Abstract
Background
Central obesity is a risk factor for chronic kidney disease (CKD). However, the exact correlation between the cardiometabolic index (CMI), an indicator of central obesity, and CKD remains unclear. Here, we aimed to investigate the correlation between the CMI and CKD in the general American population.
Methods
This cross-sectional study involved 64,313 members of the general population (≥ 20 years of age) with data in the National Health and Nutrition Examination Survey (NHANES) 1999–2020. The individuals were grouped into three categories by CMI tertile: T1 group (n = 7,029), T2 group (n = 7,356), and T3 group (n = 7,380). Logistic regression analysis was performed, with NHANES recommended weights, to assess the association between the CMI and CKD.
Results
A total of 21,765 participants were included; the overall prevalence of CKD was 12.2%. From the low to the high CMI tertile, the prevalence of CKD increased from 8.9% to 16.0% (P < 0.001). After full adjustment for confounders, the higher tertile of CMI (OR: 1.08, 95% CI: 1.03 − 1.13, P = 0.002) had the higher risk of CKD. Compared with the T1 group, the groups with higher CMI levels had a higher CKD risk (T2: OR: 1.01, 95%CI: 0.87–1.18, P = 0.812; T3: OR: 1.22, 95%CI: 1.05–1.43, P = 0.013).
Conclusions
Higher CMI was independently associated with higher CKD risk in the general population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is an irreversible and progressive condition [1] that is emerging as a serious and widely recognized public health issue [2]. More than 850 million individuals have been estimated to have kidney disease worldwide [3]. To mitigate the disease burden, CKD must be prevented in the general population.
Central obesity is strongly associated with CKD [4]. Moreover, central fat distribution has been associated with diminished estimated glomerular filtration rate (eGFR) and effective renal plasma flow, and elevated glomerular hyperfiltration [5]. The cardiometabolic index (CMI), an indicator used to assess central obesity, comprises the waist-to-height ratio (WHtR), and triglyceride (TG) and high-density lipoprotein cholesterol (HDL-C), and was first developed by Wakabayashi et al. in 2015 [6]. In the past several years, studies have focused on association of the clinical CMI value with atherosclerosis, hypertension, ischemic stroke, left ventricular dilation, diabetes, and fatty liver disease, and CMI has been found to perform well in assessing the emergence and advancement of these illnesses [6,7,8,9,10,11]. However, few studies have investigated the relationship between CMI and CKD, which remains unclear.
Therefore, our study aim was to explore the relationship between CMI and CKD in the general American population, to provide insights into CKD prevention and control.
Methods
Study design and population
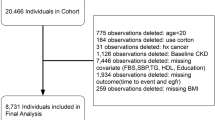
The objective of the National Health and Nutrition Examination Survey (NHANES) is to comprehensively assess the health and nutrition of individuals across all age groups in the United States. Using a complex weighted analysis, the survey ensures representation of the entire United States population by sampling approximately 5,000 individuals from different counties across the country every 2 years. We identified 64,313 individuals from United States communities who voluntarily participated in the NHANES survey between 1999 and 2020, and were above 20 years of age. Of these, 7,440 were excluded from the analysis because of insufficient data for diagnosing CKD, and 30,671 were excluded because of a lack of data for calculating the CMI. Furthermore, 2,390 individuals with a history of cancer, 667 pregnant people, and 1,380 people with a sampling weight of 0 were also excluded from the analysis. Thus, the analysis herein was based on 21,765 included individuals (Fig. 1).
Calculation of the CMI
The WHtR refers to the waist circumference in centimeters divided by the height in centimeters. The TG/HDL-C ratio represents the relationship between TG and HDL-C levels. The WHtR is multiplied by TG/HDL-C to calculate the CMI. On the basis of CMI tertiles, the participants were categorized into three groups: T1 group (CMI ≤ 0.8), T2 group (0.8 < CMI ≤ 1.7), and T3 group (CMI > 1.7).
Primary outcome
The primary endpoint was CKD. As recommended by Kidney Disease: Improving Global Outcomes (KDIGO), CKD is defined by a consistently low eGFR below 60 mL per minute per 1.73 m2 body surface area, or excretion of ≥ 30 mg urinary albumin per day [12].
Definitions of variables of interest
Participants provided self-reported information on their age, gender, ethnicity, smoking habits, and alcohol consumption. A physical examination was conducted to obtain waist and height measurements. Individuals whose body mass index (BMI) exceeded 30 were considered obese. Diabetes mellitus was characterized by either a self-reported diabetes history or a fasting blood glucose level ≥ 7.0 mmol/L documented during the survey. Individuals were categorized as having hypertension if they had been previously diagnosed with hypertension or if their blood pressure measurements during the field survey indicated a resting systolic blood pressure of 130 mmHg or higher and/or a diastolic blood pressure of 80 mmHg or higher. Automated hematological analysis equipment was used to collect laboratory measurements, including total cholesterol (TC), TG, low-density lipoprotein cholesterol (LDL-C), and HDL-C.
Statistical analyses
The calculation of weights for specific groups was performed according to the NHANES recommended weights. Since NHANES is a sampling design and needs to reflect the error between the sample and the whole in terms of the standard error, the baseline characteristics are to be presented in terms of the mean (standard error). Whereas counts (and percentages) were used to present categorical variables. Analysis of variance was used to compare the baseline characteristics among groups in terms of continuous variables, whereas a χ2 test was used for categorical variables. To evaluate the link between CMI and CKD, we performed logistic regression analyses. According to the recommended weights from NHANES, complex sampling weighted analysis was used for all statistical analyses. To improve the reliability of the findings, we examined three models. The first model, model 1, was the unadjusted model. Model 2 included adjustment for age, gender, and race, whereas model 3 included comprehensive adjustment for potential confounding factors such as age, gender, race, smoking habits, alcohol consumption behaviors, BMI, TC, LDL-C, hypertension, and diabetes mellitus (DM). To analyze the possible nonlinear correlation between CMI and CKD, we also used regression cubic spline (RCS) analysis. The RCS was adjusted for the same variables as those in model 3, to ensure consistency. Furthermore, we performed a stratified analysis according to age, gender, obesity, smoking, alcohol consumption, hypertension, and diabetes, and investigated the potential interplay between CMI and these subsets. The Survey package in R software was used for data analyses (version 4.2.2; R Foundation for Statistical Computing, Vienna, Austria). For all analyses, a significance threshold < 0.05 was considered statistically significant.
Results
Participant characteristics
Table 1 presents the baseline clinical characteristics. In this study, 21,765 participants were included, with an average age of 45.5 (0.2) years, 10,825 (49.9%) of whom were men. In total, 3,521 (12.2%) had CKD, 10,759 (45.2%) had hypertension, and 3,974 (13.2%) had DM. Participants were divided into three groups by CMI tertile [T1 (n = 7,029); T2 (n = 7,356); and T3 (n = 7,380)]. Compared with the T1 group, the T3 group had higher LDL-C [T1: 104.09 mg/dl vs. T3: 122.31 mg/dl, P < 0.001], TG [T1: 63.10 mg/dl vs. T3: 216.26 mg/dl, P < 0.001], body obesity [T1: 13.5% vs. T3: 56.9%, P < 0.001], and WHtR [T1: 0.52 vs. T3: 0.64, P < 0.001]. Furthermore, groups with higher CMI had higher proportions of hypertension [T1: 30.8% vs. T2: 45.5% vs. T3: 59.4%, P < 0.001] and DM [T1: 5.2% vs. T2: 11.7% vs T3: 22.7%, P < 0.001]. Additional comprehensive data are provided in Table 1.
Association between CMI and CKD
With increasing CMI, the incidence rate of CKD gradually increased [T1: 8.9% vs. T2: 11.8% vs. T3: 16.0%, P < 0.001] (Fig. 2). Univariate logistic regression analysis indicated that CMI was positively correlated with CKD in the participants (OR: 1.15, 95% CI: 1.12 − 1.18, P < 0.001). Compared with the T1 group, the groups with higher CMI had a higher risk of CKD (T2: OR: 1.36, 95% CI: 1.21–1.53, P < 0.001; T3: OR: 1.95, 95% CI: 1.71–2.21, P < 0.001). After adjustment for age, gender, race, smoking status, alcohol consumption status, BMI, TC, LDL-C, hypertension, and DM, the association between CMI (OR: 1.08, 95% CI: 1.03 − 1.13, P = 0.002) and CKD did not change. Moreover, the groups with higher CMI also had a higher risk of CKD (T2: OR: 1.01, 95% CI: 0.87–1.18, P = 0.812; T3: OR: 1.22, 95% CI: 1.05–1.43, P = 0.013) (Table 2).
Subgroup analysis
After stratification of participants by age (P for interaction = 0.709), obesity (P for interaction = 0.436), smoking status (P for interaction = 0.827), alcohol consumption status (P for interaction = 0.247), hypertension (P for interaction = 0.803), diabetes (P for interaction = 0.464), and sex [male (OR: 1.11, 95% CI: 1.03 − 1.19); female (OR: 1.04, 95% CI: 0.97 − 1.13), P for interaction = 0.039], the only observed interaction was between gender and CMI. Men, but not women, showed an association of CKD with CMI. Compared with the T1 group, groups with higher CMI had a greater risk of developing CKD (Fig. 3).
Regression cubic splines
RCS indicated a J-shaped relationship between CMI and CKD. Elevated CMI in participants was associated with higher risk of developing CKD. A similar trend was observed in the relationship between extremely low CMI and CKD (non-linear P = 0.087) (Fig. 4).
Discussion
In this cross-sectional study, the CMI was associated with CKD risk, such that higher CMI was associated with greater CKD risk. We observed no significant change in the association between CMI and CKD in the general American population after stratification according to age, obesity, smoking status and alcohol consumption status, hypertension, and diabetes. In the analysis of sex subgroups, we found that CMI was not significantly correlated with CKD in women.
CMI, a measure of central obesity, has been used to assess the occurrence of atherosclerosis, fatty liver associated with metabolism, and hyperuricemia, with promising results [7, 11, 13]. However, relatively less research has been conducted on the association between CMI and CKD, although CKD has been correlated with central obesity [14, 15].
To our knowledge, only two previous studies have examined the association between CMI and the development of CKD [16, 17]. First, a research project in rural areas of China has described the utility of considering diminished eGFR with CMI [16]. Chinese and American populations differ in blood lipids and body types: Misra et al. have reported that Asian populations exhibit distinct body composition characteristics characterized by greater abdominal adiposity than observed in numerous other ethnic groups [18]. Therefore further studies are needed to determine whether this conclusion applies to the United States population, given that a previous study has suggested that CMI was negatively associated with kidney function in the Asian population. Moreover, another study has found that CMI was negatively correlated with renal function in people older than 60 years in America [17]. Nevertheless, because the incidence of CKD is increasingly affecting younger people, exploring only older people is inadequate [19,20,21]. To our knowledge, this study is the first to explore the association of CMI with CKD in the general American population.
Although a potential causative mechanism between CMI and CKD remains unclear, our findings are supported by previous research. Hou et al. have identified a positive association between TG level and diminished eGFR [22]. TG catabolism is delayed by downregulation of hydrolysis of very low density lipoproteins and chylomicrons by lipoprotein lipase, thus leading to lipid accumulation in renal tissues [23], and consequently inflammation, oxidative stress, and autophagy. Ultimately, substantial proliferation of glomerular basement membrane cells occurs, thus exacerbating glomerulosclerosis and tubulointerstitial injury, and promoting the onset of proteinuria [21, 24, 25]. Tozawa et al. have demonstrated that HDL-C was positively associated with eGFR [26]. Moreover, Ho et al. have demonstrated that the TG/HDL-C ratio was an independent risk factor for CKD [27]. The role of obesity in the occurrence of focal segmental glomerulosclerosis and glomerulomegaly has been examined in previous studies [28, 29]. A population-based study has suggested global use of WHtR to assess CKD across ethnic groups [30]. Odagiri et al. have identified the WHtR as a stand-alone factor that could reliably predict the incidence of CKD [31]. These findings have established a theoretical foundation for the prevention of CKD through the regulation of CMI levels.
In subgroup analyses stratified by age, obese, smoking status, alcohol consumption status, hypertension, and diabetes, the outcome of the regression analysis did not show any significant alteration. Elevated CMI was an independent risk factor for CKD. Furthermore, an interaction between CMI and gender was observed. We speculated that this interaction might have arisen from differences in hormones between sexes and a protective effect of estrogen in inhibiting renal injury induced by dyslipidemia [32,33,34,35]. We further investigated a potential nonlinear correlation between CMI and CKD with restricted RCS analysis. No evidence of a potential non-linear relationship was detected in the RCS data, which indicated a J-shaped relationship. Very low CMI appeared to have no notable protective influence on participants with CKD. These findings might be explained as follows. The CMI was calculated as TG/HDL-C ratio × WHtR [6]. Patients with very low CMI also have low level of lipids and WHtR, thus potentially leading to malnutrition or cachexia [36, 37], thereby increasing CKD risk [38,39,40]. Thus, individuals with very low CMI might have faced an elevated risk of malnutrition that obscured the advantages of very low CMI. However, future studies are necessary to validate this hypothesis.
This study had several limitations. First, due to the inherent limitations of cross-sectional analysis. The link between CMI and CKD could be perceived only as correlative rather than causative. Second, as with other cross-sectional studies, our study might have been susceptible to the influences of additional variables that could have confounded the results. Third, subject to the limitations of public databases, there was no information on kidney transplantation in NHANES, and the impact of kidney transplantation needs to be further explored in future prospective studies. Finally, additional longitudinal follow-up studies are necessary to validate whether CMI might serve as an early screening tool for the prevention of CKD in patients.
Conclusion
CMI was positively associated with CKD risk. We advocate for the widespread implementation of CMI to evaluate potential CKD risk in the general population. Moreover, our study found that the contribution of very low CMI to CKD prevention may be limited.
Data availability
Publicly available datasets were analyzed in this study. This data can be found in NHANES’s official website, at http://www.cdc.gov/nchs/nhanes.htm.
Abbreviations
- CKD:
-
Chronic kidney disease
- eGFR:
-
Estimated glomerular filtration rate
- CMI:
-
Cardiometabolic index
- WHtR:
-
Waist-to-height ratio
- TG:
-
Triglyceride
- HDL-C:
-
High-Density Lipoprotein Cholesterol
- NHANES:
-
National Health and Nutrition Examination Survey
- KDIGO:
-
Kidney Disease: Improving Global Outcomes
- BMI:
-
Body mass index
- TC:
-
Total cholesterol
- LDL-C:
-
Low-Density Lipoprotein Cholesterol
- DM:
-
Diabetes mellitus
- RCS:
-
Regression cubic spline
References
Liu T, Guan Y, Li J, Mao H, Zhan Y (2020) Thyroid dysfunction and cardiovascular events in patients with chronic kidney disease: a protocol of systematic review and meta-analysis. Medicine 99(47):e23218. https://doi.org/10.1097/md.0000000000023218
Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS (2007) Prevalence of chronic kidney disease in the United States. JAMA 298(17):2038–2047. https://doi.org/10.1001/jama.298.17.2038
Jager KJ, Kovesdy C, Langham R, Rosenberg M, Jha V, Zoccali C (2019) A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int 96(5):1048–1050. https://doi.org/10.1016/j.kint.2019.07.012
Lakkis JI, Weir MR (2018) Obesity and kidney disease. Prog Cardiovasc Dis 61(2):157–167. https://doi.org/10.1016/j.pcad.2018.07.005
Kwakernaak AJ, Zelle DM, Bakker SJ, Navis G (2013) Central body fat distribution associates with unfavorable renal hemodynamics independent of body mass index. J Am Soc Nephrol JASN 24(6):987–994. https://doi.org/10.1681/asn.2012050460
Wakabayashi I, Daimon T (2015) The : “cardiometabolic index” as a new marker determined by adiposity and blood lipids for discrimination of diabetes mellitus. Clinica Chimica Acta Int J Clin Chem 438:274–278. https://doi.org/10.1016/j.cca.2014.08.042
Wakabayashi I, Sotoda Y, Hirooka S, Orita H (2015) Association between cardiometabolic index and atherosclerotic progression in patients with peripheral arterial disease. Clinica Chimica Acta Int J Clin Chem 446:231–236. https://doi.org/10.1016/j.cca.2015.04.020
Wang H, Chen Y, Guo X, Chang Y, Sun Y (2017) Usefulness of cardiometabolic index for the estimation of ischemic stroke risk among general population in rural China. Postgrad Med 129(8):834–841. https://doi.org/10.1080/00325481.2017.1375714
Wang H, Chen Y, Sun G, Jia P, Qian H, Sun Y (2018) Validity of cardiometabolic index, lipid accumulation product, and body adiposity index in predicting the risk of hypertension in Chinese population. Postgrad Med 130(3):325–333. https://doi.org/10.1080/00325481.2018.1444901
Wang H, Sun Y, Li Z, Guo X, Chen S, Ye N, Tian Y, Zhang L (2018) Gender-specific contribution of cardiometabolic index and lipid accumulation product to left ventricular geometry change in general population of rural China. BMC Cardiovasc Disord 18(1):62. https://doi.org/10.1186/s12872-018-0798-0
Duan S, Yang D, Xia H, Ren Z, Chen J, Yao S (2022) Cardiometabolic index: a new predictor for metabolic associated fatty liver disease in Chinese adults. Front Endocrinol 13:1004855. https://doi.org/10.3389/fendo.2022.1004855
August P (2023) Chronic kidney disease-another step forward. N Engl J Med 388(2):179–180. https://doi.org/10.1056/NEJMe2215286
Zuo YQ, Gao ZH, Yin YL, Yang X, Feng PY (2021) Association between the cardiometabolic index and hyperuricemia in an asymptomatic population with normal body mass index. Int J Gen Med 14:8603–8610. https://doi.org/10.2147/ijgm.S340595
Lin CH, Chou CY, Lin CC, Huang CC, Liu CS, Lai SW (2007) Waist-to-height ratio is the best index of obesity in association with chronic kidney disease. Nutrition (Burbank, Los Angeles County, Calif) 23(11–12):788–793. https://doi.org/10.1016/j.nut.2007.08.007
He Y, Li F, Wang F, Ma X, Zhao X, Zeng Q (2016) The association of chronic kidney disease and waist circumference and waist-to-height ratio in Chinese urban adults. Medicine 95(25):e3769. https://doi.org/10.1097/md.0000000000003769
Wang HY, Shi WR, Yi X, Wang SZ, Luan SY, Sun YX (2018) Value of reduced glomerular filtration rate assessment with cardiometabolic index: insights from a population-based Chinese cohort. BMC Nephrol 19(1):294. https://doi.org/10.1186/s12882-018-1098-8
Miao M, Deng X, Wang Z, Jiang D, Lai S, Yu S, Yan L (2023) Cardiometabolic index is associated with urinary albumin excretion and renal function in aged person over 60: data from NHANES 2011–2018. Int J Cardiol 384:76–81. https://doi.org/10.1016/j.ijcard.2023.04.017
Misra A, Wasir JS, Vikram NK (2005) Waist circumference criteria for the diagnosis of abdominal obesity are not applicable uniformly to all populations and ethnic groups. Nutrition (Burbank, Los Angeles County, Calif) 21(9):969–976. https://doi.org/10.1016/j.nut.2005.01.007
Langman CB, Alon U, Ingelfinger J, Englund M, Saland JM, Somers MJ, Stapleton FB, Sibú NO, Cochat P, Wong W, Eke FU, Satlin L, Salusky I (2009) A position statement on kidney disease from powdered infant formula-based melamine exposure in Chinese infants. Pediatr Nephrol (Berlin, Germany) 24(7):1263–1266. https://doi.org/10.1007/s00467-009-1129-6
Fuhrman DY, Schneider MF, Dell KM, Blydt-Hansen TD, Mak R, Saland JM, Furth SL, Warady BA, Moxey-Mims MM, Schwartz GJ (2017) Albuminuria, proteinuria, and renal disease progression in children with CKD. Clin J Am Soc Nephrol CJASN 12(6):912–920. https://doi.org/10.2215/cjn.11971116
Saland JM, Kupferman JC, Pierce CB, Flynn JT, Mitsnefes MM, Warady BA, Furth SL (2019) Change in dyslipidemia with declining glomerular filtration rate and increasing proteinuria in children with CKD. Clin J Am Soc Nephrol CJASN 14(12):1711–1718. https://doi.org/10.2215/cjn.03110319
Hou X, Wang C, Zhang X, Zhao X, Wang Y, Li C, Li M, Wang S, Yang W, Ma Z, Ma A, Zheng H, Wu J, Sun Y, Song J, Lin P, Liang K, Gong L, Wang M, Liu F, Li W, Xiao J, Yan F, Yang J, Wang L, Tian M, Liu J, Zh-ao R, Chen S, Chen L (2014) Triglyceride levels are closely associated with mild declines in estimated glomerular filtration rates in middle-aged and elderly Chinese with normal serum lipid levels. PloS One 9(9):e106778. https://doi.org/10.1371/journal.pone.0106778
Vaziri ND, Wang XQ, Liang K (1997) Secondary hyperparathyroidism downregulates lipoprotein lipase expression in chronic renal failure. Am J Physiol 273(6):F925–F930. https://doi.org/10.1152/ajprenal.1997.273.6.F925
Aghadavod E, Khodadadi S, Baradaran A, Nasri P, Bahmani M, Rafieian- Kopaei M (2016) Role of oxidative stress and inflammatory factors in diabetic kidney disease. Iran J Kidney Dis 10(6):337–343
Russo G, Piscitelli P, Giandalia A, Viazzi F, Pontremoli R, Fioretto P, De Cosmo S (2020) Atherogenic dyslipidemia and diabetic nephropathy. J Nephrol 33(5):1001–1008. https://doi.org/10.1007/s40620-020-00739-8
Tozawa M, Iseki K, Iseki C, Oshiro S, Ikemiya Y, Takishita S (2002) Trigly-ceride, but not total cholesterol or low-density lipoprotein cholesterol levels, predict development of proteinuria. Kidney Int 62(5):1743–1749. https://doi.org/10.1046/j.1523-1755.2002.00626.x
Ho CI, Chen JY, Chen SY, Tsai YW, Weng YM, Tsao YC, Li WC (2015) Relationship between TG/HDL-C ratio and metabolic syndrome risk factors with chronic kidney disease in healthy adult population. Clin Nutr (Edinburgh, Scotland) 34(5):874–880. https://doi.org/10.1016/j.clnu.2014.09.007
Verani RR (1992) Obesity-associated focal segmental glomerulosclerosis: pathological features of the lesion and relationship with cardiomegaly and hyperlipidemia. Am J Kidney Dis Off J Natl Kidney Found 20(6):629–634. https://doi.org/10.1016/s0272-6386(12)70230-5
Weisinger JR, Kempson RL, Eldridge FL, Swenson RS (1974) The nephrotic syndrome: a complication of massive obesity. Ann Intern Med 81(4):440–447. https://doi.org/10.7326/0003-4819-81-4-440
van Valkengoed IG, Agyemang C, Krediet RT, Stronks K (2012) Ethnic differences in the association between waist-to-height ratio and albumin-creatinine ratio: the observational SUNSET study. BMC Nephrol 13:26. https://doi.org/10.1186/1471-2369-13-26
Odagiri K, Mizuta I, Yamamoto M, Miyazaki Y, Watanabe H, Uehara A (2014) Waist to height ratio is an independent predictor for the incidence of chronic kidney disease. PloS One 9(2):e88873. https://doi.org/10.1371/journal.po-ne.0088873
Cortez-Dias N, Robalo Martins S, Belo A, Fiúza M (2013) Characterization of lipid profile in primary health care users in Portugal. Revista Portuguesa-de Cardiologia Orgao Oficial da Sociedade Portuguesa de Cardiologia Portuguese J Cardiol Official J Portuguese Soc Cardiol 32(12):987–996. https://doi.org/10.1016/j.repc.2013.06.008
Garovic VD, August P (2016) Sex differences and renal protection: keeping in touch with your feminine side. J Am Soc Nephrol JASN 27(10):2921–2924. https://doi.org/10.1681/asn.2016040454
Xia Y, Zou J, Xu H, Yi H, Guan J, Yin S (2019) Effect modification by gender of the influence of obstructive sleep apnoea characteristics on dyslipidaemia in China: a cross-sectional study. BMJ Open 9(9):e028509. https://doi.org/10.1136/bmjopen-2018-028509
Ahn SY, Choi YJ, Kim J, Ko GJ, Kwon YJ, Han K (2021) The beneficial effects of menopausal hormone therapy on renal survival in postmenopausal Korean women from a nationwide health survey. Sci Rep 11(1):15418. https://doi.org/10.1038/s41598-021-93847-9
Ferreira HDS (2020) Anthropometric assessment of children’s nutritional status: a new approach based on an adaptation of Waterlow’s classification. BMC Pediatr 20(1):65. https://doi.org/10.1186/s12887-020-1940-6
Yue J, Xu H, Zhou Y, Liu W, Han X, Mao Q, Li S, Tam LS, Ma J, Liu W (2021) Dyslipidemia is related to mortality in critical patients with coronavirus disease 2019: a retrospective study. Front Endocrinol 12:611526. https://doi.org/10.3389/fendo.2021.611526
Kalantar-Zadeh K (2005) Recent advances in understanding the malnutrition-inflammation-cachexia syndrome in chronic kidney disease patients: what is next? Semin Dial 18(5):365–369. https://doi.org/10.1111/j.1525-139X.2005.00074.x
Iorember FM (2018) Malnutrition in chronic kidney disease. Front Pediatr 6:161. https://doi.org/10.3389/fped.2018.00161
Kanda T, Murai-Takeda A, Kawabe H, Itoh H (2020) Low birth weight tren-ds: possible impacts on the prevalences of hypertension and chronic kidney disease. Hypertens Res Off J Japanese Soc Hypertens 43(9):859–868. https://doi.org/10.1038/s41440-020-0451-z
Acknowledgements
The authors thank all team members and participants in the NHANES study.
Funding
This research was funded and sponsored by the Fujian Province Health of Young and Middle-aged Backbone Personnel Training Project (Grant Numbers: 2022GGA057) and the Longyan City Science and Technology Plan Project (Grant Numbers: 2021LYF17025). The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
QG, YNW, YCL, LD, LHL, MXW, MRS, and XQL were responsible for the study concept and YL, YW, QG, YNW, XQL and YCL for study design. Data extraction was undertaken by YL, QG, YNW, YCL and LD, XQL were responsible for data analysis. Drafting of the manuscript: QG, YNW and YCL. Critical revision of the manuscript for important intellectual content: QG, YNW, YCL, LD, LHL, XQL, MXW, MRS, YL, and YW. All authors read and approved the fnal manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was performed in accordance with the principals of the Declaration of Helsinki. The NHANES protocol was revised, approved by the Ethics Review Committee of the National Center for Health Statistics.
Informed consent
The patients/participants provided their written informed consent to participate in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, Q., Wang, Y., Liu, Y. et al. Association between the cardiometabolic index and chronic kidney disease: a cross-sectional study. Int Urol Nephrol 56, 1733–1741 (2024). https://doi.org/10.1007/s11255-023-03888-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03888-4