Abstract
Range marginal populations are often perceived to have lower conservation value compared to those in the core area. The allocation of resources to maintain peripheral populations is therefore often questioned. The sage-leaved rockrose (Cistus salviifolius L.) is a self-incompatible and obligate seeder widely distributed in the Mediterranean area but rare and patchily distributed in Switzerland at its range margin on the southern slopes of the Alps. Here, we combined analysis of genetic diversity with pollinator surveys and field studies of reproductive ecology to compare peripheral Cistus populations in the Alps with range central populations in the Mediterranean. Our results showed no differences in genetic diversity between peripheral and central populations and between fragmented and connected ones at its range margin in the Alps. Although the fragmented populations were visited by more abundant and species richer pollinators (bees and wasps), they showed lower number of seeds and higher self-compatibility compared to the connected ones, which excludes the pollination limitation hypothesis. Overall, our study highlights that peripheral populations of C. salviifolius in the Alps are likely to contribute to maintain genetic diversity, while showing variation in reproductive ecology, and are therefore important for the conservation of this species.




Similar content being viewed by others
Change history
05 April 2020
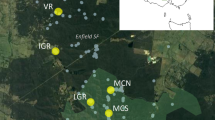
Figure 1 has been published incorrectly in the original article. The correct version of Figure 1 is provided in this correction.
References
Ågren J (1996) Population size, pollinator limitation, and seed set in the self-incompatible herb Lythrum salicaria. Ecology 77:1779–1790
Andrello M, Devaux C, Quétier F, Till-Bottraud I (2018) Paying for conservation: a bioeconomic analysis of the effects of land management options on the viability of an endangered species, Eryngium alpinum. J Appl Ecol 55:1940–1950
Beffa C (2006) Successional patterns and population development in fragmented Cistus salviifolius stands on southern slopes of the Swiss Alps. ETH, Zürich, Environmental Science, p 27
Berger S, Walther GR (2006) Distribution of evergreen broad-leaved woody species in Insubria in relation to bedrock and precipitation. Bot Helvetica 116:65–77
Bornand C, Eggenberg S, Gygax A, Juillerat P, Jutzi M, Möhl A, Rometsch S, Sager L, Santiago H (2016) Rote Liste Gefässpflanzen. Gefährdete Arten der Schweiz, Bundesamt für Umwelt, Bern und Info Flora, Genf
Bosch J (1992) Floral biology and pollinators of 3 cooccurring cistus species (Cistaceae). Bot J Linn Soc 109:39–55
Bratteler M, Lexer C, Widmer A (2006) A genetic linkage map of Silene vulgaris based on AFLP markers. Genome 49:320–327
Brown JH, Stevens GC, Kaufman DM (1996) The geographic range: size, shape, boundaries, and internal structure. Annu Rev Ecol Syst 27:597–623
Cerabolini B, Ceriani RM, Caccianiga M, Andreis RD, Raimondi B (2003) Seed size, shape and persistence in soil: a test on Italian flora from Alps to Mediterranean coasts. Seed Sci Res 13:75–85
Ceschi I (1995) La distribuzione del Cisto femmina (Cistus salviifolius L.) nel Cantone Ticino. Bollettino Società ticinese Scienze naturali 83:107–111
Conedera M, Stanga P, Oester B, Bachmann P (2001) Different post-culture dynamics in abandoned chestnut orchards and coppices. For Snow Landsc Res 76:487–492
Dai Q, Fu JZ (2011) When central populations exhibit more genetic diversity than peripheral populations: a simulation study. Chin Sci Bull 56:2531–2540
Delarze R, Caldelari D, Hainard P (1992) Effects of fire on forest dynamics in southern Switzerland. J Veg Sci 3:55–60
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Duffy KJ, Scopece G, Cozzolino S, Fay MF, Smith RJ, Stout JC (2009) Ecology and genetic diversity of the dense-flowered orchid, Neotinea maculata, at the centre and edge of its range. Ann Bot 104:507–516
Durka W (1999) Genetic diversity in peripheral and subcentral populations of Corrigiola litoralis L. (Illecebraceae). Heredity 83:476–484
Eckert CG, Samis KE, Lougheed SC (2008) Genetic variation across species’ geographical ranges: the central–marginal hypothesis and beyond. Mol Ecol 17:1170–1188
Eckstein RL, O'Neill RA, Danihelka J, Otte A, Kohler W (2006) Genetic structure among and within peripheral and central populations of three endangered floodplain violets. Mol Ecol 15:2367–2379
Excoffier L, Laval G, Schneider S (2005) Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evolut Bioinform 1:47–50
Farley RA, McNeilly T (2000) Diversity and divergence in Cistus salvifolius (L.) populations from contrasting habitats. Hereditas 132:183–192
Faugeron S, Martinez EA, Correa JA, Cardenas L, Destombe C, Valero M (2004) Reduced genetic diversity and increased population differentiation in peripheral and overharvested populations of Gigartina skottsbergii (Rhodophyta, Gigartinales) in southern Chile. J Phycol 40:454–462
Franzoni A (1890) Le piante fanerogame della Svizzera insubrica: enumerate secondo il methodo decandolliano. Nouveaux Mémoires de la Société helvétique des sciences naturelles 30:1–256
Gaudin J (1833) Flora Helvetica I-VI and VII. Topgraphia Botanica, Zürich
Glémin S, Gaude T, Guillemin M-L, Lourmas M, Olivieri I, Mignot A (2005) Balancing selection in the wild: testing population genetics theory of self-incompatibility in the rare species Brassica insularis. Genetics 171:279–289
Haller A.V. 1768. Historia stripium indigenarum helvetiae inchoata. Sumptibus Societas Typographica, Bern.
Hampe A, Petit RJ (2005) Conserving biodiversity under climate change: the rear edge matters. Ecol Lett 8:461–467
Hewitt G (2000) The genetic legacy of the Quaternary ice ages. Nature 405:907–913
Hoffmann AA, Blows MW (1994) Species borders: ecological and evolutionary perspectives. Trends Ecol Evol 9:223–227
Holt RD, Keitt TH (2000) Alternative causes for range limits: a metapopulation perspective. Ecol Lett 3:41–47
Honnay O, Coart E, Butaye J, Adriaens D, Van Glabeke S, Roldan-Ruiz I (2006) Low impact of present and historical landscape configuration on the genetics of fragmented Anthyllis vulneraria populations. Biol Conserv 127:411–419
Jennersten O (1988) Pollination in dianthus-deltoides (Caryophyllaceae): effects of habitat fragmentation on visitation and seed set. Conserv Biol 2:359–366
Kropf M (2012) Genetic variation, biogeographical history, and conservation of Anthyllis Montana L. Ssp Jacquinii (Kern) Hayek (Fabaceae) at Its Northern Distribution Limit. Int J Plant Sci 173:789–801
Lammi A, Siikamaki P, Mustajarvi K (1999) Genetic diversity, population size, and fitness in central and peripheral populations of a rare plant Lychnis viscaria. Conserv Biol 13:1069–1078
Lesica P, Allendorf FW (1995) When are peripheral-populations valuable for conservation. Conserv Biol 9:753–760
McCauley RA, Ballard HE (2002) Inferring nativity and biogeographic affinities of central and marginal populations of Froelichia floridana (Amaranthaceae) from Inter-Simple Sequence Repeat (ISSR) markers. J Torrey Bot Soc 129:311–325
Moeller DA (2006) Geographic structure of pollinator communities, reproductive assurance, and the evolution of self-pollination. Ecology 87:1510–1522
Moretti M, Legg C (2009) Combining plant and animal traits to assess community functional responses to disturbance. Ecography 32:299–309
Moretti M, Conedera M, Moresi R, Guisan A (2006) Modelling the influence of change in fire regime on the local distribution of a Mediterranean pyrophytic plant species (Cistus salviifolius) at its northern range limit. J Biogeogr 33:1492–1502
Moretti M, Staehli C, Gillet F (2008) Determinants for the conservation of a vulnerable fire-dependent species at its marginal range. Plant Ecol 199:89–98
Nunney L (2002) The effective size of annual plant populations: the interaction of a seed bank with fluctuating population size in maintaining genetic variation. Am Nat 160:195–204
Pannell JR, Auld JR, Brandvain Y, Burd M, Busch JW, Cheptou P-O, Conner JK, Goldberg EE, Grant A-G, Grossenbacher DL, Hovick SM, Igic B, Kalisz S, Petanidou T, Randle AM, de Casas RR, Pauw A, Vamosi JC, Winn AA (2015) The scope of Baker's law. New Phytol 208:656–667
Pezzatti GB, Reinhard M, Conedera M (2010) Swissfire: die neue schweizerische Waldbranddatenbank. Schweizerische Zeitschrift fur Forstwesen 161:465–469
Pironon S, Papuga G, Villellas J, Angert AL, García MB, Thompson JD (2017) Geographic variation in genetic and demographic performance: new insights from an old biogeographical paradigm. Biol Rev 92:1877–1909
Plenk K, Bardy K, Hohn M, Thiv M, Kropf M (2017) No obvious genetic erosion, but evident relict status at the westernmost range edge of the Pontic-Pannonian steppe plant Linum flavum L. (Linaceae) in Central Europe. Ecol Evol 7:6527–6539
R Core Team (2005) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org. Accessed 7 Dec 2005
Razgour O, Juste J, Ibáñez C, Kiefer A, Rebelo H, Puechmaille SJ, Arlettaz R, Burke T, Dawson DA, Beaumont M, Jones G (2013) The shaping of genetic variation in edge-of-range populations under past and future climate change. Ecol Lett 16:1258–1266
Roy J, Sonie L (1992) Germination and population-dynamics of cistus species in relation to fire. J Appl Ecol 29:647–655
Sagarin RD, Gaines SD (2002) The 'abundant centre' distribution: to what extent is it a biogeographical rule? Ecol Lett 5:137–147
Schmidt K, Jensen K (2000) Genetic structure and AFLP variation of remnant populations in the rare plant Pedicularis palustris (Scrophulariaceae) and its relation to population size and reproductive components. Am J Bot 87:678–689
Silva JL, Brennan AC, Mejías JA (2016) Population genetics of self-incompatibility in a clade of relict cliff-dwelling plant species. AoB Plants 8:29
Smouse PE, Peakall R (1999) Spatial autocorrelation analysis of individual multiallele and multilocus genetic structure. Heredity 82:561–573
Staehli C (2005) Ecologie et phytosociologie du Cistus salviifolius L au Tessin (Southern Switzerland). University of Neuchâtel, Neuchâtel
Thanos CA, Georghiou K, Kadis C, Pantazi C (1992) Cistaceae: a plant family with hard seeds. Isr J Bot 41:251–263
Torroni C (2008) Facteurs importants pour la germination et la persistance de Cistus salviifolius en Suisse. In: Thesis M. (ed), University of Neuchâtel and Swiss Federal Research Institute WSL, Bellinzona
Tramer O, Ammann P, Franscella C, Frey E (1975/76) Ricerche ecologiche concernenti specie mediterranee della zona insubrica minacciate nella loro esistenza; in particolare ii cisto bianco (Cistus salvifolius L.) I parte. Bollettino della Società ticinese di Scienze naturali 65: 29–61
Tramer O, Ammann P, Franscella C, Frey E (1977/78) Ricerche ecologiche concernenti specie mediterranee della zona insubrica minacciate nella loro esistenza; in particolare ii cisto bianco (Cistus salvifolius L.) II parte. Bollettino della Società ticinese di Scienze naturali 66: 85–98
Travis SE, Maschinski J, Keim P (1996) An analysis of genetic variation in Astragalus cremnophylax var cremnophylax a critically endangered plant, using AFLP markers. Mol Ecol 5:735–745
Vakkari P, Blom A, Rusanen M, Raisio J, Toivonen H (2006) Genetic variability of fragmented stands of pedunculate oak (Quercus robur) in Finland. Genetica 127:231–241
van Heerwaarden B, Kellermann V, Schiffer M, Blacket M, Sgro CM, Hoffmann AA (2009) Testing evolutionary hypotheses about species borders: patterns of genetic variation towards the southern borders of two rainforest Drosophila and a related habitat generalist. Proc R Soc B 276:1517–1526
Van Rossum F, Vekemans X, Gratia E, Meerts P (2003) A comparative study of allozyme variation of peripheral and central populations of Silene nutans L. (Caryophyllaceae) from Western Europe: implications for conservation. Plant Syst Evol 242:49–61
Vitalis R, Glemin S, Olivieri I (2004) When genes go to sleep: the population genetic consequences of seed dormancy and monocarpic perenniality. Am Nat 163:295–311
Vos P, Hogers R, Bleeker M, Reijans M, Vandelee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) Aflp: a new technique for DNA-fingerprinting. Nucl Acids Res 23:4407–4414
Westphal C, Bommarco R, Carre G, Lamborn E, Morison N, Petanidou T, Potts SG, Roberts SPM, Szentgyoergyi H, Tscheulin T, Vaissiere BE, Woyciechowski M, Biesmeijer JC, Kunin WE, Settele J, Steffan-Dewenter I (2008) Measuring bee diversity in different European habitats and biogeographical regions. Ecol Monogr 78:653–671
Young A, Boyle T, Brown T (1996) The population genetic consequences of habitat fragmentation for plants. Trends Ecol Evol 11:413–418
Acknowledgements
Special thanks to Prof. Dr. Alex Widmer, ETH Zurich for his suggestions and great help design the study and analyze the data. S. Karrenberg van der Nat, A. Minder, M.D. Moccia and C. Michel for helping with the manuscript and in the lab. Thanks also to all the team of the Plant Ecological Genetics Institute of Integrative Biology ETH Zurich and the Research group at the WSL Cadenazzo for the map and the helpful suggestions and discussions.
Author information
Authors and Affiliations
Contributions
MM and PC conceived and designed the study. PC collected the plant material in the field and ran the genetic analyses. AB and MM surveyed pollinators and ran the pollination experiment. PC, AB and MA analyzed data. All authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Since Cistus salviifolius a vulnerable species in Switzerland, the authors declare that, during the study, they treated the species and population with the greatest respect.
Informed consent
The investigation at national park was conducted by obtaining due permission.
Additional information
Communicated by Marjana Westergren.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moretti, M., Caretti, P., Bricalli, A. et al. Genetic diversity and reproductive ecology of the sage-leaved rockrose, Cistus salviifolius L., in the Swiss Alps. Plant Ecol 221, 361–374 (2020). https://doi.org/10.1007/s11258-020-01013-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-020-01013-4