Abstract
Deltamethrin (DM) is one of the extensively used pyrethroids for controlling ectoparasites. Unfortunately, DM is highly toxic to fish as it primarily targets the sodium channels of the plasma membrane thereby affecting their cardiac and nervous systems. The present study investigated the protective efficacy of Shatavari (Asparagus racemosus) against DM-induced cardiotoxicity in Nile tilapia (Oreochromis niloticus). The fish were segregated into nine groups having 36 fish/group maintained in triplicates exposed to DM (1 µg/L) and fed with a diet containing three different concentrations (10 g, 20 g, and 30 g/kg feed) of aqueous extract of A. racemosus (ARE) for 21 days. DM caused significant alterations in the blood and serum parameters, and expression of cardiac and apoptotic genes compared to the control group. The ARE cotreatment significantly reduced the increase in serum transaminases, creatine kinase, and lactate dehydrogenase levels induced by DM. ARE facilitated the regain of electrolyte (sodium, potassium, chloride) homeostasis and antioxidants such as catalase, superoxide dismutase, glutathione peroxidase, and glutathione in DM-exposed fish. The cardiac histology exhibited loose separation of the cardiomyocytes and myofibrillar loss in the DM group which was ameliorated in the DM-ARE cotreatment group. Significant modulations were observed in the expression of cardiac-specific genes (gata4, myh6, tnT, cox1) and apoptosis signaling genes and proteins (HSP70, bax, bcl-2, caspase3), in the cotreatment group compared to the DM-exposed group. The current study suggests that ARE possesses potential cardioprotective properties that are effective in mitigating the toxic effects induced by DM via ameliorating oxidative stress, electrolyte imbalance, and apoptosis in tilapia.


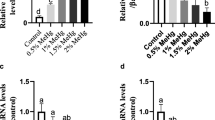
WBC - white blood cell, Hb - hemoglobin, RBC - red blood cell, HCT - hematocrit, MCV - mean corpuscular volume, MCH - mean corpuscular hemoglobin, MCHC - mean corpuscular hemoglobin concentration, RDW - red cell distribution width, PLT – platelet. C- Control group (normal fishes fed with control diet); VC- Vehicle control (normal fishes fed with control diet exposed to acetone 25 µL); DM- Deltamethrin in 25 µL acetone mixed in tank water to a final concentration of 1 µg/L and fed with control diet; ARE1- Group fed with 10 g ARE/kg feed (1% ARE diet); ARE2- Group fed with 20 g ARE/kg feed (2% ARE diet); ARE3- Group fed with 30 g ARE/kg feed (3% ARE diet); DM + ARE1- Group exposed to DM (1 µg/L) and fed with 1% ARE diet; DM + ARE2- Group exposed to DM (1 µg/L) and fed with 2% ARE diet; DM + ARE3- Group exposed to DM (1 µg/L) and fed with 3% ARE diet; ARE - aqueous root extract of Asparagus racemosus. Values are means ± SEM of three replicates (n = 15), different letters indicate statistical difference at p < 0.05 in ANOVA. The means of groups in homogeneous subsets have been indicated with the same letters

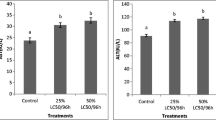
(A) ALT- Alanine transaminase; (B) AST- Aspartate transaminase; (C) CK- Creatine kinase; (D) LDH- Lactate dehydrogenase. C- Control group (normal fishes fed with control diet); VC- Vehicle control (normal fishes fed with control diet exposed to acetone 25 µL); DM- Deltamethrin in 25 µL acetone mixed in tank water to a final concentration of 1 µg/L and fed with control diet; ARE1- Group fed with 10 g ARE/kg feed (1% ARE diet); ARE2- Group fed with 20 g ARE/kg feed (2% ARE diet); ARE3- Group fed with 30 g ARE/kg feed (3% ARE diet); DM + ARE1- Group exposed to DM (1 µg/L) and fed with 1% ARE diet; DM + ARE2- Group exposed to DM (1 µg/L) and fed with 2% ARE diet; DM + ARE3- Group exposed to DM (1 µg/L) and fed with 3% ARE diet; ARE - aqueous root extract of Asparagus racemosus. Values are means ± SEM of three replicates (n = 15 for serum samples, n = 6 for tissue samples), different letters indicate statistical difference at p < 0.05 in ANOVA. The means of groups in homogeneous subsets have been indicated with the same letters

(A) SOD- Superoxide dismutase; (B) CAT- Catalase; (C) GSH- Reduced glutathione; (D) GPx- Glutathione peroxidase. C- Control group (normal fishes fed with control diet); VC- Vehicle control (normal fishes fed with control diet exposed to acetone 25 µL); DM- Deltamethrin in 25 µL acetone mixed in tank water to a final concentration of 1 µg/L and fed with control diet; ARE1- Group fed with 10 g ARE/kg feed (1% ARE diet); ARE2- Group fed with 20 g ARE/kg feed (2% ARE diet); ARE3- Group fed with 30 g ARE/kg feed (3% ARE diet); DM + ARE1- Group exposed to DM (1 µg/L) and fed with 1% ARE diet; DM + ARE2- Group exposed to DM (1 µg/L) and fed with 2% ARE diet; DM + ARE3- Group exposed to DM (1 µg/L) and fed with 3% ARE diet; ARE - aqueous root extract of Asparagus racemosus. Values are means ± SEM of three replicates (n = 6), different letters indicate statistical difference at p < 0.05 in ANOVA. The means of groups in homogeneous subsets have been indicated with the same letters

MDA- Malondialdehyde; ROS- Reactive oxygen species; C- Control group (normal fishes fed with control diet); VC- Vehicle control (normal fishes fed with control diet exposed to acetone 25 µL); DM- Deltamethrin in 25 µL acetone mixed in tank water to a final concentration of 1 µg/L and fed with control diet; ARE1- Group fed with 10 g ARE/kg feed (1% ARE diet); ARE2- Group fed with 20 g ARE/kg feed (2% ARE diet); ARE3- Group fed with 30 g ARE/kg feed (3% ARE diet); DM + ARE1- Group exposed to DM (1 µg/L) and fed with 1% ARE diet; DM + ARE2- Group exposed to DM (1 µg/L) and fed with 2% ARE diet; DM + ARE3- Group exposed to DM (1 µg/L) and fed with 3% ARE diet; ARE - aqueous root extract of Asparagus racemosus. Values are means ± SEM of three replicates (n = 6), different letters indicate statistical difference at p < 0.05 in ANOVA. The means of groups in homogeneous subsets have been indicated with the same letters

Na+- Sodium; K+- Potassium; Cl−- Chloride; C- Control group (normal fishes fed with control diet); VC- Vehicle control (normal fishes fed with control diet exposed to acetone 25 µL); DM- Deltamethrin in 25 µL acetone mixed in tank water to a final concentration of 1 µg/L and fed with control diet; ARE1- Group fed with 10 g ARE/kg feed (1% ARE diet); ARE2- Group fed with 20 g ARE/kg feed (2% ARE diet); ARE3- Group fed with 30 g ARE/kg feed (3% ARE diet); DM + ARE1- Group exposed to DM (1 µg/L) and fed with 1% ARE diet; DM + ARE2- Group exposed to DM (1 µg/L) and fed with 2% ARE diet; DM + ARE3- Group exposed to DM (1 µg/L) and fed with 3% ARE diet; ARE - aqueous root extract of Asparagus racemosus. Values are means ± SEM of three replicates (n = 6), different letters indicate statistical difference at p < 0.05 in ANOVA. The means of groups in homogeneous subsets have been indicated with the same letters

The C, ARE3, and DM + ARE3 groups had normal compactly arranged cardiomyocytes with no evident pathology; the DM group had loosely separated cardiomyocytes (LC), and myofibrillar loss (FL) without any significant pathological alterations. C- Control group (normal fishes fed with control diet); DM- Deltamethrin in 25 µL acetone mixed in tank water to a final concentration of 1 µg/L and fed with control diet; ARE3- Group fed with 30 g ARE/kg feed (3% ARE diet); DM + ARE3- Group exposed to DM (1 µg/L) and fed with 3% ARE diet; ARE - aqueous root extract of Asparagus racemosus

gata4- GATA binding protein 4; myh6- myosin heavy chain 6; tnT- troponin T; cox1- cyclooxygenase 1; bax- bcl2 associated X protein; bcl2- B cell lymphoma 2; casp3a- cysteine-aspartic acid protease 3; C- Control group (normal fishes fed with control diet); VC- Vehicle control (normal fishes fed with control diet exposed to acetone 25 µL); DM- Deltamethrin in 25 µL acetone mixed in tank water to a final concentration of 1 µg/L and fed with control diet; ARE1- Group fed with 10 g ARE/kg feed (1% ARE diet); ARE2- Group fed with 20 g ARE/kg feed (2% ARE diet); ARE3- Group fed with 30 g ARE/kg feed (3% ARE diet); DM + ARE1- Group exposed to DM (1 µg/L) and fed with 1% ARE diet; DM + ARE2- Group exposed to DM (1 µg/L) and fed with 2% ARE diet; DM + ARE3- Group exposed to DM (1 µg/L) and fed with 3% ARE diet; ARE - aqueous root extract of Asparagus racemosus. Values are means ± SEM of three replicates (n = 6), different letters indicate statistical difference at p < 0.05 in ANOVA. The means of groups in homogeneous subsets have been indicated with the same letters

C- Control group (normal fishes fed with control diet); DM- Deltamethrin in 25 µL acetone mixed in tank water to a final concentration of 1 µg/L and fed with control diet; ARE3- Group fed with 30 g ARE/kg feed (3% ARE diet); DM + ARE3- Group exposed to DM (1 µg/L) and fed with 3% ARE diet; ARE - aqueous root extract of Asparagus racemosus
Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article and the raw data will be made available on request.
References
Adams JM, Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Science 281(5381):1322–1326
Afouda BA (2022) Towards understanding the gene-specific roles of GATA Factors in Heart Development: does GATA4 lead the way? Int J Mol Sci 23(9):5255
Aiello F, Simons MG, van Velde JW, Dani P (2021) New insights into the degradation path of deltamethrin. Molecules 26(13):3811
Akinmoladun AC, Ibukun EO, Afor E, Akinrinlola BL, Onibon TR, Akinboboye AO, Obuotor EM, Farombi EO (2007) Chemical constituents and antioxidant activity of Alstonia boonei. Afr J Biotechnol 6(10):1197–1201
Allam A, Abdeen A, Devkota HP, Ibrahim SS, Youssef G, Soliman A, Abdel-Daim MM, Alzahrani KJ, Shoghy K, Ibrahim SF, Aboubakr M (2022) N-Acetylcysteine alleviated the Deltamethrin-Induced oxidative Cascade and apoptosis in liver and kidney tissues. Int J Environ Res Public Health 19(2):638
Awoyemi OM, Kumar N, Schmitt C, Subbiah S, Crago J (2019) Behavioral, molecular and physiological responses of embryo-larval zebrafish exposed to types I and II pyrethroids. Chemosphere 219:526–537
Baden KN, Murray J, Capaldi RA, Karen Guillemin K (2007) Early Developmental Pathology due to cytochrome c Oxidase Deficiency is revealed by a new zebrafish model. J Biol Chem 282(48):34839–34849
Badr A, Vornanen M (2023) Deltamethrin and Retene toxicity to excitability of ventricular myocytes in Rainbow Trout (Oncorhynchus mykiss). Sohag J Sci 8(2):111–116. https://doi.org/10.21608/sjsci.2023.180890.1047
Banaee M, Mohammadipour S, Madhani S (2015) Effects of sublethal concentrations of permethrin on bioaccumulation of cadmium in zebra cichlid (Cichlasoma nigrofasciatum). Toxicol Environ Chem 97(2):200–207
Belzunces LP, Tchamitchian S, Brunet JL (2012) Neural aspects of insecticide in the honeybee. Apidologie 43(3):348–370
Bhattacharjee P, Borah A, Das S (2020) Quercetin-induced amelioration of deltamethrin stress in freshwater teleost, Channa punctata: multiple biomarker analysis. Comp Biochem Physiol C Toxicol Pharmacol 227:108626. https://doi.org/10.1016/j.cbpc.2019.108626
Borkar SB, Rathod SH, Kulkarni KM, Tantarpale VT (2014) Impact of shatavari and ashwagandha on average body weight of freshwater fish Channa punctatus. J Global Biosci 3:582–585
Bothe SN, Lampert A (2021) The insecticide deltamethrin enhances sodium channel slow inactivation of human Nav1.9, Nav1.8 and Nav1.7. Toxicol Appl Pharmacol 428:115676. https://doi.org/10.1016/j.taap.2021.115676
Branch TN (2011) Sub-lethal toxicity impacts of endosulfan on some biochemical parameters of the freshwater crayfish (Astacus leptodactylus). Res J Environ Sci 5(11):827–835
Burgos-Aceves MA, Lionetti L, Faggio C (2019) Multidisciplinary haematology as prognostic device in environmental and xenobiotic stress-induced response in fish. Sci Total Environ 670:1170–1183. https://doi.org/10.1016/j.scitotenv.2019.03.275
Chebbi SG, David M (2010) Quinalphos induced alterations in the levels of ions and whole animal oxygen consumption of freshwater fish, Cyprinus Carpio (Linnaeus, 1758). J Vet Sci Technol 1(1):102. https://doi.org/10.4172/2157-7579.1000102
Das R, Abraham TJ, Singha J, Bardhan A, Patil PK (2022) Dietary emamectin benzoate induces dose-dependent variations in haemato-biochemical and erythrocyte-metric parameters of Oreochromis niloticus (L). Aquaculture 561:738680. https://doi.org/10.1016/j.aquaculture.2022.738680
Das S, Pradhan C, Singh AK, Vineetha VP, Pillai D (2023) Dietary coriander (Coriandrum sativum L) oil improves growth, nutrient utilization, antioxidant status, tissue histomorphology and reduces omega-3 fatty acid production in Nile tilapia (Oreochromis niloticus). Anim Feed Sci Technol 305:115774. https://doi.org/10.1016/j.anifeedsci.2023.115774
Datta M, Kaviraj A (2003) Ascorbic acid supplementation of diet for reduction of deltamethrin induced stress in freshwater catfish Clarias gariepinus. Chemosphere 53(8):883–888
David M, Sangeetha J, Shrinivas J, Harish ER, Naik VR (2014) Alterations in the levels of ions in tissues of freshwater fish Cirrhinus mrigala exposed to deltamethrin. Int J Pharm Biol Arch 5(1):36–40
Dawood MAO, Moustafa EM, Gewaily MS, Abdo SE, AbdEl-kader MF, SaadAllah MS, Hamouda AH (2020) Ameliorative effects of Lactobacillus plantarum L-137 on Nile tilapia (Oreochromis niloticus) exposed to deltamethrin toxicity in rearing water. Aquat Toxicol 219:105377. https://doi.org/10.1016/j.aquatox.2019.105377
Dinu D, Marinescu D, Munteanu MC, Staicu AC, Costache M, Dinischiotu A (2010) Modulatory effects of deltamethrin on antioxidant defense mechanisms and lipid peroxidation in Carassius auratus Gibelio liver and intestine. Arch Environ Contam Toxicol 58(3):757–764
Doroshow JH (1983) Effect of anthracycline antibiotics on oxygen radical formation in rat heart. Cancer Res 43(2):460–472
El-Sappah AH, Seif MM, Abdel-Kader HH, Soaud SA, Elhamid MAA, Abdelghaffar AM, El-Sappah HH, Sarwar H, Yadav V, Maitra P, Zhao X, Yan K, Li J, Abbas M (2022) Genotoxicity and Trace Elements Contents Analysis in Nile Tilapia (Oreochromis niloticus) indicated the levels of aquatic contamination at three Egyptian areas. Front Vet Sci 9:818866. https://doi.org/10.3389/fvets.2022.818866
Erstfeld KM (1999) Environmental fate of synthetic pyrethroids during spray drift and field runoff treatments in aquatic microcosms. Chemosphere 39(10):1737–1769. https://doi.org/10.1016/s0045-6535(99)00064-8
Everson JL, Jones DR, Taylor AK, Rutan BJ, Leeds TD, Langwig KE, Wargo AR, Wiens GD (2021) Aquaculture reuse water, genetic line, and vaccination affect Rainbow Trout (Oncorhynchus mykiss) Disease susceptibility and Infection dynamics. Front Immunol 12:721048. https://doi.org/10.3389/fimmu.2021.721048
FAO (2020) The state of World fisheries and aquaculture 2020—Sustainability in action. FAO, Rome, Italy
Farag MR, Alagawany M, Bilal RM, Gewida AGA, Dhama K, Abdel-Latif HMR, Amer MS, Rivero-Perez N, Zaragoza-Bastida A, Binnaser YS, Batiha GE, Naiel MAE (2021) An overview on the potential hazards of pyrethroid insecticides in Fish, with special emphasis on Cypermethrin Toxicity. Anim (Basel) 11(7):1880. https://doi.org/10.3390/ani11071880
Gabriel UU, Jack IR, Edori OS, Egobueze E (2009) Electrolytes in selected tissues of Heterobranchus bidorsalis treated with sublethal levels of Cypermethrin. Ethiop J Environ Stud Manag 2(3):83–87
Ghosh R, Gilda JE, Gomes AV (2014) The necessity of and strategies for improving confidence in the accuracy of western blots. Expert Rev Proteomics 11(5):549–560. https://doi.org/10.1586/14789450.2014.939635
Haverinen J, Vornanen M (2014) Effects of deltamethrin on excitability and contractility of the rainbow trout (Oncorhynchus mykiss) heart, comp Biochem Physiol Part C. Toxicol Pharmacol 159:1–9. https://doi.org/10.1016/j.cbpc.2013.09.004
Haverinen J, Vornanen M (2016) Deltamethrin is toxic to the fish (crucian carp, Carassius carassius) heart. Pestic Biochem Phys 129:36–42
Jayaprakash C, Shettu N (2013) Changes in the hematology of the freshwater fish, Channa punctatus (Bloch) exposed to the toxicity of deltamethrin. J Chem Pharm Res 5(6):178–183
John PJ (2007) Alteration of certain blood parameters of freshwater teleost Mystus vittatus after chronic exposure to Metasystox and Sevin. Fish Physiol Biochem 33(1):15–20
Keer NR, Chadha NK, Saini VP, Ojha ML, Sawant PB (2020) Dietary shatavari, Asparagus racemosus root extract promotes growth, feed conversion and nutrient utilization in Labeo rajasthanicus. J Environ Biol 41:1464–1469
Khalatbary AR, Ghabaee DNZ, Ahmadvand H, Amiri FT, Lehi ST (2017) Deltamethrin-Induced Hepatotoxicity and Virgin Olive Oil Consumption: an experimental study. Iran J Med Sci 42(6):586–592
Lawrence MJ, Raby GD, Teffer AK, Jeffries KM, Danylchuk AJ, Eliason EJ, Hasler CT, Clark TD, Cooke SJ (2020) Best practices for non-lethal blood sampling of fish via the caudal vasculature. J Fish Biol 97(1):4–15
Li M, Liu X, Feng X (2019) Cardiovascular toxicity and anxiety-like behavior induced by deltamethrin in zebrafish (Danio rerio) larvae. Chemosphere 219:155–164. https://doi.org/10.1016/j.chemosphere.2018.12.011
Liu X, Gao Q, Feng Z, Tang Y, Zhao X, Chen D, Feng X (2021) Protective effects of spermidine and melatonin on deltamethrin-induced cardiotoxicity and neurotoxicity in zebrafish. Cardiovasc Toxicol 21(1):29–41. https://doi.org/10.1007/s12012-020-09591-5
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using realtime quantitative PCR and the 2-∆∆C T method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Lu Q, Sun Y, Ares I, Anadón A, Martínez M, Martínez-Larrañaga M-Z, Yuan Z, Wang X, Martínez M-A (2019) Deltamethrin toxicity: a review of oxidative stress and metabolism. Environ Res 170:260–281. https://doi.org/10.1016/j.envres.2018.12.045
Miao W, Jiang Y, Hong Q, Sheng H, Liu P, Huang Y, Cheng J, Pan X, Yu Q, Wu Y, Zhu X, Zhang Y, Zhang T, Xiao H, Ye J (2023) Systematic evaluation of the toxicological effects of deltamethrin exposure in zebrafish larvae. Environ Toxicol Pharmacol 100:104155. https://doi.org/10.1016/j.etap.2023.104155
Mongi S, Mahfoud M, Amel B, Kamel J, Abdelfattah el F (2011) Protective effects of vitamin C against haematological and biochemical toxicity induced by deltamethrin in male Wistar rats. Ecotoxicol Environ Saf 74(6):1765–1769. https://doi.org/10.1016/j.ecoenv.2011.04.003
Mukherjee D, Ghosal I, Chakraborty SB (2015) Application of Asparagus racemosus roots for production of Monosex Nile tilapia, Oreochromis niloticus. Int J Adv Res 3:828–833
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Pamila D, Subbaiyan PS, Ramaswamy M (1991) Toxic effects of chromium and cobalt on Sarotherodon mossambicus (Peters). Indian J Environ Health 33(2):218–224
Posch MG, Waldmuller S, Müller M, Scheffold T, Fournier D, Andrade-Navarro MA, De Geeter B, Guillaumont S, Dauphin C, Yousseff D, Schmitt KR, Perrot A, Berger F, Hetzer R, Bouvagnet P, Özcelik C (2011) Cardiac alpha-myosin (MYH6) is the predominant sarcomeric Disease gene for familial atrial septal defects. PLoS ONE 6(12):e28872. https://doi.org/10.1371/journal.pone.0028872
Poznyak AV, Grechko AV, Orekhova VA, Chegodaev YS, Wu WK, Orekhov AN (2020) Oxidative stress and antioxidants in Atherosclerosis development and treatment. Biology 9(3):60. https://doi.org/10.3390/biology9030060
Prendiville TW, Guo H, Lin Z, Zhou P, Stevens SM, He A, VanDusen N, Chen J, Zhong L, Wang DZ, Gao G, Pu WT (2015) Novel roles of GATA4/6 in the postnatal heart identified through temporally controlled, cardiomyocyte-specific gene inactivation by adeno-associated virus delivery of cre recombinase. PLoS ONE 10(5):e0128105
Rajesh KS, Bushra I, Ashi S, Arun R (2022) Impact of deltamethrin (2.8% EC) on serum biochemistry and histopathology of fish, Channa punctatus (Bloch 1793). Int J Dev Res 12(08):58223–58226
Rathi BS, Kumar PS, Vo D-VN (2021) Critical review on hazardous pollutants in water environment: occurrence, monitoring, fate, removal technologies and risk assessment. Sci Total Environ 797:149134. https://doi.org/10.1016/j.scitotenv.2021.149134
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Meth Enzymol 233:357–363. https://doi.org/10.1016/s0076-6879(94)33041-7
Sasse S, Brand NJ, Kyprianou P, Dhoot GK, Wade R, Arai M, Periasamy M, Yacoub MH, Barton PJ (1993) Troponin I gene expression during human cardiac development and in end-stage Heart Failure. Circ Res 72:932–938
Shen C, He J, Zhu K, Zheng N, Yu Y, He C, Yang C, Zuo Z (2023) Mepanipyrim induces cardiotoxicity of zebrafish (Danio rerio) larvae via promoting AhR-regulated COX expression pathway. J Environ Sci 125:650–661
Singha J, Abraham TJ, Roy A, Bardhan A, Sar TK, Rajisha R, Krishna EKN, Kumar KA, Patil PK (2022) Influence of dietary emamectin benzoate on the biological responses of monosex (all-male) Oreochromis niloticus (L.) fries. Comp Biochem Physiol Part C: Toxicol Pharmacol 252:109223. https://doi.org/10.1016/j.cbpc.2021.109223
Song J, Qiao L, Ji L, Ren B, Hu Y, Zhao R, Ren Z (2018) Toxic responses of zebrafish (Danio rerio) to thallium and deltamethrin characterized in the electrocardiogram. Chemosphere 212:1085–1094. https://doi.org/10.1016/j.chemosphere.2018.09.014
Svoboda M, Lusková V, Drastichová J, Žlábek V (2001) The effect of diazinon on haematological indices of common carp (Cyprinus carpio L). Acta Vet Brno 70(4):457–465
Svoboda J, Pech P, Heneberg P (2023) Low concentrations of acetamiprid, deltamethrin, and sulfoxaflor, three commonly used insecticides, adversely affect ant queen survival and egg laying. Sci Rep 13:14893. https://doi.org/10.1038/s41598-023-42129-7
Velisek J, Dobsíková R, Svobodová Z, Modrá H, Lusková V (2006) Effect of deltamethrin on the biochemical profile of common carp (Cyprinus carpio L). Bull Environ Contam Toxicol 76(6):992–998
Velisek J, Jurčíková J, Dobšíková R, Svobodová Z, Piackova V, Máchová J, Novotny L (2007) Effects of DM on rainbow trout (Oncorhynchus mykiss). Environ Toxicol Pharmacol 23(3):297–301. https://doi.org/10.1016/j.etap.2006.11.006
Vig K, Singh DK, Agarwal HC, Dhawan AK, Dureja P (2001) Insecticide residues in Cotton Crop Soil. J Environ Sci Health B 36(4):421–434. https://doi.org/10.1081/PFC-100104186
Vineetha VP, Girija S, Soumya RS, Raghu KG (2014) Polyphenol-rich apple (Malus domestica L.) peel extract attenuates arsenic trioxide induced cardiotoxicity in H9c2 cells via its antioxidant activity. Food Func 5(3):502–511. https://doi.org/10.1039/c3fo60470e
Vineetha VP, Soumya RS, Raghu KG (2015) Phloretin ameliorates arsenic trioxide induced mitochondrial dysfunction in H9c2 cardiomyoblasts mediated via alterations in membrane permeability and ETC complexes. Eur J Pharmacol 754:162–172. https://doi.org/10.1016/j.ejphar.2015.02.036
Vineetha VP, Asha G, Devika P (2021a) Withania somnifera attenuates Tilapia lake virus (TiLV)-induced mortality by inhibiting stress and strengthening the innate antioxidant defence system. Aquac Res 52(11):5493–5505. https://doi.org/10.1111/are.15423
Vineetha VP, Devika P, Prasitha K, Anilkumar TV (2021b) Tinospora cordifolia ameliorated titanium dioxide nanoparticle-induced toxicity via regulating oxidative stress-activated MAPK and NRF2/Keap1 signaling pathways in Nile tilapia (Oreochromis niloticus) Comp Biochem Physiol Part C – Toxicol Pharmacol. 240:108908. https://doi.org/10.1016/j.cbpc.2020.108908
Vineetha VP, Tejaswi HN, Suresh K, Lekshmi H, Sneha KG, Rakesh CG, Devika P (2022) Asparagus racemosus improves immune-related parameters in Nile tilapia (Oreochromis niloticus) and mitigates deltamethrin-induced toxicity. Fish Shellfish Immunol 130:283–293. https://doi.org/10.1016/j.fsi.2022.09.028
Widmark J, Sundström G, Ocampo Daza D, Larhammar D (2011) Differential evolution of voltage-gated sodium channels in tetrapods and teleost fishes. Mol Biol Evol 28(1):859–871
Wilfred-Ekprikpo PC (2021) Changes in electrolytes in Heterobranchus longifilis exposed to sub lethal levels of different chemicals in the laboratory. J Agricultural Res Pesticides Biofertilizers 1(2):1–5
Yousef MI, Awad TI, Mohamed EH, Toxicology (2006) 227(3): 240–247. https://doi.org/10.1016/j.tox.2006.08.008
Yuan X, Wu H, Gao J, Geng X, Xie M, Song R, Zheng J, Wu Y, Ou D (2023) Acute deltamethrin exposure induces oxidative stress, triggers endoplasmic reticulum stress, and impairs hypoxic resistance of crucian carp. Comp Biochem Physiol Part C: Toxicol Pharmacol 263:109508. https://doi.org/10.1016/j.cbpc.2022.109508
Zhang C, Zhang Q, Pang Y, Song X, Zhou N, Wang J, He L, Lv J, Song Y, Cheng Y, Yang X (2019) The protective effects of melatonin on oxidative damage and the immune system of the Chinese mitten crab (Eriocheir sinensis) exposed to deltamethrin. Sci Total Environ 653:1426–1434. https://doi.org/10.1016/j.scitotenv.2018.11.063
Zhang L, Hong X, Zhao X, Yan S, Ma X, Zha J (2020) Exposure to environmentally relevant concentrations of deltamethrin renders the Chinese rare minnow (Gobiocypris rarus) vulnerable to Pseudomonas fluorescens Infection. Sci Total Environ 715:136943. https://doi.org/10.1016/j.scitotenv.2020.136943
Zhou S, Dong J, Liu Y, Yang Q, Xu N, Yang Y, Ai X (2021) Effects of acute deltamethrin exposure on kidney transcriptome and intestinal microbiota in goldfish (Carassius auratus). Ecotoxicol Environ Saf 225:112716
Acknowledgements
The authors are thankful for the support received from the Plan fund, Kerala University of Fisheries and Ocean Studies and the Kerala State Council for Higher Education for the smooth conductance of the experiments. The authors acknowledge ChatGPT and Grammarly for language assistance.
Funding
This research did not receive external funding.
Author information
Authors and Affiliations
Contributions
V.P.V.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing- original draft, review & editing; H.N.T.: Investigation, Methodology, Writing- original draft; N.S.S.: Methodology; S.D.: Writing- review & editing; D.P.: Conceptualization, Project administration, Resources, Writing- review & editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
The entire investigation was conducted with full adherence to the guidelines of the Committee for the purpose of control and supervision of experiments on animals (CPCSEA) registration number: 1174/ac/08/CPCSEA. The protocol was reviewed and approved by the institutional animal ethics committee of Kerala University of Fisheries and Ocean Studies, India.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Vadavanath Prabhakaran Vineetha and Hemla Naik Tejaswi contributed equally to this work.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vineetha, V.P., Tejaswi, H.N., Sooraj, N.S. et al. Implications of deltamethrin on hematology, cardiac pathology, and gene expression in Nile tilapia (Oreochromis niloticus) and its possible amelioration with Shatavari (Asparagus racemosus). Vet Res Commun 48, 811–826 (2024). https://doi.org/10.1007/s11259-023-10251-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-023-10251-6