Abstract
Purpose
Benign prostatic hyperplasia (BPH) is a urogenital disorder that affects approximately 85% of males who are over 50 years of age. Hydrocotyle ramiflora (HR), belonging to Apiaceae family, is used to treat urinary system diseases such as urine retention in traditional Chinese herbal medicine. In this study, we evaluated the effects of HR in the BPH animal model.
Methods
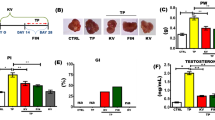
We induced BPH in rats via subcutaneous (sc) injections of testosterone propionate (TP, 3 mg/kg). Rats were also administered HR (150 mg/kg), finasteride (10 mg/kg), or vehicle via oral gavage. After induction, prostate glands were collected, weighed, and processed for further analysis, including histopathological examination and immunohistochemistry. In addition, the mRNA expression of inflammatory cytokines in prostatic tissues was determined by quantitative real-time PCR (qRT-PCR). The protein expression of pro-apoptotic markers was examined using western blotting.
Results
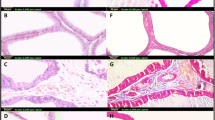
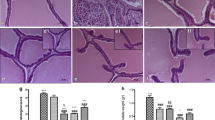
HR treatment significantly reduced the prostate weight, epithelial thickness, and proliferating cell nuclear antigen (PCNA) expression, with the levels of cleaved caspase-3 and Bcl-2-associated X (Bax) protein considerably increased compared to BPH group. HR also decreased inflammatory cell infiltration and pro-inflammatory cytokine levels compared with BPH group. Furthermore, the expression of phosphor-nuclear factor-κB (NF-κB), cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS) were reduced by HR treatment.
Conclusion
These results indicate that HR suppresses the development of BPH associated with anti-proliferative, pro-apoptotic, and anti-inflammatory effects, suggesting it is a potential alternative therapeutic agent for BPH.







Similar content being viewed by others
Data availability statement
Not applicable.
References
Minutoli L et al (2016) Apoptotic pathways linked to endocrine system as potential therapeutic targets for benign prostatic hyperplasia. Int J Mol Sci 17:1311. https://doi.org/10.3390/ijms17081311
Abdelwahab O et al (2012) Evaluation of the resistive index of prostatic blood flow in benign prostatic hyperplasia. Int Braz J Urol 38:250–257. https://doi.org/10.1590/S1677-55382012000200014
Patel ND, Parsons JK (2014) Epidemiology and etiology of benign prostatic hyperplasia and bladder outlet obstruction. Indian J Urol 30:170–176. https://doi.org/10.4103/0970-1591.126900
Ub Wijerathne C et al (2017) Quisqualis indica improves benign prostatic hyperplasia by regulating prostate cell proliferation and apoptosis. Biol Pharm Bull 40:2125–2133. https://doi.org/10.1248/bpb.b17-00468
Izumi K et al (2013) Androgen receptor roles in the development of benign prostate hyperplasia. Am J Pathol 182:1942–1949. https://doi.org/10.1016/j.ajpath.2013.02.028
Andriole G et al (2004) Dihydrotestosterone and the prostate: the scientific rationale for 5alpha-reductase inhibitors in the treatment of benign prostatic hyperplasia. J Urol 172:1399–1403. https://doi.org/10.1097/01.ju.0000139539.94828.29
Ishimaru T, Pages L, Horton R (1977) Altered metabolism of androgens in elderly men with benign prostatic hyperplasia. J Clin Endocrinol Metab 45:695–701. https://doi.org/10.1210/jcem-45-4-695
Feldman HA et al (2002) Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 87:589–598. https://doi.org/10.1210/jcem.87.2.8201
Liao CH et al (2012) Significant association between serum dihydrotestosterone level and prostate volume among Taiwanese men aged 40–79 years. Aging Male 15:28–33. https://doi.org/10.3109/13685538.2010.550660
Gandaglia G et al (2013) The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH). BJU Int 112:432–441. https://doi.org/10.1111/bju.12118
Bostanci Y et al (2013) Correlation between benign prostatic hyperplasia and inflammation. Curr Opin Urol 23:5–10. https://doi.org/10.1097/MOU.0b013e32835abd4a
Ren H et al (2015) The effects of ROS in prostatic stromal cells under hypoxic environment. Aging Male 18:84–88. https://doi.org/10.3109/13685538.2015.1018159
Verze P, Cai T, Lorenzetti S (2016) The role of the prostate in male fertility, health and disease. Nat Rev Urol 13:379–386. https://doi.org/10.1038/nrurol.2016.89
Huang SS et al (2008) Antioxidant and antiproliferative activities of the four Hydrocotyle species from Taiwan. Bot Stud 49:311–322
Kwon HC, Zee OP, Lee KR (1998) Two new monogalactosylacylglycerols from Hydrocotyle ramiflora. Planta Med 64:477–479. https://doi.org/10.1055/s-2006-957491
Yang JS et al (2007) Effect of alpha-spinasterol extracted from Phytolacca americanna on the apoptosis of U937 cell line. J Physiol Pathol Korean Med 21:1108–1117
Jeong SI et al (2004) Alpha-spinasterol isolated from the root of Phytolacca americana and its pharmacological property on diabetic nephropathy. Planta Med 70:736–739. https://doi.org/10.1055/s-2004-827204
Borges FR et al (2014) Anti-inflammatory action of hydroalcoholic extract, dichloromethane fraction and steroid α-spinasterol from Polygala sabulosa in LPS-induced peritonitis in mice. J Ethnopharmacol 151:144–150. https://doi.org/10.1016/j.jep.2013.10.009
Bailey JA, Vincent GG, Burden RS (1976) The antifungal activity of glutinosone and capsidiol and their accumulation in virus-infected tobacco species. Physiol Plant Pathol 8:35–41. https://doi.org/10.1016/0048-4059(76)90005-9
Yang EJ, Song KS (2021) The ameliorative effects of capsidiol isolated from elicited Capsicum annuum on mouse splenocyte immune responses and neuroinflammation. Phytother Res 35:1597–1608. https://doi.org/10.1002/ptr.6927
Kim YJ et al (2021) Inhibitory effects of Gyeji-tang on MMP-9 activity and the expression of adhesion molecules in IL-4- and TNF-α-stimulated BEAS-2B cells. Plants (Basel) 10:951. https://doi.org/10.3390/plants10050951
Kumari S et al (2016) Rapid screening and identification of phenolic antioxidants in Hydrocotyle sibthorpioides Lam. by UPLC–ESI-MS/MS. Food Chem 203:521–529. https://doi.org/10.1016/j.foodchem.2016.02.101
Hoke GP, McWilliams GW (2008) Epidemiology of benign prostatic hyperplasia and comorbidities in racial and ethnic minority populations. Am J Med 121:S3–S10. https://doi.org/10.1016/j.amjmed.2008.05.021
Wilson EM, French FS (1976) Binding properties of androgen receptors. Evidence for identical receptors in rat testis, epididymis, and prostate. J Biol Chem 251:5620–5629. https://doi.org/10.1016/S0021-9258(17)33103-4
Mcconnell JD (1991) The pathophysiology of benign prostatic hyperplasia. J Androl 12:356–363. https://doi.org/10.1002/j.1939-4640.1991.tb00272.x
Roehrborn CG (2008) Pathology of benign prostatic hyperplasia. Int J Impot Res 20:S11–S18. https://doi.org/10.1038/ijir.2008.55
Azzouni F et al (2012) The 5 alpha-reductase isozyme family: a review of basic biology and their role in human diseases. Adv Urol 2012:530121. https://doi.org/10.1155/2012/530121
La Vignera S et al (2016) Endocrine control of benign prostatic hyperplasia. Andrology 4:404–411. https://doi.org/10.1111/andr.12186
Kyprianou N, Tu H, Jacobs SC (1996) Apoptotic versus proliferative activities in human benign prostatic hyperplasia. Hum Pathol 27:668–675. https://doi.org/10.1016/S0046-8177(96)90396-2
Schönenberger F et al (2015) Discrimination of cell cycle phases in PCNA-immunolabeled cells. BMC Bioinformatics 16:180. https://doi.org/10.1186/s12859-015-0618-9
Barman J et al (2018) Apoptosis: mediator molecules, interplay with other cell death processes and therapeutic potentials. Curr Pharm Biotechnol 19:644–663. https://doi.org/10.2174/1389201019666180821093239
Ribal MJ (2013) The link between benign prostatic hyperplasia and inflammation. Eur Urol Suppl 12:103–109. https://doi.org/10.1016/j.eursup.2013.08.001
Wang L et al (2008) Chronic inflammation in benign prostatic hyperplasia: implications for therapy. Med Hypotheses 70:1021–1023. https://doi.org/10.1016/j.mehy.2007.08.022
McLaren ID, Jerde TJ, Bushman W (2011) Role of interleukins, IGF and stem cells in BPH. Differentiation 82:237–243. https://doi.org/10.1016/j.diff.2011.06.001
Yang X et al (2014) Abacopteris penangiana exerts testosterone-induced benign prostatic hyperplasia protective effect through regulating inflammatory responses, reducing oxidative stress and anti-proliferative. J Ethnopharmacol 157:105–113. https://doi.org/10.1016/j.jep.2014.09.025
De Nunzio C, Presicce F, Tubaro A (2016) Inflammatory mediators in the development and progression of benign prostatic hyperplasia. Nat Rev Urol 13:613–626. https://doi.org/10.1038/nrurol.2016.168
Chughtai B et al (2011) Role of inflammation in benign prostatic hyperplasia. Rev Urol 13:147–150
Baltaci S et al (2001) Inducible nitric oxide synthase expression in benign prostatic hyperplasia, low-and high-grade prostatic intraepithelial neoplasia and prostatic carcinoma. BJU Int 88:100–103. https://doi.org/10.1046/j.1464-410x.2001.02231.x
Wang W, Bergh A, Damber JE (2004) Chronic inflammation in benign prostate hyperplasia is associated with focal upregulation of cyclooxygenase-2, Bcl-2, and cell proliferation in the glandular epithelium. Prostate 61:60–72. https://doi.org/10.1002/pros.20061
Nakanishi Y et al (2001) Inhibitors of cyclooxygenase-2 (COX-2) suppressed the proliferation and differentiation of human leukaemia cell lines. Eur J Cancer 37:1570–1578. https://doi.org/10.1016/S0959-8049(01)00160-5
Altavilla D et al (2012) Effects of flavocoxid, a dual inhibitor of COX and 5-lipoxygenase enzymes, on benign prostatic hyperplasia. Br J Pharmacol 167:95–108. https://doi.org/10.1111/j.1476-5381.2012.01969.x
Inouye BM et al (2018) The emerging role of inflammasomes as central mediators in inflammatory bladder pathology. Curr Urol 11:57–72. https://doi.org/10.1159/000447196
Jiang MY et al (2017) Mitochondrion-associated protein peroxiredoxin 3 promotes benign prostatic hyperplasia through autophagy suppression and pyroptosis activation. Oncotarget 8:80295–80302. https://doi.org/10.18632/oncotarget.17927
Funding
This work was supported by research fund of Chungnam National University.
Author information
Authors and Affiliations
Contributions
Conceptualization: SP, Y-SW, and H-JK. Preparation and analysis of extracts: Y-HH. Data curation: SP, E-BB, and E-JH. Investigation: SP, Y-HH, Y-SW, and H-JK. Writing—original draft preparation: SP, Y-HH, and Y-SW. Writing—review and editing: SP, Y-SW, and H-JK. Supervision: Y-SW and H-JK. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
All protocols for the studies were approved by the Animal Experimental Ethics Committee of Chungnam National University (Daejeon, South Korea; Approval No. CNU-00711).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, S., Hwang, YH., Baek, EB. et al. Inhibitory effects of Hydrocotyle ramiflora on testosterone-induced benign prostatic hyperplasia in rats. Int Urol Nephrol 55, 17–28 (2023). https://doi.org/10.1007/s11255-022-03362-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03362-7