Abstract
Background
Few works have analyzed factors associated with urine output in ADPKD patients taking tolvaptan (TVP).
Methods
We selected 24-h urine samples from ADPKD patients treated with TVP. Urine osmolality/creatinine ratio was used as estimator of urinary osmolar load.
Results
We included 127 urine samples from 61 patients. After TVP, urine output doubled with a parallel reduction in urine solute concentration. However, when expressed as urine solute/creatinine ratios, no significant changes were observed. Daily osmolar load and osmolality/creatinine ratio did not change significantly.
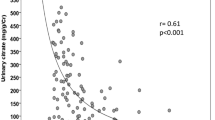
Before TVP, urine output was positively correlated with body weight and urine osmolality/creatinine ratio and negatively with eGFR, urine morning osmolality, and 24-h urine-calculated osmolality. After TVP, urine output was positively correlated with body weight, eGFR and negatively with age. There was a poor correlation with urine-calculated osmolality.
We constructed a predictor model using mixed-effects modeling and we found that urine output was related to lower age, higher body weight, higher eGFR, and greater doses of TVP. When body weight was removed, urine output was also related to male sex and a higher daily osmolar excretion. Equation of prediction was: Urine output (mL/day) = 2771–52.9 × Age (years) + 58.4 × Weight (kg) + 18.7 × eGFR (mL/min) + 870 (if TVP = 90/30 mg) + 517 (if TVP = 60/30 mg).
Conclusion
Patients taking TVP will undergo an increase about twice in urine production from baseline. Greater doses of TVP cause a progressive increase in urine production. GFR, age, and body weight are the main predictors of future urine output in patients taking TVP.



Similar content being viewed by others
Data availability
Date are not available for authors not participating in this study.
References
Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E et al (2012) Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367:2407–2418
Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G et al (2017) Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med 377(20):1930–1942
Yamamura Y, Nakamura S, Itoh S et al (1998) OPC-41061, a highly potent human vasopressin V2-receptor antagonist: pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J Pharmacol Exp Ther 287:860–867
Boertien WE, Meijer E, Li J, Bost JE, Struck J, Flessner MF, Gansevoort RT, Torres VE (2013) Relationship of copeptin, a surrogate marker for arginine vasopressin, with change in total kidney volume and GFR decline in autosomal dominant polycystic kidney disease: results from the CRISP cohort. Am J Kidney Dis 61(3):420–429
Torres VE, Harris PC (2014) Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J Am Soc Nephrol 25(1):18–32
Perrone RD, Chapman AB, Oberdhan D, Czerwiec FS et al (2020) The NOCTURNE randomized trial comparing 2 tolvaptan formulations. Kidney Int Rep 5(6):801–812
Perrone RD, Chapman AB, Oberdhan D, Czerwiec FS, Sergeyeva O et al (2020) A randomized trial of modified-release versus immediate-release tolvaptan in ADPKD. Kidney Int Rep 5(6):790–800
Devuyst O, Chapman AB, Shoaf SE, Czerwiec FS, Blais JD (2017) Tolerability of aquaretic-related symptoms following tolvaptan for autosomal dominant polycystic kidney disease: results from TEMPO 3:4. Kidney Int Rep 2(6):1132–1140
Devuyst O, Chapman AB, Gansevoort RT, Higashihara E, Perrone RD et al (2017) Urine osmolality, response to tolvaptan, and outcome in autosomal dominant polycystic kidney disease: results from the TEMPO 3:4 Trial. J Am Soc Nephrol 28:1592–1602
Irazabal MV, Torres VE, Hogan MC, Glockner J et al (2011) Short-term effects of tolvaptan on renal function and volume in patients with autosomal dominant polycystic kidney disease. Kidney Int 80(3):295–301
Boertien WE, Meijer E, de Jong PE, ter Horst GJ, Renken RJ et al (2015) Short-term effects of tolvaptan in individuals with autosomal dominant polycystic kidney disease at various levels of kidney function. Am J Kidney Dis 65(6):833–841
Kramers BJ, van Gastel MDA, Boertien WE, Meijer E, Gansevoort RT (2019) Determinants of urine volume in ADPKD patients using the vasopressin V2 receptor antagonist tolvaptan. Am J Kidney Dis 73(3):354–362
Borrego Utiel FJ, Merino GE (2020) Glomerular filtration rate is the main predictor of urine volume in autosomal dominant polycystic kidney disease patients treated with tolvaptan when daily osmolar excretion is expressed as urinary osmolality/creatinine ratio. Clin Kidney J 14:1031–1033
Levey AS, Stevens LA, Schmid CH, Zhang YL et al (2009) CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Bhasin B, Velez JC (2016) Evaluation of polyuria: the roles of solute loading and water diuresis. Am J Kidney Dis 67:507–511
Casteleijn NF, Zittema D, Bakker SJ, Boertien WE et al (2015) Urine and plasma osmolality in patients with autosomal dominant polycystic kidney disease: reliable indicators of vasopressin activity and disease prognosis? Am J Nephrol 41(3):248–256
Borrego Utiel FJ, Herrera Contreras I, Merino García E et al (2022) Urinary citrate as a marker of renal function in patients with autosomal dominant polycystic kidney disease. Int Urol Nephrol 54:873–881
Gabow PA, Kaehny WD, Johnson AM, Duley IT et al (1989) The clinical utility of renal concentrating capacity in polycystic kidney disease. Kidney Int 35:675–680
Ferry M (2005) Strategies for ensuring good hydration in the elderly. Nutr Rev 63:S22–S29
Stachenfeld NS, DiPietro L, Nadel ER, Mack GW (1997) Mechanism of attenuated thirst in aging: role of central volume receptors. Am J Physiol 272:R148–R157
Phillips PA, Rolls BJ, Ledingham JG, Forsling ML, Morton JJ, Crowe MJ, Wollner L (1984) Reduced thirst after water deprivation in healthy elderly men. N Engl J Med 311:753–759
Maughan RJ, Watson P, Shirreffs SM (2015) Implications of active lifestyles and environmental factors for water needs and consequences of failure to meet those needs. Nutr Rev 73(Suppl 2):130–140
Manz F, Johner SA, Wentz A, Boeing H, Remer T (2012) Water balance throughout the adult life span in a German population. Br J Nutr 107:1673–1681
Sawka MN, Cheuvront SN, Carter R 3rd (2005) Human water needs. Nutr Rev 63:S30–S39
Armstrong LE, Johnson EC (2018) water intake, water balance, and the elusive daily water requirement. Nutrients 10(12):1928
Rosinger AY, Lawman HG, Akinbami LJ, Ogden CL (2016) The role of obesity in the relation between total water intake and urine osmolality in US adults, 2009–2012. Am J Clin Nutr 104(6):1554–1561
Laja García AI, Moráis-Moreno C, Samaniego-Vaesken ML, Puga AM et al (2019) Influence of water intake and balance on body composition in healthy young adults from Spain. Nutrients 11(8):1923
Amro OW, Paulus JK, Noubary F, Perrone RD (2016) Low-osmolar diet and adjusted water intake for vasopressin reduction in autosomal dominant polycystic kidney disease: a pilot randomized controlled trial. Am J Kidney Dis 68(6):882–891
Ecelbarger CA, Kim GH, Terris J, Masilamani S et al (2000) Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney. Am J Physiol Renal Physiol 279(1):F46-53
Bankir LT, Trinh-Trang-Tan MM (2000) Renal urea transporters. Direct and indirect regulation by vasopressin. Exp Physiol 85:243S-252S
Minami S, Hamano T, Iwatani H, Mizui M et al (2018) Tolvaptan promotes urinary excretion of sodium and urea: a retrospective cohort study. Clin Exp Nephrol 22(3):550–561
Imamura T, Kinugawa K, Minatsuki S, Muraoka H et al (2014) Urine sodium excretion after tolvaptan administration is dependent upon baseline serum sodium levels: a possible explanation for the improvement of hyponatremia with scarce chance of hypernatremia by a vasopressin receptor antagonist. Int Heart J 55(2):131–137
Al Therwani S, Rosenbæk JB, Mose FH, Bech JN, Pedersen EB (2017) Effect of tolvaptan on renal water and sodium excretion and blood pressure during nitric oxide inhibition: a dose-response study in healthy subjects. BMC Nephrol 18(1):86
Shoaf SE, Bricmont P, Mallikaarjun S (2014) Pharmacokinetics and pharmacodynamics of oral tolvaptan in patients with varying degrees of renal function. Kidney Int 85(4):953–961
Matsuzaki M, Hori M, Izumi T, Asanoi H, Tsutamoto T (2011) Effects of tolvaptan on volume overload in Japanese patients with heart failure: results of a phase II, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Cardiovasc Drugs Ther 25(Suppl 1):S19-31
Al Therwani S, Malmberg MES, Rosenbaek JB, Bech JN, Pedersen EB (2017) Effect of tolvaptan on renal handling of water and sodium, GFR and central hemodynamics in autosomal dominant polycystic kidney disease during inhibition of the nitric oxide system: a randomized, placebo-controlled, double blind, crossover study. BMC Nephrol 18(1):268
Kim SR, Hasunuma T, Sato O, Okada T et al (2011) Pharmacokinetics, pharmacodynamics and safety of tolvaptan, a novel, oral, selective nonpeptide AVP V2-receptor antagonist: results of single- and multiple-dose studies in healthy Japanese male volunteers. Cardiovasc Drugs Ther 25(Suppl 1):S5-17
Côté G, Asselin-Thompstone L, Mac-Way F, René de Cotret P et al (2020) Sodium and urea excretion as determinants of urine output in autosomal dominant polycystic kidney disease patients on V2 receptor antagonists: impact of dietary intervention. Int Urol Nephrol 52(2):343–349
Higashihara E, Nutahara K, Tanbo M et al (2014) Does increased water intake prevent disease progression in autosomal dominant polycystic kidney disease? Nephrol Dial Transplant 29(9):1710–1719
Imamura T, Kinugawa K, Minatsuki S, Muraoka H et al (2013) Urine osmolality estimated using urine urea nitrogen, sodium and creatinine can effectively predict response to tolvaptan in decompensated heart failure patients. Circ J 77(5):1208–1213
Iwatani H, Kawabata H, Sakaguchi Y, Yamamoto R et al (2015) Urine osmolarity predicts the body weight-reduction response to tolvaptan in chronic kidney disease patients: a retrospective, observational study. Nephron 130(1):8–12
Shimizu K, Doi K, Imamura T, Noiri E et al (2015) Ratio of urine and blood urea nitrogen concentration predicts the response of tolvaptan in congestive heart failure. Nephrology (Carlton) 20(6):405–412
Imamura T, Kinugawa K, Fujino T, Inaba T et al (2014) Increased urine aquaporin-2 relative to plasma arginine vasopressin is a novel marker of response to tolvaptan in patients with decompensated heart failure. Circ J 78(9):2240–2249
Kwon TH, Frokiaer J, Knepper MA, Nielsen S (1998) Reduced AQP1, 2, and 3 levels in kidneys of rats with CRF induced by surgical reduction in renal mass. Am J Physiol 275(5 Pt 2):F724–F741
Czarzasta K, Cudnoch-Jedrzejewska A, Niemczyk L et al (2018) Effect of chronic kidney disease on changes in vasopressin system expression in the kidney cortex in rats with nephrectomy. Biomed Res Int 2018:2607928
Shoaf SE, Chapman AB, Torres VE, Ouyang J, Czerwiec FS (2017) Pharmacokinetics and pharmacodynamics of tolvaptan in autosomal dominant polycystic kidney disease: phase 2 trials for dose selection in the pivotal phase 3 trial. J Clin Pharmacol 57(7):906–917
Bedford JJ, Leader JP, Walker RJ (2003) Aquaporin expression in normal human kidney and in renal disease. J Am Soc Nephrol 14(10):2581–2587
Shoaf SE, Wang Z, Bricmont P, Mallikaarjun S (2007) Pharmacokinetics, pharmacodynamics, and safety of tolvaptan, a nonpeptide AVP antagonist, during ascending single-dose studies in healthy subjects. J Clin Pharmacol 47(12):1498–1507
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Contributions
FJBU conceived the study. FJBU, AIMG, APM, FRO, EMG, and RER collected the data. FJBU analyzed the data. FJBU and RER wrote the manuscript. All authors provided critical feedback and helped shape the research. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The results presented in this article have not been published previously in whole or part, except in abstract format. F.J.B.U. has received lecture fees and travel support from Otsuka. R.E.R. has received grant funding for meeting organizations and travel support from Otsuka.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11255_2023_3555_MOESM1_ESM.pptx
Supplementary file1 Twenty-four hour-urine output in ADPKD patients treated with tolvaptan. Urine output increased significantly from baseline with each dose of tolvaptan, but with a high dispersion in individual response. (PPTX 61 KB)
11255_2023_3555_MOESM2_ESM.pptx
Supplementary file2 Twenty-four hour-urine output increased progressively after treatment with tolvaptan in ADPKD patients in all doses in both sexes. There were no differences in urine production between sex when considering each dose separately. (PPTX 61 KB)
11255_2023_3555_MOESM3_ESM.pptx
Supplementary file3 A. Urine output was midly correlated with daily osmolar load expressed as OsmU/CrU in 24 h-urine at baseline in ADPKD patients. B. After tolvaptan, urine output did not correlated with OsmU/CrU ratio. (PPTX 110 KB)
11255_2023_3555_MOESM4_ESM.pptx
Supplementary file4 A. Before treatment with tolvaptan, ADPKD patients showed a negatively correlation between 24 h-urine output and calculated urinary osmolality in 24 h-urine. B. After starting treatment with tolvaptan, we observed a poor correlation between both variables. (PPTX 106 KB)
11255_2023_3555_MOESM5_ESM.pptx
Supplementary file5 A. At baseline, 24-urine output was negatively correlated with morning urinary osmolality in ADPKD patients. B. After tolvaptan treatment, there was no correlation between both variables. (PPTX 98 KB)
11255_2023_3555_MOESM6_ESM.pptx
Supplementary file6 Predicted values of 24 h-urine output using equation built with linear mixed-effects modelling (see Table 2) for several values of sex, age, glomerular filtration rate, weight and doses of tolvaptan. (PPTX 53 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Utiel, F.J.B., García, A.I.M., Moyano, A.P. et al. Identifying the main predictors of urine output in autosomal-dominant polycystic kidney disease (ADPKD) patients taking tolvaptan. Int Urol Nephrol 55, 2629–2637 (2023). https://doi.org/10.1007/s11255-023-03555-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03555-8