriocins (mostly lantibiotics – nisin (Nisaplin®, Aplin and Barret, United Kingdom) and gallidermin (Enzo Life Sci. Corporation USA, MW2069.4), but also Ents) against MRS and MDR bacteria was repeatedly confirmed, also in the animal (murine) model (Al Atya et al. 2016; Mathur et al. 2018; Belguesmia et al. 2021; Benítez-Chao et al. 2021), showing their ability to eliminate/reduce, particularly MR bacteria. Ents have also great potential during their application in animal farms and veterinary medicine with both, preventive and medicinal effects against bacterial infections (Simons et al. 2020). Despite these findings, knowledge regarding in vivo anti-staphylococcal effect against MRS/MDRS in food animals is limited, resp. has not yet been presented and new studies/experiments to expand these data are required. Therefore, this study aimed to test and compare the preventive and medicinal effect of dipeptide Enterocin (Ent) A/P against methicillin-resistant (MR) Staphylococcus epidermidis SEP3Tr2a strain in a rabbit model (food animal model) and their influence on growth performance, glutathione-peroxidase enzyme activity, immune response (phagocytic activity, sIgA), and jejunal morphometry was evaluated. The in vivo testing of the medicinal effect of a new EntA/P on the intestinal morphometry is the novelty of this study.
Materials and methods
Animal model
A total of 88 rabbits (meat lines M91 and P91, weaned at 35 days, both sexes, equal male-to-female ratio per treatment) were divided into three experimental groups: S, E, S + E and one control group (C), 22 animals in each. The average live weight of rabbits at the start of the experiment was 1091.7 g ± 149.3. Animals were kept in standard cages (type D-KV-72; 0.61 m x 0.34 cm x 0.33 m; Kovobel company, Domažlice, Czech Republic), two rabbits per cage. A cycle of 16 h light and 8 h dark was used throughout the experiment. The temperature and humidity in the building were recorded continuously by a digital thermograph positioned at the same level as the cages. The heating and ventilation systems allowed the building air temperature maintained within 16 ± 4 °C and the relative humidity to about 70 ± 5% throughout the experiment. Data were recorded continuously with a digital thermograph positioned at the same level as the cages. The experiment was performed in co-operation with our colleagues in Nitra (National Agricultural and Food Centre—NAFC). All care and experimental procedures involving animals followed the guidelines stated in the Guide for the Care and Use of Laboratory Animals approved by the Slovakian State Veterinary and Food Administration and the Ethical Committees of both institutions (permission code: SK CH 17,016 and SK U 18,016).
Preparation of tested substances
The MR S. epidermidis SEP3/Tr2a strain was marked by rifampicin to differentiate it from the total staphylococci and prepared as described previously by Strompfová et al. (2006). The EntA/P (previously named EntEK13, produced by the E. faecium EK13 strain, deponed to the Czech Collection of Microorganisms, number CCM7319 (Lauková et al. 2006) was prepared according to Mareková et al. (2007). The activity of EntA/P was tested using the agar spot test according to De Vuyst et al. (1996) against the principal indicator strain E. avium EA5 (isolated from piglet feces in our laboratory). Doses of additives and their manner of application were decided on the results of our previous in vivo experiment with rabbit-derived bacteriocin-producing strain E. faecium EF2019 (CCM7420; Pogány Simonová et al. 2009).
Experimental design – treatments
As both tested substances are water-soluble, they were applied to the drinking water of rabbits, using nipple drinkers in all cages. Rabbits in group E received the EntA/P at a dose of 50 µL/animal/day, with activity of 25,600 AU/mL in concentration 0.4 g/L. To test and compare the preventive and also the medicinal effect of the EntA/P, it was added in two intervals to rabbits: during the first 14 days (between days 0 and 14) to control the preventive effect, and between days 28 and 42 to evaluate the medicinal effect. Rabbits in group S received only the MR S. epidermidis SEP3/Tr2a strain (1.0 × 105 CFU/mL; Pogány Simonová et al. 2021a) at a dose of 500µL/animal/day for 7 days (between days 14 and 21), to simulate the spoilage/pathogen attack in rabbits. Rabbits in the E + S group firstly consumed the Ent A/P for 14 days (between 0 and 14 days), and after it the SEP3Tr2a strain was applied to animals for 7 days, (between 14 and 21 days of the experiment), to test the preventive effect of EntA/P. After a one-week break, at day 28, to rabbits in the E + S group the EntA/P was applied for 14 days (between 28 and 42 days) to detect the medicinal effect of EntA/P. Control rabbits (C) did not receive any additives in their drinking water. The rabbits were fed with a commercial pelleted diet for growing rabbits (KV, Tekro-Nitra, Ltd., Slovakia; Table 1), with free access to drinking water.
Growth performance
Body weight (BW; g) and feed consumption (g) were measured every week during the experiment; average daily weight gain (ADWG; the difference between the initial and current weight of animals, divided by the number of days that occurred between weights; g/day) and feed conversion ratio (FCR; feed intake divided by weight gain for a period;.g/g) were calculated mathematically. Health status and mortality were recorded daily throughout the whole experiment.
Blood sampling and testing of glutathione-peroxidase and phagocytic activity
Blood was sampled from the marginal ear vein (Vena auricularis) into dry heparinized Eppendorf tubes at days 0, 14, 21 and 42 for analyses (n = 8/group). The activity of glutathione-peroxidase (GPx; µkat/L) was determined by the colorimetric method (Spectrophotometer UV-2550 Shimadzu, Japan) using commercial kit Randox RS 504 (Randox Laboratories Ltd., UK).
The phagocytic activity (PA) was measured by a direct microscopic counting procedure, according to the modified test described by Vetvička et al. (1982): 50 µL of MSH particle suspension (ARTIM, Prague, Czech Republic) was mixed with 100 µL of blood in an Eppendorf-type test tube and incubated at 37 °C for 1 h. Blood smears were then prepared and stained by May-Grünwald and Giemsa-Romanowski. PA was calculated as the number of white cells containing at least three engulfed particles per 100 white cells (monocytes/granulocytes).
Slaughtering, intestinal IgA and morphometry testing
At days 21 and 42, rabbits were randomly selected for slaughter (n = 8), stunned with electronarcosis (50 Hz, 0.3 A/rabbit/4s), immediately hung by the hind legs on the processing line and quickly bled by cutting jugular veins and carotid arteries. The concentration of immunoglobulin A (IgA) in the intestinal wall was measured using the competitive inhibition enzyme immunoassay technique (Rabbit Immunoglobulin A, IgA ELISA kit, Cusabio, Houston, TX, USA). Samples of the intestinal wall were prepared according to Nikawa et al. (1999) and analyzed using the Multireader Synergy HTX (Biotek USA), at the wavelength 450 nm according to the manufacturer of the Cusabio kit.
To test morphometry (villus cut surface, villus circumference, villus height, crypt depth and villus height:crypt depth ratio), intestinal tissue (1 cm2) of proximal jejeunum was sampled and treated as previously described by Žitňan et al. (2008). Briefly, intestinal tissue (1 cm2) from the proximal jejunum was fixed in a 4% neutral formaldehyde solution. After being rinsed in water, samples were dehydrated in a graded series of ethanol (30%, 50%, 70%, 90%, and absolute ethanol), cleared in benzene and embedded in paraffin. Sections of 5 μm thickness (10 slices of each sample) were stained with hematoxylin/eosin and observed under a light microscope. The height, circumference, and cut surface area of 30 villi and depth of 30 crypts were determined by the computer-operated Image C picture analysis system (Imtronic GmbH, Berlin, Germany) and the interactive measurement (IMES) analysis program, by using a color video camera (SONY 3 CCD, Sony Electronics Ltd., Tokyo, Japan) and a light microscope (Axiolab, Carl Zeiss AG, Jena, Germany).
Statistical analysis
Treatment effects on tested parameters were analyzed using two-way ANOVA, followed by a Bonferroni post-hoc test for pair‐wise comparisons, where appropriate. The statistical model included the time, treatment effects and their interaction. All statistical analyses were performed by the GraphPad Prism statistical software (GraphPad Prism version 6.0, GraphPad Software, San Diego, CA, USA). Differences between the mean values of the different dietary treatments were considered statistically significant at p < 0.05. Data are expressed as means and standard deviations of the mean (SD).
Results
The animals were in good health throughout the experiment. All tested zootechnical parameters were influenced by time (BW, FCR), treatment (BW, ADWG) and their interaction (ADWG; Table 2). Higher BW was recorded in all experimental groups (p < 0.001) compared to control data. The ADWG was affected by both additives, but mostly the EntA/P (days 0–14; E, S + E vs. C: p < 0.001; S vs. C: p < 0.01; days 14–21: E vs. C, S: p < 0.05).
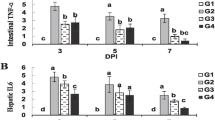
While PA (days 14, 21, 42; E vs. S, E + S, C: p < 0.001; Fig. 1) and sIgA levels (day 21; S + E vs. S, E, C: p < 0.001; day 42; E vs. S, S + E: p < 0.001; S, S + E vs. C: p < 0.05; Fig. 2) were influenced by time, treatment and their interaction, the blood GPx enzyme activity was affected by time and treatment (day 14; S vs., S + E: p < 0.001; day 42; S vs. E: p < 0.05; Fig. 3).
The effect of methicillin-resistant S. epidermidis SEP3/Tr2a, EntA/P and their combinative application on phagocytic activity using a direct microscopic counting procedure, according to the modified test of Vetvička et al. (1982). S - S. epidermidis SEP3/Tr2a, E – EntA/P, S + E - S. epidermidis SEP3/Tr2a + EntA/P, C – control. a,b,c,d Mean values within lines with different superscript letters are significantly different (p < 0.05) using the Bonferroni post-test
The effect of methicillin-resistant S. epidermidis SEP3/Tr2a, EntA/P and their combinative application on secretory IgA production, prepared and measured using the competitive inhibition enzyme immunoassay technique. S - S. epidermidis SEP3/Tr2a, E – EntA/P, S + E - S. epidermidis SEP3/Tr2a + EntA/P, C – control. Hb – hemoglobin; a,b,Mean values within lines with different superscript letters are significantly different (p < 0.05) using the Bonferroni post-test
The effect of methicillin-resistant S. epidermidis SEP3/Tr2a, EntA/P and their combinative application on glutathione-peroxidase activity determined by colorimetric method. S - S. epidermidis SEP3/Tr2a, E – EntA/P, S + E - S. epidermidis SEP3/Tr2a + EntA/P, C – control. a,b,c,d Mean values within lines with different superscript letters are significantly different (p < 0.05) using the Bonferroni post-test
Morphometry parameters were influenced by treatment (Table 2). The lowest values of all tested parameters were measured after the MR SEP3/Tr2a strain, which reflects the negative effect of the applied strain on the intestinal epithelium and environment. The opposite results were observed in the case of the EntA/P administration to rabbits, showing the tendency to improve the jejunal morphological parameters till to the end of the experiment (day 42), with a significant increase in resp. the highest level of all tested parameters (p < 0.01) compared to control and other experimental groups. Optimized values were noted testing the medicinal effect of EntA/P after the MR SEP3/Tr2a strain application (day 42) (see Fig. 4).
The effect of methicillin-resistant S. epidermidis SEP3/Tr2a, EntA/P and their combinative application on jejunal morphometry parameters tested according to Žitňan et al. (2008) – villus cut surface A, villus circumference B, villus height C, crypt depth D, villus height and crypt depth ratio (VH:CD; E). S - S. epidermidis SEP3/Tr2a, E – EntA/P, S + E - S. epidermidis SEP3/Tr2a + EntA/P, C – control. a,b,c,dMean values within lines with different superscript letters are significantly different (p < 0.05) using the Bonferroni post-test
Discussion
Weaning is a key period associated with stress, during which rabbits are susceptible to dietary changes and infections, which have adverse impacts on their health and production. The most rapid and significant changes are seen in the growth of rabbits, such as lack of appetite, stunting, and lower gains. However, we suspected lower weight gains in rabbits after the application of the potential pathogenic MR SEP3Tr2a strain, no negative effect on the growth performance of rabbits was noted. Similar results – higher weight gain – were obtained also after the application of a potentially pathogenic biofilm-forming Enterococcus hirae Kr8+ strain to rabbits (Lauková et al. 2022). We can speculate that applied strains may affect the intestinal environment and health, but in the gut is a complex interaction of the microbiome, enzymes, epithelial barrier and immunity, which may mitigate the pathogenicity resp. the negative effect of a potential pathogenic strain. The stimulatory effect of EntA/P application on animals´ growth and weight gains was noted, in accordance with our previous results achieved after several enterocins (Ent4231, Ent7420, EntM, durancin, etc.) application to rabbits (Pogány Simonová et al. 2015, 2021b). These findings repeatedly confirm the prosperity of enterocins as feed additives with prophylactic character to improve animal production. Moreover, optimized ADWG values noted in experimentally infected rabbits with the MR SEP3/Tr2a strain receiving EntA/P after the infection underline the medicinal effect of EntA/P.
Dietary changes, manipulation and infections can induce a stress response in animals with immoderate production of reactive oxygen species (ROS), which is controlled by the antioxidant system of the host organism. Monitoring the GPx activity in blood resp. in neutrophils is one of the markers of the antioxidant defensive system and stress reaction. Chakraborty et al. (2012) present the susceptibility of neutrophils to S. aureus infection through the increased production of nitric oxide, which leads to decreased antioxidant status, similar to our findings – lower level of GPx enzyme after MR SEP3Tr2a strain application. It is known, that lactic acid bacteria (LAB), which are usually producer strains of bacteriocins have antioxidant properties. However, the mechanisms underlying the antioxidant activity of probiotics/beneficial bacteria/LAB are not completely understood; however, it has been suggested that LAB may play antioxidant roles through scavenging ROS, chelating metals, increasing antioxidant enzymes levels, and modulating the microbiota (Feng and Wang 2020). Kim et al. (2022) also documented the ability of (LAB) and their metabolites–bacteriocins to remove the ROS and maintain the intestinal oxidation–reduction balance, confirming their antioxidant activity. When LAB are attached to the intestinal lumen, their metabolites – bacteriocins increase to remove ROS, thereby maintaining the intestinal oxidation–reduction balance. Xin et al. (2014) also presented alleviated high-fat diet-induced oxidative stress and changed intestinal Firmicutes/Bacteroidetes ratio in mice after Lactobacillus johnsonii BS15 supplementation. The gut microbiota modulation by LAB and their bacteriocins can improve the host redox state and protect the neutrophils from such infection by decreasing nitrite-oxide (NO) generation, lipid, and protein damage and also by increasing the antioxidant status. Significantly increased GPx activity in rabbits receiving the EntA/P alone (E) and after the MR SEP3/Tr2a application (S + E) also confirming previous findings and the potential use of EntA/P as free radical scavenger with both, preventive and medicinal effects against staphylococcal infection in rabbit breeding. Of course, further experiments are required to verify this hypothesis.
Several studies present also the immunostimulatory effect of postbiotics – bacteriocins (Benítez-Chao et al. 2021. The immunomodulatory effect of tested bacteriocins is expressed as increased CD4 + and CD8 + T lymphocyte proliferation and cytokine IL-6 production (Hernández-González et al. 2021). Bacteriocins/enterocins can also stimulate phagocytic activity (PA), as a parameter of non-specific immunity. Enterocin therapy prevented the suppression of phagocytosis of peripheral blood mononuclear cells caused by Trichinella spiralis infection in the intestinal phase of trichinellosis and stimulated PA and oxidative burst during the migration of new-born larvae into muscles (Vargová et al. 2023). In vivo PA stimulation was noted in rabbits and horses after their dietary bacteriocins/enterocins supplementation (Lauková et al. 2018, 2022; Pogány Simonová et al. 2013, 2022). The immunostimulatory effect of EntA/P (E) was recorded during the whole experiment, similar to previous results. Optimized PA level in the S + E group after EntA/P addition to experimentally infected rabbits (day 42) has shown the immunomodulatory resp. medicinal effect of the tested dipeptide. The MR SEP3/Tr2a strain application also stimulated the PA in infected rabbits (S; day 21), but surprisingly, decreased PA was noted in S + E group (day 21), compared to other groups. Probiotics/beneficial bacteria and postbiotics can enhance the immune response of rabbits through the modulation of gut microbiota, by supporting/improving gut-associated lymphoid tissues and stimulating the IgA system (Zamojska et al. 2021). Slight increase of sIgA levels in rabbits after EntA/P supplementation can confirm this finding, i.e. to improve the intestinal health and modulate the immune response of the host organism. On the other hand, a more significant increase of sIgA level noted immediately after the MR SE strain application in the S + E group (day 21) suggested the negative attack of the potentially pathogenic MR strain on the intestinal epithelium/lymphoid tissue resp. immunity. Optimization (decrease) of high sIgA level after 2 weeks of EntA/P medicinal application (day 42 vs. day 21) underlines the protective effect of tested EntA/P against the pathological attack in the intestine.
The complex of intestinal epithelium, microbiome and immunity forms a balanced/stable gut environment and homeostasis. Dietary dysbiosis, metabolic changes and the presence of gastrointestinal infection agents – bacteria, viruses, and parasites can influence this stability, which directly disrupt gut integrity (Berkes et al. 2003). Impaired morphometric parameters in this study reflect the negative influence of resp. attack of the MR SEP3/Tr2a strain on rabbits´ intestine. These changes are closely related to intestinal microbiota composition, metabolic activities and immunity. Outgoing from decreased villus surface area, circumference and VH:CD ratio values, we expected lower nutrient resorption and weight gains in experimentally infected rabbits, but their higher body weight compared to control animals did not confirm this assumption. Therefore, we hypothesize stronger resp. more direct effect of the MR SEP3/Tr2a strain on gut lymphoid tissue, than on gastrointestinal microbiota or metabolism. Stimulated secretion of sIgA as a local defense mechanism can confirm this hypothesis, but more detailed studies about intestinal epithelial integrity and immunity are needed to extend knowledge regarding the complexity of microbiological, physiological and immunological processes in the gut. The beneficial influence of dietary probiotic and postbiotic supplementation on intestinal morphological parameters and their improvement of rabbits was reported in several previous studies (Oso et al. 2013; Pogány Simonová et al. 2015, 2020, 2022), similar to present results achieved after the EntA/P application to rabbits with preventive goal. Improved morphometry parameters suggest better intestinal functionality, strengthening of the epithelium, preventing the entry and gut colonization of pathogenic bacteria, increasing of commensal bacterial activities, mucosal immunity (IgA production), and nutrient absorption (enlargement of surface area), leading also to better health status and higher weight gains in animals (Aggarwal et al. 2022; Zhong et al. 2022). The EntA/P applied to experimentally infected rabbits before the infection was able to strengthen the jejunal epithelium, and thus prevent significant damage to the intestinal barrier, compared to untreated infected rabbits. While there are several studies on the preventive administration of postbiotics/bacteriocins in animals (Aggarwal et al. 2022; Zhong et al. 2022), studies on their in vivo therapeutic effect against infections and intestinal homeostasis are still scarce. Therefore, the novelty of this study is testing the therapeutic effect of EntA/P against staphylococcal infection in rabbits directly in the gut, through monitoring morphological changes. The optimized values of morphological parameters in rabbits receiving EntA/P after the MR SEP3/Tr2a strain infection testify to the medicinal effect of EntA/P. To the best of our current knowledge, these results are the first to present the in vivo therapeutic action of enterocins in general on jejunal morphometry.
Conclusion
The experimental application of potentially pathogenic MR SEP3Tr2a strain did not negatively influence the growth of rabbits, but significantly impaired the antioxidant and immune response of infected animals. The prophylactic application of EntA/P to untreated rabbits (without MRSEP3Tr2a) was reflected in their higher weight gains and lower feed conversion. Moreover, improved jejunal morphometry and enhanced immunity and antioxidant defense system were noted. Optimized/improved values of growth, morphometry and immunity parameters in experimentally infected rabbits receiving EntA/P after their infection underline the medicinal effect of the EntA/P. Prophylactic administration of EntA/P to rabbits before their infection enhanced the animal´s health against the MR SEP3/Tr2a strain. These results suggest the promising use of dipeptide EntA/P as a feed additive and potential therapeutic agent against staphylococcal infection in rabbits, to improve their productivity, health status and immunity.
Data Availability
All data generated or analyzed during this study are included in this article.
Code Availability
Not applicable.
Change history
19 December 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11259-023-10284-x
Abbreviations
- MR:
-
Methicillin-resistant
- Ent:
-
Enterocin
- SE:
-
Staphylococcus epidermidis
- GPx:
-
Glutathione-peroxidase
- PA:
-
Phagocytic activity
- sIgA:
-
Secretory immunoglobulin A
- JM:
-
Jejunal morphometry
- CPS:
-
Coagulase-positive staphylococci
- MDR:
-
Multidrug-resistance
- MR-CNS:
-
Methicillin-resistant coagulase-negative staphylococci
- MRS:
-
Methicillin-resistant staphylococci
- MDRS:
-
Multidrug-resistant staphylococci
- LAB:
-
Lactic acid bacteria
- NAFC:
-
National Agricultural and Food Centre
- CCM:
-
Czech Culture Collection of Microorganisms
- BW:
-
Body weight
- ADWG:
-
average daily weight gain
- FCR:
-
feed conversion ratio
- NO:
-
Nitrite-oxide
- VH:
-
Villus height
- CD:
-
Crypt depth
References
Aggarwal S, Sabharwal V, Kaushik P, Joshi A, Aayushi A, Suri M (2022) Postbiotics: from emerging concept to application. Front Sust Food Syst 6:887642. https://doi.org/10.3389/fsufs.2022.887642
Al Atya AK, Belguesmia Y, Chataigne G, Ravallec R, Vachée A, Szunerits S, Boukherroub R, Drider D (2016) Anti-MRSA activities of enterocins DD28 and DD93 and evidences on their role in the inhibition of biofilm formation. Front Microbiol 7:817. https://doi.org/10.3389/fmicb.2016.00817
Belguesmia Y, Spano G, Drider D (2021) Potentiating effects of leaderless enterocin DD14 in combination with methicillin on clinical methicillin-resistant Staphylococcus aureus S1 strain. Microbiol Res 252:126864. https://doi.org/10.1016/j.micres.2021.126864
Bemena LD, Mohamed LA, Fernandes AM, Lee BH (2014) Applications of bacteriocins in food, livestock health and medicine. Int J Curr Microbiol Appl Sci 3:924–949. https://doi.org/10.13140/RG.2.1.3426.2488
Ben Lagha B, Haas M, Gottschalk D, Grenier D (2017) Antimicrobial potential of bacteriocins in poultry and swine production. BMC Vet Res 48(1):22. https://doi.org/10.1186/s13567-017-0425-6
Benítez-Chao DF, León-Buitimea A, Lerma-Escalera JA, Morones-Ramírez JR (2021) Bacteriocins: an overview of antimicrobial, toxicity, and biosafety assessment by in vivo models. Front Microbiol 12:630695. https://doi.org/10.3389/fmicb.2021.630695
Berkes J, Viswanathan VK, Savkovic SD, Hecht G (2003) Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut 52:439–451. https://doi.org/10.1136/gut.52.3.439
Bonvegna M, Grego E, Sona B, Stella MC, Nebbia P, Mannelli A, Tomassone L (2021) Occurrence of meth cillin-resistant coagulase-negative staphylococci (MRCoNS) and methicillin-resistant Staphylococcus aureus (MRSA) from pigs and farm environment in Northwestern Italy. Antibiotics 10(6):676. https://doi.org/10.3390/antibiotics10060676
Chakraborty SP, Pramanik P, Roy S (2012) Staphylococcus aureus Infection induced oxidative imbalance in n utrophils: possible protective role of nanoconjugated Vancomycin. ISRN Pharmacol 2012:435214. https://doi.org/10.5402/2012/435214
De Vuyst L, Callewaert R, Pot B (1996) Characterization of the antagonistic activity of Lactobacillus amylovorus DCE471 and large-scale isolation of its bacteriocin amylovorin L471. Syst Appl Microbiol 9:9–20. https://doi.org/10.1016/S0723-2020(96)80003-8
EFSA (2017) Safety of nisin (E 234) as a food additive in the light of new toxicological data and the proposed extension of use. EFSA J. 15(12) e05063
Feng T, Wang J (2020) Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: a systematic review. Gut Microbes 12(1):1801944. https://doi.org/10.1080/19490976.2020.1801944
Franz CM, van Belkum MJ, Holzapfel WH, Abriouel H, Gálvez A (2007) Diversity of enterococcal bacteriocins and their grouping in a new classification scheme. FEMS Microbiol Rev 31:293–310. https://doi.org/10.1111/j.15746976.2007.00064.x
Hernández-González JC, Martínez-Tapia A, Lazcano-Hernández G, García-Pérez BE, Castrejón-Jiménez NS (2021) Bacteriocins from lactic acid bacteria. A powerful alternative as antimicrobials, probiotics, and immunomodulators in veterinary medicine. Animals 11(4):979. https://doi.org/10.3390/ani11040979
Igbinosa EO, Beshiru A, Akporehe LU, Ogofure AG (2016) Detection of methicillin resistant staphylococci isolated from food producing animals: a public health implication. Vet Sci 3(3):14. https://doi.org/10.3390/vetsci3030014
Kasela M, Ossowski M, Dzikoń E, Ignatiuk K, Wlazło Ł, Malm A (2023) The epidemiology of animal associated methicillin-resistant Staphylococcus aureus. Antibiotics 12(6):1079. https://doi.org/10.3390/antibiotics12061079
Kim S, Lee JY, Jeong Y, Kang C-H (2022) Antioxidant activity and probiotic properties of lactic acid bacteria. Fermentation. 8(1):29. https://doi.org/10.3390/fermentation8010029
Lauková A, Strompfová V, Skřivanová V, Volek Z, Jindřichová E, Marounek M (2006) Bacteriocin-producing strain of Enterococcus faecium EK 13 with probiotic character and its application in the digestive tract of rabbits. Biologia 61:779–782. https://doi.org/10.2478/s11756-006-0191-9
Lauková A, Styková E, Kubašová I, Gancarčíková S, Plachá I, Mudroňová D, Kandričáková A, Miltko R, Belzecki G, Valocký I, Strompfová V (2018) Enterocin M and its beneficial effects in horses—a pilot experiment. Probiotics Antimicrob Proteins 10:420–426. https://doi.org/10.1007/s12602-018-9390-2
Lauková A, Chrastinová Ľ, Micenková L, Bino E, Kubašová I, Kandričáková A, Gancarčíková S, Plachá I, Holodová M, Grešáková Ľ, Formelová Z, Pogány Simonová M (2022) Enterocin M in interaction in broiler rabbits with autochthonous, biofilm-forming Enterococcus hirae Kr8 strain. Probiotics Antimicrob Proteins 14:845–853. https://doi.org/10.1007/s12602-022-09941-5
Mareková M, Lauková A, Skaugen M, Nes FI (2007) Isolation and characterization of a new bacteriocins produced by enviromental isolate Enterococcus faecium AL41. J Indust Microbiol Biotechnol 34:533537. https://doi.org/10.1007/s10295-007-0226-4
Nikawa T, Odahara K, Koizumi H, Kido Y, Teshima S, Rokutan K, Kishi K (1999) Vitamin A prevents decline in immunoglobulin A and Th2 cytokine levels in small intestinal mucosa of protein-malnourished mice. J Nutr 129:934–941. https://doi.org/10.1093/jn/129.5.934
Oso AO, Idowu OMO, Haastrup AS, Ajibade AJ, Olowonefa KO, Aluko AO, Ogunade IM, Osho SO, Bamgbose AE (2013) Growth performance, apparent nutrient digestibility, caecal fermentation, ileal morphology and caecal microflora of growing rabbits fed diets containing probiotics and prebiotics. Livest Sci 157:184–190. https://doi.org/10.1016/j.livsci.2013.06.017
Pogány Simonová M, Lauková A, Chrastinová L’, Strompfová V, Faix Š, Vasilková Z, Ondruška Ľ, Jurčík R, Rafay J (2009) Enterococcus faecium CCM7420, bacteriocin PPB CCM7420 and their effect in the digestive tract of rabbits. Czech J Anim Sci 54:376–386. https://doi.org/10.17221/1659-CJAS
Pogány Simonová M, Lauková A, Plachá I, Čobanová K, Strompfová V, Szabóová R, Chrastinová Ľ (2013) Can enterocins affect phagocytosis and glutathione peroxidase in rabbits? Centr Eur J Biol 8:730–734. https://doi.org/10.2478/s11535-013-0198-x
Pogány Simonová M, Lauková A, Žitňan R, Chrastinová L’ (2015) Effect of rabbit origin enterocin-producing strain Enterococcus faecium CCM7420 application on growth performance and gut morphometry in rabbits. Czech J Anim Sci 60:509–512. https://doi.org/10.17221/8559-CJAS
Pogány Simonová M, Chrastinová Ľ, Lauková A (2020) Autochtonous strain Enterococcus faecium EF2019(CCM7420), its bacteriocin and their beneficial effects in broiler rabbits—A. Rev Anim 10:1188. https://doi.org/10.3390/Fani10071188
Pogány Simonová M, Maďar M, Lauková A (2021a) Effect of enterocins against methicillin-resistant animal derived staphylococci. Vet Res Comm 45:467–473. https://doi.org/10.1007/s11259-021-09841-z
Pogány Simonová M, Lauková A, Chrastinová Ľ, Kandričáková A, Ščerbová J, Strompfová V, Miltko R, Belzecki G (2021b) Enterocins as novel feed additives in rabbit diet: enterocin ent M and durancin ent ED26E/7, their combination, and effects on microbiota, caecal fermentation, and enzymatic activity. Antimicrob Proteins 5:1433–1442. https://doi.org/10.1007/s12602-021-09809-0
Pogány Simonová M, Lauková A, Chrastinová Ľ, Kandričáková A, Ščerbová J, Strompfová V, Gancarčíková S, Plachá I, Žitňan R (2022) Effect of enterocin M and durancin ED26E/7 supplementation on blood parameters, immune response and jejunal morphometry in rabbits. J Anim Physiol Anim Nutr 106(2):378386. https://doi.org/10.1111/jpn.13570
Silva CCG, Silva SPM, Ribeiro SC (2018) Application of bacteriocins and protective cultures in dairy food preservation. Front Microbiol 9:594. https://doi.org/10.3389/fmicb.2018.00594
Silva V, Caniça M, Ferreira E, Vieira-Pinto M, Saraiva C, Pereira JE, Capelo JL, Igrejas G, Poeta P (2022) Multidrug-resistant methicillin-resistant coagulase-negative staphylococci in healthy poultry slaughtered for human consumption. Antibiotics 11(3):365. https://doi.org/10.3390/antibiotics11030365
Simons A, Alhanout K, Duval RE (2020) Bacteriocins, antimicrobial peptides from bacterial origin: overview of their biology and their impact against multidrug-resistant bacteria. Microorganisms 8:639. https://doi.org/10.3390/microorganisms8050639
Strompfová V, Marciňáková M, Simonová M, Gancarčíková S, Jonecová Z, Sciránková Ľ, Koščová J, Buleca V, Čobanová K, Lauková A (2006) Enterococcus faecium EK13—an enterocin A-producing strain with probiotic character and its effect in piglets. Anaerobe 12:242–248. https://doi.org/10.1016/j.anaerobe.2006.09.003
Vargová M, Revajová V, Lauková A, Hurníková Z, Dvorožňáková E (2023) Modulatory effect of beneficial enterococci and their enterocins on the blood phagocytes in murine experimental trichinellosis. Life 13(9):1930. https://doi.org/10.3390/life13091930
Vera-Santander VE, Hernández-Figueroa RH, Jiménez-Munguía MT, Mani-López E, López-Malo A (2023) Health benefits of consuming foods with bacterial probiotics, postbiotics, and their metabolites: a review. Molecules 28(3):1230. https://doi.org/10.3390/molecules28031230
Vetvička V, Fornousek L, Kopeček J, Kaminkova J, Kasparek L, Vranova M (1982) Phagocytosis of human blood leukocytes, a simple micro-method. Immunol Lett 5:97–100. https://doi.org/10.1016/01652478(82)90040-2
Vidovic N, Vidovic S (2020) Antimicrobial resistance and food animals: influence of livestock environment on the emergence and dissemination of antimicrobial resistance. Antibiotics 9(2):52. https://doi.org/10.3390/antibiotics9020052
Vieco-Saiz N, Belguesmia Y, Raspoet R, Auclair E, Gancel F, Kempf I, Drider D (2019) Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food animal production. Front Microbiol 10:57. https://doi.org/10.3389/fmicb.2019.00057.
Xin J, Zeng D, Wang H, Ni X, Yi D, Pan K, Jing B (2014) Preventing non-alcoholic fatty Liver Disease through Lactobacillus johnsonii BS15 by attenuating inflammation and mitochondrial injury and improving gut environment in obese mice. Appl Microbiol Biotechnol 98:6817–6829. https://doi.org/10.1007/s00253-014-5752-1
Zamojska D, Nowak A, Nowak I, Macierzyńska-Piotrowska E (2021) Probiotics and postbiotics as substitutes of antibiotics in farm animals: a review. Animals 11(12):3431. https://doi.org/10.3390/ani11123431
Zhong Y, Wang S, Di H, Deng Z, Liu J, Wang H (2022) Gut health benefit and application of postbiotics in animal production. J Anim Sci Biotechnol 13(1):38. https://doi.org/10.1186/s40104-022-00688-1
Žitňan R, Voigt J, Kuhla S, Wegner J, Chudy A, Schönhausen U, Brna M, Župčanová M, Hagemeister H (2008) Morphology of small intestinal mucosa and intestinal weight change with metabolic type of cattle. Vet Med 53:525–532. https://doi.org/10.17221/1968-VETMED
Acknowledgements
This research was funded by the Scientific Grant Agency of the Ministry for Education, Science, Research and Sport of the Slovak Republic and the Slovak Academy of Sciences VEGA, grant number 2/0005/21. We are grateful to D. Melišová and P. Jerga for their skillful technical assistance, and to Ľ. Ondruška, R. Jurčík and T. Sládeček for blood sampling and J. Pecho for slaughtering (National Agricultural and Food Centre, Nitra). Part of the preliminary results was presented in the Proceedings from the Conference of the 20. BOKU SymposiumTierernährung in Wien: Universität für Bodenkultur, Wien, 2022 pp. 153–156 by Pogány Simonová et al. (2022) and a part of results regarding the preventive effect of EntA/P was published in the journal Animals, 2022, 12(9), by Pogány Simonová et al. (2022).
Funding
This study was financially supported by the project VEGA 2/0005/21 (Slovak Scientific Agency).
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic
Author information
Authors and Affiliations
Contributions
M.P.S.: Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Resources, Validation, Visualization, Methodology, Writing—original draft preparation, Writing—review and editing, Supervision; Ľ.Ch.: Methodology; Investigation, Data curation, J.Š: Methodology; V.F.: Methodology; I.P.: Methodology; K.T.: Methodology; R.Ž.: Methodology; A.L.: Conceptualization, Investigation, Supervision.
Corresponding author
Ethics declarations
Ethical approval
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. The authors confirm that they have followed EU standards for the protection of animals used for scientific purposes. All care and experimental procedures involving animals followed the guidelines stated in the Guide for the Care and Use of Laboratory Animals approved by the State Slovak Veterinary and Food Administration and the Ethics Committees of both institutions (permission code: SK CH 17016 and SK U 18016).
Consent to participate
All authors participated voluntarily in the research.
Consent for publication
All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: This article was originally published with data of Time effect are included in Table 2, but missing in the PDF version.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pogány Simonová, M., Chrastinová, Ľ., Ščerbová, J. et al. The effect of enterocin A/P dipeptide on growth performance, glutathione-peroxidase activity, IgA secretion and jejunal morphology in rabbits after experimental methicillin-resistant Staphylococcus epidermidis P3Tr2a Infection. Vet Res Commun 48, 507–517 (2024). https://doi.org/10.1007/s11259-023-10277-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-023-10277-w