Abstract
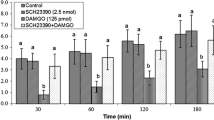
Recently, methylamine has been found as an endogenous amine, which is controling food intake in mammals. However, there is no evidence about the effect of methylamine on feeding behavior in poultry. So, the present study was designed to evaluate the effect of intracerebroventricular (ICV) injection of methylamine and involvement of central methylamine/dopaminergic systems on feeding behavior in neonatal meat type chicks. In experiment 1, chicks were ICV injected with different doses of methylamine (0.48, 0.96, 1.44, 1.92 and 2.40 μmol). In experiment 2, chicks received a dose of either the control solution, 2.40 μmol methylamine, 125 nmol L-DOPA (dopamine precursor) or a combination of methylamine plus L-DOPA. Experiments 3–7 were similar to experiment 2 except that 150 nmol 6-OHDA (dopamine synthase inhibitor), 5 nmol SCH23390 (D1 receptor antagonist), 5 nmol AMI-193 (D2 receptor antagonist), 6.4 nmol NGB2904 (D3 receptor antagonist) and 6 nmol L-741, 742 (D4 receptor antagonist) were used instead of 125 nmol L-DOPA, respectively. Cumulative food intake was determined until 2 h post-injection. According to the results, methylamine significantly decreased food intake in a dose dependent manner (p < 0.05). The inhibitory effect of methylamine on food intake was significantly attenuated by 6-OHDA, SCH23390 and AMI-193 (P < 0.05), but NGB2904 and L-741, 742 had no effect on food intake induced by methylamine. In addition, hypophagic effect of methylamine significantly amplified by L-DOPA (P < 0.05). These results suggest that methylamine decrease food intake and there is an interaction between methylamine and dopaminergic system via D1 and D2 receptors in chickens.







Similar content being viewed by others
References
Boswell T (2005) Regulation of energy balance in birds by the neuroendocrine hypothalamus. J Poult Sci 42(3):161–181
Bouthenet ML, Soult MP, Martres P, Sokoloff B, Giros S, Schwartz JC (1991) Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: Comparison with dopamine D2 receptor mRNA. Brain Res 564:203–219
Buffoni F (1995) Semicarbazide-sensitive amine oxidases: some biochemical properties and general considerations. Prog. Brain Res 106:323–331
Cadet JL, Jayanthi S, McCoy MT, Beauvais G, Cai NS (2010) Dopamine D1 receptors, regulation of gene expression in the brain, and neurodegeneration. CNS & Neurological Disorders—Drug Targets 9:526–538
Cioni L, De Siena G, Ghelardini C, Sernissi O, Alfarano C, Pirisino R, et al. (2006) Activity and expression of semicarbazide-sensitive benzylamine oxidase in a rodent model of diabetes: Interactive effects with methylamine and alpha-aminoguanidine. Eur J Pharmacol 529:179–187
Contreras F, Fouillioux C, Bolívar A, Simonovis N, Hernández-Hernández R, Armas-Hernandez MJ, Velasco M (2002) Dopamine, hypertension and obesity. J Hum Hypertens 16(Suppl 1):13–17
Cooper AJ, Plum F (1987) Biochemistry and physiology of brain ammonia. Physiol Rev 67:440–519
Cooper SJ, Al Naser HA, Clifton PG (2006) The anorectic effect of the selective dopamine D1-receptor agonist A-77636 determined by meal pattern analysis in free-feeding rats. Eur J Pharmacol 532:253–257
Davis JL, Masuoka DT, Gerbrandt LK, Cherkin A (1979) Autoradiographic distribution of L-proline in chicks after intracerebral injection. Physiol Behav 22:693–695
Furuse M, Matsumoto M, Saito N, Sugahara K, Hasegawa S (1997) The central corticotropin-releasing factor and glucagon-like peptide-1 in food intake of the neonatal chick. Eur J Pharmacol 339:211–214
Furuse M, Ando R, Bungo T, Ao R, ShimoJO M, Masuda Y (1999) Intracerebroventricular injection of orexins does not stimulate food intake in neonatal chicks. Br Poult Sci 40:698–700
Grace AA (1991) Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: A hypothesis for the eitiology of schizophrenia. Neuroscience 41(1):1–24
Hassanpour S, Zendehdel M, Babapour V, Charkhkar S (2015) Endocannabinoid and nitric oxide interaction mediates food intake in neonatal chicken. Br Poult Sci 56(4):443–451
Hrnjez BJ, Song JC, Prasad M, Mayol JM, Matthews JB (1999) Ammonia blockade of intestinal epithelial K conductance. Am J Physiol 277:521–532
Ikemoto S (2007) Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Reviews 56(1):27–78
Jonaidi H, Noori Z (2012) Neuropeptide Y-induced feeding is dependent on GABAA receptors in neonatal chicks. J Comp Physiol A 198:827–832
Kitano T, Matsumura S, Seki T, Hikida T, Sakimura K, Nagano T, et al. (2004) Characterization of N-methyl – aspartate receptor subunits involved in acute ammonia toxicity. Neurochem Int 44:83–90
Leibowitz SF, Weiss GF, Walsh UA, Viswanath D (1989) Medial hypothalamic serotonin: role in circadian patterns of feeding and macronutrient selection. Brain Res 503:132–140
Meador-Woodruff MDK, Grandy SP, Damask O, Civelli S, Watson JR (1992) Distribution of D5 dopamine receptor mRNA in rat brain. Neurosci Lett 145:209–212
Monfort P, Munoz MD, Kosenko E, Llansola M, Sanchez-Perez A, Cauli O, et al. (2004) Sequential activation of soluble guanylate cyclase, protein kinase G and cGMP-degrading phosphodiesterase is necessary for proper induction of long-term potentiation in CA1 of hippocampus: Alterations in hyperammonemia. Neurochem Int 45:895–901
Moroni A, Bardella L, Thiel G (1998) The impermeant ion methylammonium blocks Kþ and NH4þ currents through KAT1 channel differently: evidence for ion interaction in channel permeation. J Membr Biol 163:25–35
Novoseletsky N, Nussinovitch A, Friedman-Einat M (2011) Attenuation of food intake in chicks by an inverse agonist of cannabinoid receptor 1 administered by either injection or ingestion in hydrocolloid carriers. Gen Comp Endocrinol 170:522–527
Olanrewaju HA, Thaxton JP, Dozier WA, Purswell J, Roush WB, Branton SL (2006) A review of lighting programs for broiler production. Int J poultry Sci 5(4):301–308
Parada M, Hernandez L, Schwartz D, Hoebel BG (1988) Hypothalamic infusion of amphetamine increases serotonin, dopamine and norepinephrine. Physiol Behav 44:607–610
Parker KE, Johns HW, Floros TG, Will MJ (2014) Central amygdala opioid transmission is necessary for increased high-fat intake following 24-h food deprivation, but not following intra-accumbens opioid administration. Behav Brain Res 260:131–138
Pirisino R, Ghelardini C, Banchelli G, Galeotti N, Raimondi L (2001) Methylamine and benzylamine induced hypophagia in mice: modulation by semicarbazide-sensitive benzylamine oxidase inhibitors and aODN towards Kv1.1 channels. Br J Pharmacol 134:880–886
Pirisino R, Ghelardini C, Pacini A, Galeotti N, Raimondi L (2004) Methylamine, but not ammonia, is hypophagic in mouse by interaction with brain Kv1.6 channel subtype. Br J Pharmacol 142:381–389
Raimondi L, Alfarano C, Pacini A, Livi S, Ghelardini C, DeSiena G, Pirisino R (2007) Methylamine- dependent release of nitric oxide and dopamine in the CNS modulates food intake in fasting rats. Brit J Pharmacol 150:1003–1110
Saito ES, Kaiya H, Tachibana T, Tomonaga S, Denbow DM, Kangawa K, Furuse M (2005) Inhibitory effect of ghrelin on food intake is mediated by the corticotropin-releasing factor system in neonatal chicks. Regul Pept 125:201–208
Stanley S, Wynne K, McGowan B, Bloom S (2005) Hormonal regulation of food intake. Physiol Rev 85:1131–1158
Van Tienhoven A, Juhaz LP (1962) The chicken telencephalon, diencephalons and mesencephalon in stereotaxic coordinates. J Comp Neural 118:185–197
Van Tol HHM, Bunzow JR, Guan H, Sunahara RK, Seeman P, Niznik HB, Civelli O (1991) Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature 350:610–614
Wynne K, Stanley S, McGowan B, Bloom S (2005) Appetite control. J Endocrinol 184:291–318
Zeisel SH, da Costa K (1986) Increase in human exposure to methylamine precursors of N-nitrosamines after eating fish. Cancer Res 46:6136–6138
Zendehdel M, Hassanpour S (2014b) Central regulation of food intake in mammals and birds: a review. Neurotransmitter 1:1–7
Zendehdel M, Hasani K, Babapour V, Seyedali Mortezaei S, Khoshbakht Y, Hassanpour S (2014a) Dopamine-induced hypophagia is mediated by D1 and 5HT-2c receptors in chicken. Vet Res Commun 38:11–19
Zendehdel M, Ghashghayi E, Hassanpour S, Baghbanzadeh A, Jonaidi H (2015) Interaction Between Opioidergic and Dopaminergic Systems on Food Intake in Neonatal Layer Type Chicken. Int J Pept Res Ther. doi:10.1007/s10989-015-9486-4
Acknowledgments
This research was performed in the Dr. Rastegar central Lab, Faculty of Veterinary Medicine, University of Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Informed Consent
This manuscript does not contain any studies with human subjects performed by any of the authors.
Human and Animal Rights
All experiments executed according to the Guide for the Care and Use of Laboratory Animals and approved by the institutional animal ethics committee.
Rights and permissions
About this article
Cite this article
Mahzouni, M., Zendehdel, M., Babapour, V. et al. Methylamine induced hypophagia is mediated via dopamine D1 and D2 receptors in neonatal meat chicks. Vet Res Commun 40, 21–27 (2016). https://doi.org/10.1007/s11259-015-9649-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-015-9649-y