Abstract
Introduction
Acute kidney injury (AKI) is one of the main complications of COVID-19 caused by SARS-CoV-2. This study aimed to evaluate the incidence of AKI in Brazilian hospitalized patients diagnosed with COVID-19 and identify the risk factors associated with its onset and those associated with its prognosis.
Methods
A prospective cohort study of hospitalized patients diagnosed with COVID-19 at a public and tertiary university hospital in São Paulo from March to December 2020.
Results
There were 347 patients hospitalized with COVID-19, 52.4% were admitted to the intensive care unit (ICU) and 47.6% were admitted to the wards. The overall incidence of AKI was 46.4%, more frequent in the ICU (68.1% vs 22.4, p < 0.01) and the overall mortality was 36.1%. Acute kidney replacement therapy was indicated in 46.6% of patients with AKI. In the general population, the factors associated with AKI were older age (OR 1.03, CI 1–1.05, p < 0.05), mechanical ventilation (OR 1.23, CI 1.06–1.83, p < 0.05), presence of proteinuria (OR 1.46, CI 1.22–1.93, p < 0.05), and use of vasoactive drugs (OR 1.26, CI 1.07–1.92, p < 0.05). Mortality was higher in the elderly (OR 1.08, CI 1.04–1.11, p < 0.05), in those with AKI (OR 1.12, CI 1.02–2.05, p < 0.05), particularly KDIGO stage 3 AKI (OR 1.10, CI 1.22–2.05, p < 0.05) and in need of mechanical ventilation (OR 1.13, CI 1.03–1.60, p < 0.05).
Conclusion
AKI was frequent in hospitalized patients with COVID-19 and the factors associated with its development were older age, mechanical ventilation, use of vasoactive drugs, and presence of proteinuria, being a risk factor for death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronavirus disease 2019 (COVID-19) caused by SARS-Cov-2 was declared a pandemic by the World Health Organization (WHO) in March 2020. The first Latin American case was reported on February 2020 in Brazil, and by June 2020, Latin America and The Caribbean became the worlds’ latest COVID-19 epicenter with the number of deaths in the region exceeding 7 million, or over 27% of the world’s COVID-19 deaths [1, 2].
One of the main complications of the infectious condition is acute kidney injury (AKI), a syndrome characterized by a rapid decrease in renal function, with consequent accumulation of nitrogenous wastes, hydro-electrolytic, and acid–base disorders [3].
Although there is ample literature on the association between respiratory failure and AKI, within the physiopathological model of cross-talk between the organs, there are still few studies that elucidate the renal repercussions caused by the new coronavirus, in view of its recent discovery.
Diffuse alveolar damage and acute respiratory failure are the main characteristics of the disease in its severe form; however, AKI is very frequent (4–37%) [4, 5], especially among critically ill patients, and is a factor related to worse outcomes.
Therefore, this study aimed to evaluate the incidence of AKI in Brazilian hospitalized patients diagnosed with COVID-19 and to identify both the risk factors associated with its onset and those associated with its prognosis.
Materials and methods
A prospective cohort study of hospitalized patients diagnosed with COVID-19, confirmed by real-time polymerase chain reaction (RT-PCR) for SARS-Cov-2, was performed in clinical wards and intensive care units (ICUs) of a public and tertiary university hospital in São Paulo, Brazil, in the period from March to December 2020. Patients were accompanied from their hospitalization until the clinical outcome (discharge or death), the AKI diagnosis was assessed, and their risk factors were identified through the collection of information in electronic medical records, those being associated with their diagnosis, death, and indication for acute kidney replacement therapy.
We collected clinical and laboratory data during hospitalization. Renal function was evaluated daily by measuring serum creatinine and checking urine output. AKI was identified according to KDIGO definition when occurred an increase in serum creatinine level > 0.3 mg/dL within 48 h or by 50% within 7 days and staged in the three KDIGO categories [6]. For the detection of proteinuria or hematuria, the semiquantitative dipstick test was used; data were requested at admission in all patients and during hospital stay in those patients without proteinuria at admission.
Indications for acute kidney replacememnt therapy were uraemia or azotaemia (BUN > 100 mg/dL), fluid overload (after diuretics use), electrolyte imbalance (K > 6.5 mEq/L after clinical treatment), acid–base disturbances (pH < 7.1 and bicarbonate < 10 mEq/L after clinical treatment), and metabolic and fluid emand to capacity imbalance as defined by Macedo et al. in 2011 and revised by Ostermann et al. in 2016 [7, 8]. The demand is determined by the severity of the acute illness and the solute and fluid burdens. The demand capacity balance is dynamic and varies with the course of critical illness. When renal capacity decreases and fails to cope with the demands, initiation of acute kidney replacememnt therapy was considered [9].
Patients with chronic kidney disease stages IV and V, kidney transplant patients, and individuals under 18 years old were excluded.
This study was registered in the Brazilian Registry of clinical trials (ReBEC) under number RBR-62y3h7 and was approved by the local ethics committee (CAAE 30451520.6.0000.5411 and report number 4.003.880). All the research was performed following current regulations, and written informed consent was obtained from all participants or their legal guardians.
From the study protocol, the data were entered into an electronic spreadsheet, and any typing errors were eliminated. The analysis was performed with the aid of IBM SPSS 20 or Sigma Stat 3.5. Frequency or central tendency and dispersion measures were calculated for the categorical or continuous variables, respectively, being AKI established as outcome variable. The Chi-square test was used for comparison of categorical variables and the t test for continuous variables.
Afterward, multivariate analysis was performed through the construction of a logistic regression model with calculations of the odds ratio (OR), including in the model all the independent variables that showed association with the outcome, with p ≤ 0.20. A similar procedure was performed by establishing the occurrence of death and the indication of acute renal replacement therapy as a dependent variable.
Results
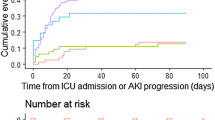
During 2020, 347 patients were hospitalized at our HC with confirmed diagnosis of COVID-19. The mean age was 59.8 ± 16.1, with a predominance of males (57.6%), the majority being hypertensive (60.5%), 52.4% admitted to the intensive care unit (ICU), and 47.6% admitted to the wards. The overall incidence of AKI was 46.4%, more frequent among patients admitted to ICU than in those in wards (68.1% vs 22.4, p < 0.01). The mean time for the AKI diagnosis was 6 days and KDIGO stage 3 AKI was the most frequent (53.4%). Upon hospital admission, 235 patients underwent Urine I examination, of which 31.1% had proteinuria (43.5% of AKI patients) and 36.9% had hematuria (49.1% of AKI patients). Acute renal replacement therapy was indicated for 75 patients (46.6% of AKI patients).
The factors associated with the development of AKI were mechanical ventilation (11.3 vs 72%, p < 0.0001), use of vasoactive drugs (VAD, 12.9 vs 72%, p < 0.0001), and ICU admission (31.2 vs 77%, p < 0.0001). Based on logistic regression analysis, mechanical ventilation (OR 1.23, CI 1.06–1.83, p < 0.05), presence of proteinuria (OR 1.46, CI 1.22–1.93, p < 0.05), VAD use (OR 1.26, CI 1.07–1.92, p < 0.05), and older age (OR 1.03, CI 1–1.05 p < 0.05) were AKI risk factors. Tables 1 and 2 show, respectively, the factors associated with AKI in the univariate and multivariate analysis.
The overall mortality was 36.1% (125 patients), being higher in ICU patients (32.9 vs 87.2%, p < 0.0001). The factors associated with mortality were ICU admission (32.9 vs 87.2%, p < 0.0001), the need for mechanical ventilation (14.9 vs 83.2%, p < 0.0001), the use of VAD (16.2 vs 83.2%, p < 0.0001), and the presence of AKI (26.6 vs 81.6%, p < 0.0001), mainly KDIGO stage 3 AKI (9 vs 52.8%, p < 0.0001), as identified in Table 3.
Based on logistic regression analysis, the presence of AKI (OR 1.12, CI 1.02–2.05, p < 0.05), mechanical ventilation (OR 1.13, CI 1.03–1.60, p < 0.05), and KDIGO stage 3 AKI (OR 1.10, CI 1.22–2.05, p < 0.05) remained as variables associated with death, with the addition of higher age (OR 1.08, CI 1.04–1.11, p < 0.05), as shown in Table 4.
When analyzing only patients admitted to intensive care beds, AKI occurred in 68.3% of patients and the overall mortality was 61.1%. The mean time for the diagnosis of AKI was 5 days and KDIGO stage 3 AKI was the most frequent (57.7%). Acute renal replacement therapy was indicated for 41.6% of patients who were under intensive care.
The factors associated with the development of AKI in ICU patients, as identified in Table 5, were the need for mechanical ventilation (36.8 vs 91.1%, p < 0.001), use of VAD (40.4 vs 89.4%, p < 0.001), presence of CKD (3.5 vs 13%, p = 0.048), hematuria (33.3 vs 52.8%, p = 0.006), proteinuria (28.1 vs 45.5%, p = 0.022), increased baseline creatinine dosages (0.72 ± 0.26 vs 0.92 ± 0.44, p = 0.009), and D-dimer (5723.3 ± 5013.4 vs 10,249.8 ± 5905, p < 0.001), as well as APACHE II and SOFA scores (14.6 ± 6.4 vs 22.3 ± 6, p < 0.001 and 5.9 ± 3.9 vs 9.6 ± 3.6, p < 0.001, respectively). CPK dosage was also associated with AKI (168 (69.25–688)).
In the logistic regression (Table 6), the risk factors for AKI were the need for mechanical ventilation (OR 1.1, CI 1.02–1.4, p < 0.05), older age (OR 1.06, CI 1–1.11, p < 0.05), and the presence of proteinuria (OR 1.2, CI 1.06–1.7, p < 0.05).
The mortality of ICU patients was 61.1%, being higher in older patients (56.4 ± 14 vs 64.4 ± 13. 7, p < 0.001), in those requiring mechanical ventilation (47.1 vs 90.9%, p < 0.001), use of VAD (47.1 vs 90.9%, p < 0.001), presence of CKD (5.7 vs 12.7%, p = 0.12), presence of AKI (38.6 vs 87.3%, p < 0.001), mainly KDIGO stage 3 AKI (11.4 vs 57.3%, p < 0.001), undergoing acute renal replacement therapy (12.9 vs 60, p < 0.001) and with higher APACHE II and SOFA scores (15. 5 ± 6.7 vs 23.2 ± 5.4, p < 0.001 and 6.2 ± 3.6 vs 10.1 ± 3.5, p < 0.001, respectively), as identified in Table 7.
At logistic regression analysis, the variables associated with death were older age (OR 1, CI 1.03–1.1, p < 0.0001), VAD use (OR 1.15, CI 1.05–1.43, p < 0.0001), need for acute renal replacement therapy (OR 1.26, CI 1.09–1.72, p = 0.01), and presence of AKI (OR 3.4, CI 1.35–8.6, p = 0.01), as shown in Table 8.
Discussion
This study describes the first wave of the COVID-19 pandemic in a public university hospital in the inland of São Paulo, Brazil, which is a reference for 28 municipalities in the region with more than 2 million inhabitants. During this period, 347 patients diagnosed with COVID-19 were hospitalized, with a mean age of 59.8 ± 16.1, 52.4% admitted to the ICU and 47.6% admitted to the ward. The overall incidence of AKI was 46.4% with a mean time for diagnosis of 6 days. This incidence of AKI is higher than that reported so far in the literature.
Chinese studies [10,11,12,13,14,15,16,17] have reported a low and variable AKI incidence (0.5–7%), higher in severe COVID-19 cases (2.9–19%). European and North American studies [18,19,20] have reported an AKI incidence of 20–40% of patients hospitalized with COVID-19. In all cohorts, AKI occurred between the 7th and the 14th day of illness; it was associated with higher hospital mortality and decisive in the prognosis of these patients [9,10,11,12,13,14,15,16,17,18,19,20,21]. Brazil is a large country with several vulnerable groups, in addition to an emerging economy and fragile social protection, which may have contributed to more demand for health services and increased development of serious forms of COVID-19 [23].
SARS-CoV-2 has a protein, called Spike (S), which binds to the ACE2 receptor present in host cells, enabling its activation and cleavage by transmembrane proteases and culminating in the release of fusion peptides by the virus. ACE2 is highly expressed in the mouth and tongue, in addition to alveolar epithelial cells. In the kidneys, it is highly expressed in proximal tubule cells and to a lesser extent in podocytes [13, 22,23,24]. Thus, the higher AKI incidence in western countries could be associated with the higher expression of angiotensin-converting enzyme 2 (ACE2) in podocytes and proximal tubule in western individuals compared with eastern individuals, as identified in normal kidneys and described by Pan et al. [22]. However, other studies did not find the SARS-CoV-2 virus in the renal biopsy/autopsy issue samples [25, 26].
AKI is a complex disorder characterized by the degradation of renal function over a period of hours to days, resulting in a temporary decrease or interruption of the renal capacity to promote the excretion of nitrogenous products and the hydro-electrolytic homeostasis of the body, also resulting in volume overload [27, 28]. Its incidence in hospitalized patients varies between 5 and 7%; it is higher in ICU patients, around 50%, according to other studies. Despite the technological advances that have occurred and the reduction in the mortality rate in the last decade, the AKI prognosis remains severe and the death rate remains high, especially in patients in need of dialysis (up to 62%) [29,30,31].
In logistic regression, we identified that the factors associated with the development of AKI in patients hospitalized with COVID-19 were mechanical ventilation, use of VAD, proteinuria, and older age. In the case of ICU patients with COVID-19, the factors associated with the development of AKI were mechanical ventilation, proteinuria, and older age. In this last regression, we removed the APACHE and SOFA scores because of collinearity.
In a Brazilian study, Bucuvic et al. [33] showed that 62% of patients diagnosed with AKI were male, 65.2% were elderly, diabetes mellitus occurred in 61.9%, hypertension in 44.4%, and CKD in 21.9%. Ponce et al. [34] performed a large retrospective observational study that looked into the epidemiology of AKI and its effect on patient outcomes across time periods. For comparison purposes, patients were divided into two groups according to the year of follow up: 2011–2014 and 2015–2018. The authors evaluated 5,428 AKI patients. The AKI stage 3 was 3 (50.6%), and there was a mortality rate of 34.3% (1,865 patients). Dialysis treatment was indicated in 928 patients (17.1%). Patient survival improved along the study periods, and patients treated in 2015–2018 had a relative risk death reduction of 0.89 (95% CI 0.81–0.98, p = 0.02). The independent risk factors for mortality were sepsis, > 65 years of age, admission to the ICU, AKI-KDIGO 3, recurrent AKI, no metabolic and fluid demand to capacity imbalance (as a dialysis indication), and the period of treatment.
We already know from the non-COVID-19 literature that AKI is associated with worse clinical outcomes. An international multicenter study from 2015 [35] with 1,032 ICU patients showed that AKI was independently associated with higher mortality at all stages, with the following odds ratios: 1.7 for KDIGO stage 1 and 6.9 for KDIGO stage 3. In ICU patients, AKI is also associated with longer duration of mechanical ventilation, use of vasoactive drugs, and increased length of hospital stay, with acute renal replacement therapy being necessary in 50% of cases.
The data presented by our study identified an overall mortality of 36.1%, being higher in ICU patients (32.9 vs 87.2%, p < 0.0001). The factors associated with mortality were the presence of AKI, mainly KDIGO stage 3, older age, use of VAD, and mechanical ventilation. Mortality was 61.1% in ICU patients, higher in individuals with older age, in VAD use, presence of AKI, and need for dialysis.
Many factors contribute to the maintenance of high mortality of AKI in COVID-19 patients, especially the lack of identification of risk factors for the development of this pathology, as well as the lack of knowledge about factors associated with mortality.
Still, it is important to emphasize that mechanical ventilation, mainly associated with renal and/or pulmonary involvement, may predispose hospital infections that contribute to a higher mortality of these patients. When intubated patients are under mechanical ventilation, the lung defense mechanisms are altered by the underlying disease or by the loss of protection of the upper airways, such as the loss of an intact cough reflex, which may result in pulmonary hypersecretion or an increase in the frequency of respiratory infections, with a high morbidity and mortality rate..
Upon hospital admission, 235 patients underwent Urine I, of whom 36.9% had hematuria (49.1% of AKI patients) and 31.1% had proteinuria (43.5% of AKI patients). Data published to date [8,9,10,11,12,13,14,15,16,17,18,19,20] reported that, at COVID-19 diagnosis, 27–64% of the patients presented hematuria and/or proteinuria.
It is worth mentioning that proteinuria, as highlighted in our results, showed to be an important risk factor associated with the development of AKI, having as main pathophysiological basis the hypothesis of direct damage of SARS-Cov-2 in tubular epithelial cells and renal podocytes. Therefore, proteinuria in patients with COVID-19 may be associated with the viral cytopathic effects, which reduces filtration and protein reabsorption, as well as results in tubular injury; or even have a glomerular origin, especially in those patients who develop acute glomerulopathies [5].
This study has some limitations: data were collected from a single center, by means of electronic medical records and have included patients only during the first pandemic wave in the country. However, this study represents an important datapoint on the epidemiological profile of AKI associated with COVID-19 in Brazil, a country with continental characteristics and a heterogeneous and vulnerable population.
Conclusion
Thus, the analysis of our results allows us to conclude that AKI associated with severe COVID-19 is more frequent than the already published data from the Chinese, European, and North American cohorts, and the risk factors associated with the development of AKI are the older age, presence of proteinuria, use of mechanical ventilation, and VAD. Mortality is high in this population, being higher in elderly patients with greater clinical severity, especially in those requiring dialysis and who develop KDIGO stage 3 AKI.
More and larger studies are needed to determine the etiology of AKI in patients with COVID-19, as well as the factors associated with its development and prognosis. The second wave occurred in the country from December 2020 to July 2021 and was even more devastating in both incidence and severity, despite the onset of vaccination. Studies comparing the incidence of AKI during both waves, as well as the risk factors for its development and its impact on patient survival are needed.
Data availability
The authors declare the data may be available.
References
Burki T (2020) COVID-19 in Latin America. Lancet Infect Dis 20:547–578. https://doi.org/10.1016/S1473-099(20)30303-0
Dyer O (2020) COVID-19 hot spot appear across Latin America. BMJ 369:m2182. https://doi.org/10.1136/bmj.m2182
Singer M, Deutschman CS, Seymour CW et al (2016) The third international consensus definitions for sepsis and septic shock (Sepsis3). JAMA 315(8):801–810. https://doi.org/10.1001/jama.2016.028
Ronco C, Reis T, Husain-Syed F (2020) Management of acute kidney injury in patients with COVID-19. Lancet Respir Med 8:738–742. https://doi.org/10.1016/S2213-2600(20)30229-0
Pecly IMD et al (2021) Uma revisão da Covid-19 e lesão renal aguda: da fisiopatologia aos resultados clínicos. Braz J Nephrol (BJN) 43(4):551–571. https://doi.org/10.1590/2175-8239-JBN-2020-0204
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138. https://doi.org/10.1038/kisup.2012.1
Macedo E, Mehta RL (2011) When should renal replacement therapy be initiated for acute kidney injury. Semin Dial 24:132–137. https://doi.org/10.1111/j.1525-139X.2011.00838.x
Ostermann M, Joannidis M, Pani A, Floris M, De Rosa S, Kellum JA, Ronco C (2016) Patient selection and timing of continuous renal replacement therapy. Blood Purif 42:224–237. https://doi.org/10.1159/000448506
Annigeri RA, Ostermann M, Tolwani A, Vazquez-Rangel A, Ponce D, Bagga A, Chakravarthi R, Mehta RL (2017) Renal support for acute kidney injury in the developing world. Kidney Int Rep 2:559–578. https://doi.org/10.1016/j.ekir.2017.04.006
Li R, Pei S, Chen B, Song Y, Zhang T, Yang W et al (2020) Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2). Science 368:489–493
Ghinai I, McPherson TD, Hunter JC, Kirking HL, Christiansen D, Joshi K, Illinois COVID-19 Investigation Team et al (2020) First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet 395:1137–1144
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J et al (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323:1061
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S et al (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 180:934–943
Cheng Y, Luo R, Wang K et al (2020) Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. https://doi.org/10.1016/j.kint.2020.03.005
Zhou F, Yu T, Du R et al (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395:1054–1062. https://doi.org/10.1016/S01406736(20)30566-3
Chen N, Zhou M, Dong X et al (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395:507–513. https://doi.org/10.1016/S0140-6736(20)30211-7
Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Cao M, Zhang D, Wang O et al (2020) Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in Shanghai, China. medRxiv preprint. https://doi.org/10.1101/2020.03.04.20030395
Arentz M, Yim E, Klaff L et al (2020) Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 323(16):1612–1614. https://doi.org/10.1001/jama.2020.4326
Cummings MJ, Baldwin MR, Abrams D et al (2020) Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 395:1763–1770. https://doi.org/10.1016/S0140-6736(20)31189-2
Hirsch JS, Ng JH, Ross DW et al (2020) Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98:209–218. https://doi.org/10.1016/j.kint.2020.05.006
Pan XW, Xu D, Zhang H, Zhou W, Wang LH, Cui XG (2020) Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med 46(6):1114–1116. https://doi.org/10.1007/s00134-020-06026-1
Perico L, Benigni A, Remuzzi G (2020) Should COVID-19 Concern Nephrologists? Why and to what extent? The emerging impasse of angiotensin blockade. Nephron. https://doi.org/10.1159/000507305
Ponce D (2020) The impact of coronavirus in Brazil: politics and the pandemic. Nat Rev Nephrol 16:483. https://doi.org/10.1038/s41581-020-0327-0
Kudose S, Batal I, Santoriello D, Xu K, Barasch J, Peleg Y et al (2020) Achados de biópsia renal em pacientes com COVID-19. JASN 31:1959–1968. https://doi.org/10.1681/ASN.2020060802
Santoriello D, Khairallah P, Bomback AS, Xu K, Kudose S, Batal I et al (2020) Achados de patologia renal pós-morte em pacientes com COVID-19. JASN 31:2158–2167. https://doi.org/10.1681/ASN.2020050744
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG et al (2007) Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11(2):R31. https://doi.org/10.1186/cc5713
Chang CH, Fan PC, Chang MY, Tian YC, Hung CC, Fang JT et al (2014) Acute kidney injury enhances outcome prediction ability of sequential organ failure assessment score in critically ill patients. PloS One 9(10):e109649. https://doi.org/10.1371/journal.pone.0109649
Caires RA, Abdulkader RC, Costa e Silva VT, Ferreira GS, Burdmann EA, Yu L et al (2016) Sustained low-efficiency extended dialysis (SLED) with single-pass batch system in critically-ill patients with acute kidney injury (AKI). J Nephrol 29(3):401–409. https://doi.org/10.1007/s40620-015-0224-yy
Ikizler TA, Himmelfarb J (2007) Acute kidney injury: changing lexicography, definitions, and epidemiology. Kidney Int 71:971–976. https://doi.org/10.1038/sj.ki.5002224
Bellomo R, Ronco C, Mehta RL, Asfar P, Boisrame-Helms J, Darmon M et al (2017) Acute Kidney Injury in the ICU: from injury to recovery: reports from the 5th Paris International Conference. Ann Intensive Care. https://doi.org/10.1186/s13613-017-0260-y
Bellomo R, Kellum JA, Ronco C, Wald R, Martensson J, Maiden M et al (2017) acute kidney injury in sepsis. Intensive Care Med 43:816–828. https://doi.org/10.1007/s00134-017-4755-7
Bucuvic EM, Ponce D, Balbi AL (2011) Fatores de risco para mortalidade na lesão renal aguda. Rev Assoc Med Bras 57(2):158–163. https://doi.org/10.1590/S0104-42302011000200012
Ponce D, Zamoner W, Batistoco MM et al (2020) Changing epidemiology and outcomes of acute kidney injury in Brazilian patients: a retrospective study from a teaching hospital. Int Urol Nephrol 52:1915–1922. https://doi.org/10.1007/s11255-020-02512-z
Hoste EA, Bagshaw SM, Bellomo R et al (2015) Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 41(8):1411–1423. https://doi.org/10.1007/s00134-015-3934-7. (Epub 2015 Jul 11)
Funding
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) [grant number 2020/11766-8].
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to conception and design, acquisition of data, and/or analysis and interpretation of data, took part in drafting the article or revising it critically for important intellectual content, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. LEM: funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Magalhães, L.E., de Oliveira, P.G.S., Favarin, A.J. et al. Acute kidney injury in coronavirus infectious disease: a study of incidence, risk factors, and prognosis during the first wave of the disease in Brazil. Int Urol Nephrol 55, 1501–1508 (2023). https://doi.org/10.1007/s11255-022-03454-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03454-4