Abstract
Background
Hydrophilic coated catheters are recommended to reduce the side effects of intermittent catheterization (IC) in patients with bladder dysfunction. However, there is lack of Level one evidence to support the use of this intervention.
Search methods
Several electronic databases were systematically searched to evaluate complication incidences for hydrophilic coated (HC) and non-hydrophilic catheters (NHC).
Results
Twelve studies were eligible for inclusion in the review. The meta-analyses exploring microscopic hematuria frequencies (RR = 0.69; 95% CI 0.52–0.90) and urethral stricture frequencies (RR = 0.28; 95% CI 0.13–0.60) showed a lower risk ratio associated with HC in comparison to NHC, whereas gross hematuria was no statistically significant difference in two groups. Subgroup analyses of gross hematuria which was grouped according to "catheterization frequency", "single/multiple catheterization" and "self/other catheterization” were performed and the values of combined RR were also no statistically significant difference.
Conclusions
Compared with non-hydrophilic catheters, the hydrophilic coated catheters have positive significance in reducing the incidence of urethral microtrauma and the urethral stricture. However, more studies are warranted for evaluating effects of hydrophilic coated catheters on the incidence of gross hematuria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Causes of bladder dysfunction are neurogenic or non-neurogenic. Neurogenic bladder dysfunction is often secondary to spinal cord injury and central nervous system disease (multiple sclerosis or spina bifida), of which complications often manifest as urinary tract infections (UTI), urinary incontinence and upper urinary tract lesion [1]. Common non-neurogenic bladder dysfunction includes outlet obstruction, such as benign prostatic hyperplasia and postoperative urinary retention, which probably leads to vesicoureteral reflux. Bladder dysfunction hinders urine discharge, increases pressure in bladder, eventually causes urinary retention, which aggravates the risk of renal failure [2]. The treatment of bladder dysfunction is aimed at alleviating urinary incontinence, protecting the upper urinary tract, and improving bladder function as well as patients' quality of life.
Intermittent catheterization (IC) is a preferred treatment for patients with significant urination problems [3] which is used in 56% spinal cord injury patients for bladder management in the United States [4]. IC makes the bladder store a reasonable amount of urine at low pressure and empty it at appropriate intervals, which simulates physiological urinary function. Thereby, IC prevents overdistention and decreases pressure of bladder [5], improves blood circulation in bladder wall [6], reduces the incidence of urinary retention, and ultimately prevents deterioration of upper urinary tract [7].
However, there are non-negligible side effects of IC, such as inducible urethral trauma, microtrauma, urethral stricture, bladder stone and false passages formation [8,9,10]. In recent years, several types of conduits are gradually available for IC to solve these disadvantages, including especially gel pre-lubricated polyvinyl chloride (external lubricant at most) and hydrophilic-coated catheter (polyvinylpyrrolidone coated at most) [10]. Compared with gel pre-lubricated polyvinyl chloride, HC is increasingly used to reduce intubation friction, urethral injury and urethral adhesion due to its special hydrophilic lubrication characteristics and non-sensitization [11].
Three previously published meta-analyses investigated the effects of HC and non-hydrophilic catheters (NHC) on urethral bleeding morbidity in IC patients [3, 12, 13], however, the results were contradictory. In addition, these studies provide few reliable evidence of urethral microtrauma and urethral stricture which are also important outcomes in the early and late stages of IC, respectively, except for gross hematuria. Consequently, the aim of our study is to evaluate whether HC improves the direct adverse effects compared with NHC, especially in urethral trauma, microtrauma, urethral stricture and rare adverse events.
Materials and methods
Inclusion/exclusion criteria
Population Studies considering adults (over 18 years old), adolescents (12–18 years old) and children (less than 12 years old) population with bladder dysfunction requiring IC.
Intervention Hydrophilic catheters—single-use.
Control Non-hydrophilic catheters—single-use or multiple-use.
Outcomes Gross hematuria, urethral microtrauma (microscopic hematuria), urethral stricture, false passages, bladder stone.
Study Randomized controlled trials, controlled before-and-after study, prospective cohort studies and cross-over trials.
Availability English; full text.
Data sources
We searched the following electronic databases to identify studies: Embase, PubMed, The Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science, Medline, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), British Nursing Index and three Chinese databases (The CNKI, Wan Fang Database and the VIP). The database has been established until December 31, 2021 and the search has been carried out by combining subject words with free words. English search terms include: 1. hydrophilic urethral catheters, hydrophilic-Coated Catheters, hydrophilic coated catheter. 2. Self-lubricated urethral catheters, pre-lubricated catheter, ultra-slippery, aqueous lubrication, surface wettability and lubrication, lubricant, aqueous lubrication, hydrogel coatings hydrogels, aqueous. 3. Reducing friction. 4. Urethra trauma, urethral micro trauma, urinary tract trauma, urethral epithelial micro-trauma. 5. Long-term follow-up study, long-term follow-up, reduce treatment-related complications, adverse events, false passages, urethral stricture, bladder stone. At the same time, the references of the included literatures have been manually retrieved to supplement the relevant literatures.
Literature screening
Two evaluators read the obtained literature independently. After excluding the trials that clearly did not meet the inclusion criteria, the full text of the trials that might meet the inclusion criteria was read to determine whether they really met the inclusion criteria. After the cross-check, if there is a disagreement, a third party will assist in adjudication. Data extraction was performed using standardized forms of the Cochrane Collaboration. The extracted contents include: ① basic information of the included study, ② baseline characteristics included in the study, ③ specific details of the intervention including catheter material/catheter brand, the coating type and the lubrication mode, ④ key factors for the risk of bias include catheter size, self-catheterization or other-catheterization, single-use or multiple-use of catheterization, daily frequency of intubation, ⑤ Outcome indicators and outcome measures.
Bias risk assessment for included studies
Methodologic quality was independently assessed by 2 reviewers using Cochrane.
Statistical analysis
Risk Ratios (RRs) were used as a measure of the relationship between hydrophilic or non-hydrophilic catheters and outcome indicators. The 95% confidence interval (CI) for the dichotomous data was calculated. The pooled RRs were adopted the Mantel–Haenszel method. If there were no events in one or both arms, the Peto method was used. The percentage of variability of each study attributable to heterogeneity beyond chance was evaluated by the chi-square test (P < 0.10) and I2 statistics. According to heterogeneity test, we adopted the random effects model (I2 > 50%, P < 0.10) or the fixed effects model. Then, the probability of publication bias was evaluated with Egger’s test and funnel plots. All statistical analyses were conducted with Stata15.0.
Results
Literature screening process and results
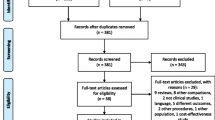
Figure 1 shows the selection process at each step and the reasons for excluded studies. Finally, 12 papers containing 850 participants met the inclusion criteria [14,15,16,17,18,19,20,21,22,23,24,25], including 9 randomized controlled trials [14, 15, 17, 19, 21,22,23, 25], 1 controlled before-and-after study [20], 1 prospective cohort studies [18], and 1 cross-over trials [16]. Table 1 illustrated patients’ characteristics (age and gender), catheter materials and catheter size. Meta-regression was performed with the year of publication, male proportion and age as independent variables, and the results showed that the regression equation had no statistical significance (p > 0.05).
Risk bias assessment form for included studies
In these studies, blinding of participants and interveners were not possible, but even unblinded methods were considered unlikely to have an impact on objective evaluation indicators. Therefore, they were classified as low risk. Patient withdrawal (an average of 17.71%) was common in the literature [14,15,16,17, 19, 22,23,24,25], which was an unbalanced and potentially biased factors (Fig. 2).
The results of the study
Gross hematuria
Studies have used different terms such as urethral bleeding, hematuria and gross hematuria to describe the same condition. A total of eight trials reported the number of patients with gross hematuria [14,15,16,17, 19,20,21,22]. The incidence of gross hematuria was 17.9% (57/318) in patients using hydrophilic catheters and 21.0% (73/347) in patients using non-hydrophilic catheters (RR = 0.80; 95% CI 0.45–1.42) (Fig. 3). The risk of gross hematuria was not statistically significant between two groups. As "catheterization frequency", "single/multiple catheterization" and "self/other catheterization" are key indicators for gross hematuria incidence, we performed subgroup analysis for the three aspects. Figure 4 shows that there was still no statistically significant difference in the risk of gross hematuria incidence. In addition, the proportion of male was found that it did not affect the results of the final forest plot of gross hematuria by meta-regression (additional Fig. 3④). Moreover, there was also no evidence of heterogeneity (p = 0.060; I2 = 55.8%) or publication bias (t = − 1.94, P = 0.148) (additional Fig. 3②). For the results of the sensitivity analysis, all the included studies were within the confidence interval except one study at the lower limit of the 95% CI (additional Fig. 3③). In brief, HC did not significantly improve the incidence of gross hematuria compared with NHC.
Microscopic hematuria
In this study, we considered microscopic hematuria as the following definition: the presence of red blood cells (RBC) in high power field under the microscope. There were 3 trials in 12 studies for microscopic hematuria in our study [19, 23, 24]. The incidence of microscopic hematuria was 41.7% (53/127) in patients using hydrophilic catheters and 56.3% (49/87) in patients using non-hydrophilic catheters (RR = 0.69; 95% CI, 0.52–0.90) (Fig. 5). The difference between two groups was statistically significant, indicating that the risk of microscopic hematuria with hydrophilic catheters was only 69% of that in non-hydrophilic group. There was also no evidence of heterogeneity (p = 0.678; I2 = 0.0%) or publication bias (t = − 0.65, P = 0.633) (additional Fig. 5②). For the results of the sensitivity analysis, the included studies were all within the CI (additional Fig. 5③). In short, HC significantly improved the incidence of microscopic hematuria compared with NHC.
Urethral stricture
The method for stricture evaluation is maximum flow rate < 14 mL/s or endoscopic or radiographic examination. A total of five trials reported the number of patients with urethral stricture [14, 15, 21, 22, 25]. The incidence of urethral stricture was 3.1% (6/194) in patients using hydrophilic catheters and 11.5% (23/200) in patients using non-hydrophilic catheters (RR = 0.28; 95% CI 0.13–0.60) (Fig. 6). The difference between two groups was statistically significant, suggesting that the risk of urethral stricture with hydrophilic catheters was only 28% of that in the non-hydrophilic group. There was also no evidence of heterogeneity (P = 0.983; I2 = 0.0%) or publication bias (t = 0.69, P = 0.617) (additional Fig. 6②). Five studies were all within the 95% CI about the sensitivity analysis (additional Fig. 6③). In a word, HC significantly improved the incidence of urethral stricture compared with NHC.
Rare adverse events
In addition to hematuria and urethral stricture, false passages and bladder stone are also rare adverse reactions after intubation in patients with bladder dysfunction. There were two studies focusing on the incidence of false passages [14, 20] and another two studies on bladder stone morbidity [18, 23]. Wyndaele [20] enrolled 39 patients who had been using NHC for IC over a number of years and switched to urocath-gel hydrophilic lubricated catheter for 1 month. It was found that only NHC group had one false passage. William [14] included children with neurogenic bladder dysfunction and divided them into 41 patients with NHC and 37 patients with HC. There were no false passages patients found in both groups. Jonathan [23] included 30 patients with HC and 31 patients with NHC for neurogenic bladder dysfunction, and found that one patient in each group had bladder stone. Tariq [18] included 101 children with spina bifida and divided them into HC and NHC groups. There were no bladder stones in the two groups. The incidences of both indicators were low after IC, and there was no difference between the two groups.
Discussion
Since Dr. Lapides proposed that using of IC as an alternative way to urinary diversion in《Urology》in 1972 [6], IC has become the globally recognized standard for the treatment of neurogenic bladder dysfunction and has been usually used in managements for various urinary system disease [26]. Generally, IC improves the quality of patients’ life through removing long-standing drainage tubes and drainage bags [2]. Initially, catheters for IC were mainly made of latex and rubber. However, these catheters were gradually taken placed by polyvinyl chloride (PVC) catheters due to their sensitization, hardness and difficulty in catheterization [27]. In addition, the practice of re-using catheters with same tube in IC has changed over the past 10 years, for example most patients with intermittent self-catheterization (ISC) were required to use disposable catheters during catheterization [2].
Under the guidance of healthcare workers, almost all patients with bladder dysfunction could get benefits from IC [2]. IC changes the pattern of urinary management in patients with bladder dysfunction because of its various advantages. In addition to decreasing mortality caused by kidney deterioration [28], IC also reduces the harmful effects of long-term indwelling urinary catheters, including urinary tract infections (UTIs) [29], traumatic hypospadias, urinary fistula and even bladder cancer [30]. However, there are still unavoidable complications including mechanical stimulation and mucosa injuries for IC, such as pain and urethral injury. Applying external lubricant is a traditional method to reduce mucosa friction and adhesion during catheterization. Common external lubricants cover Vaseline, paraffin oil, gel, lidocaine cream, amiodarone and ketamine [31]. Nevertheless, the application of external lubricant on the surface of urinary duct has plentiful limitations such as uneven application, cumbersome operation, weak lubrication effect and short residence time. In addition, anesthetic lubricant such as lidocaine cream contains additives that cause allergic reactions [32].
In recent years, water lubrication, which is an ideal solution to ultralow friction of medical catheter has received growing attentions. Hydrophilic coated catheters are usually made of PVC material and polyvinylpyrrolidone coated (PVP coated). PVP is a polymer with hydrophilic groups [33]. After the PVP hydrophilic groups are combined with a lubricating fluid (such as water or saline), the interface between the surface of catheter and the urethral mucosa forms a smooth area composed mainly of water molecules [24]. Direct contact between the surfaces is avoided during sliding process, thus greatly reducing friction coefficient and mucosal injury [24, 34, 35]. Furthermore, PVP coated possibly reduce a potential risk of urethral stricture caused by repeatedly intubation [22, 36]. Meanwhile, PVP coated is able to reduce the adsorption of fibrinogen and fibronectin, as well as the deposition of hydroxyapatite on the tube surface [34], potentially resulting in lower incidence of bladder stone.
Generally, gross hematuria is used as an indicator to estimate urethral trauma. However, the results of previous researches were contradictory in regard to whether gross hematuria could be reduced by HC [3, 12, 13]. Two meta-analyses concluded that HC was associated with a reduced risk of urethral bleeding compared with NHC [12, 13], but another research suggested a higher risk of hematuria in the HC group [3]. Simultaneously, the results from the three meta-analyses were challenged due to their inclusion, heterogeneity and bias risk analysis.
Gross hematuria is a more serious outcome indicator, so it is not a favorable indicator for reflecting the early condition of urethral damage. Innovatively, our study assessed urethral microtrauma using microscopic hematuria. Except for urethral bleeding, there are few studies evaluating whether HC reduce the incidence of adverse events, such as urethral stricture, false passages and bladder stone. In our study, HC made positive contributions to reducing the incidence of urethral microtrauma and urethral stricture compared with NHC, whereas gross hematuria was no significant difference. More studies are needed to further confirm the association between HC and these indicators in the future.
Implications for clinical practice
Due to the limitations of the study population and relevant intervention measures, the results of previous studies were contradictory and difficult to be generalized. Our study included a broad population of men and women of all ages with IC. There were no strict restrictions on the influencing factors, including catheterization frequency, self-catheterization or other-catheterization, single-use or multiple-use and the intubation environment. Therefore, our results regarding the complications of HC have broad adaptability to guide clinical practice.
Call for future studies
More high-quality, large-scale RCT studies are urgently needed. Recommendations for future research are as follows: ① The inclusion and exclusion criteria of study subjects should be clarified; ② The specific details of the intervention should be clarified including catheter material/catheter brand, the coating type and the way of lubrication; ③ key factors for the risk of bias need to be controlled including catheter size, total duration of intubation, time to start catheterization, self-catheterization or other catheterization, single-use or multiple-use of catheterization and catheterization frequency; ④ Call for clear definition of outcome indicators and specification of outcome measures.
Limitations
Our study still had some aspects for improving: ① Due to the wide heterogeneity of study subjects, study design, outcome measurement methods, as well as the small number of included literatures, it was difficult to conduct meta-subgroup analysis about long term adverse events such as urethral stricture. Therefore, we only performed subgroup analysis for gross hematuria; ② Risk of bias covers “little blinding of participants and interveners” and “the differences in patient drop-off between the two groups”, which perhaps impact study results; ③ The majority of our data was in males and it would be a non-negligible influence factor for IC. However, the objects are only men in the current literature which was eligible for inclusion in these two indicators of microscopic hematuria and urethral stricture.
Conclusion
This meta-analysis supports the benefits of using hydrophilic coated catheters for IC in patients with bladder dysfunction, including reduced incidence of microscopic hematuria and urethral stricture. However, whether HC reduces the risk of gross hematuria has not been proven. While waiting for more evidence, it is recommended to select a more appropriate catheter type of IC combined safety, efficacy, cost effectiveness and patient satisfaction. Patients are advised to use hydrophilic coated catheter as the first treatment option when the condition permits to reduce urethral complications and offers higher comfort [20]. In this study, we evaluated the effects of HC and NHC on urethral trauma, microtrauma, urethral stricture and rare adverse events, demonstrating that HC is a better intubation method for patients with bladder dysfunction.
References
Shamout S, Biardeau X, Corcos J et al (2017) Outcome comparison of different approaches to self-intermittent catheterization in neurogenic patients: a systematic review. Spinal Cord 55:629–643
Lamin E, Newman DK (2016) Clean intermittent catheterization revisited. Int Urol Nephrol 48(6):931–939
Rognoni C, Tarricone R (2017) Intermittent catheterization with hydrophilic and non-hydrophilic urinary catheters: systematic literature review and meta-analyses. BMC Urol 17:4
Cameron AP, Wallner LP, Tate DG et al (2010) Bladder management after spinal cord injury in the United States 1972 to 2005. J Urol 184:213–217
Giannantoni A, Scivoletto G, Di Stasi SM et al (1998) Clean intermittent catheterization and prevention of renal disease in spinal cord injury patients. Spinal Cord 36(1):29–32
Lapides J, Diokno AC, Silber SJ et al (1972) Clean, intermittent self-catheterization in the treatment of urinary tract disease. J Urol 107(3):458–461
Spinu A, Onose G, Daia C et al (2012) Intermittent catheterization in the management of post spinal cord injury (SCI) neurogenic bladder using new hydrophilic, with lubrication in close circuit devices-our own preliminary results. J Med Life 5:21–28
Lindehall B, Abrahamsson K, Hjälmås K et al (2004) Complications of clean intermittent catheterization in boys and young males with neurogenic bladder dysfunction. J Urol 172:1686–1688
Wyndaele JJ, Maes D (1990) Clean intermittent self-catheterization: A 12-year follow-up. J Urol 143:906–908
Emmanuel CK, Pierre D (2011) Intermittent catheterization with hydrophilic catheters as a treatment of chronic neurogenic urinary retention. Neurourol Urodyn 30:21–31
Eliza L, Diane KN (2016) Clean intermittent catheterization revisited. Int Urol Nephrol 48:931–939
Li L, Wenqin Y, Hong R et al (2013) Impact of hydrophilic catheters on urinary tract infections in people with spinal cord injury: systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil 94:782–787
Dechao F, Liang C, Yunjin B et al (2020) Outcomes comparison of hydrophilic and non-hydrophilic catheters for patients with intermittent catheterization: an updated meta-analysis. Asian J Surg 43:633–635
William DF, Pramod R, Melissa R et al (2017) Results of a prospective randomized control trial comparing hydrophilic to uncoated catheters in children with neurogenic bladder. J Pediatr Urol 13(373):e1-373.e5
Ridder DJMKD, Everaert K, Fernández LG et al (2005) Intermittent catheterisation with hydrophilic-coated catheters (SpeediCath) reduces the risk of clinical urinary tract infection in spinal cord injured patients-a prospective randomised parallel comparative trial. Eur Urol 48:991–995
Pachler J, Frimodt MC (1999) A comparison of prelubricated hydrophilic and non-hydrophilic polyvinyl chloride catheters for urethral catheterization. BJU Int 83:767–769
Diana DC, Katherine NM, Amy DM et al (2011) Intermittent catheterization with a hydrophilic-coated catheter delays urinary tract infections in acute spinal cord injury: a prospective, randomized. Multicenter Trial PM R 3:408–411
Burki T, Abasher A, Alshahrani A et al (2019) Complications and patient satisfaction with urethral clean intermittent catheterization in spina bifida patients: comparing coated vs uncoated catheters. J Pediatr Urol 15:646–650
Sutherland RS, Kogan BA, Baskin LS et al (1996) Clean intermittent catheterization in boys using the LoFric catheter. J Urol 156:2041–2043
Wyndaele J, Ridder DD, Everaert K et al (2000) Evaluation of the use of Urocath-Gel catheters for intermittent self-catheterization by male patients using conventional catheters for a long time. Spinal Cord 38:97–99
Luca C, Emiliano AP, Riccardo A et al (2004) Standard versus hydrophilic catheterization in the adjuvant treatment of patients with superficial bladder cancer. Urol Int 73:19–22
Sallami S, Mouine Y, Rhouma SB et al (2011) Clean intermittent catheterization following urethral stricture surgery using a low friction catheter versus conventional plastic catheter: a prospective: a randomized trial. Urol Today Int J 4:7
Jonathan MV, Frederick MM, Jiensup K (2003) A prospective randomized trial of the LoFric hydrophilic coated catheter versus conventional plastic catheter for clean intermittent catheterization. J Urol 169:994–998
Stensballe J, Looms D, Nielsen PN et al (2005) Hydrophilic-coated catheters for intermittent catheterisation reduce urethral micro-trauma: a prospective, randomised, participant BliMicro-trauma: a prospective, randomised, participant-blinded, crossover study of three different types of catheters. Eur Urol 48:978–983
Kjaergaard B, Walter S, Bartholin J et al (1994) Prevention of urethral stricture recurrence using clean intermittent self-catheterization. Br J Urol 73:692–695
Hedlund H, Hjelmås K, Jonsson O et al (2001) Hydrophilic versus non-coated catheters for intermittent catheterization. Scand J Urol Nephrol 35(1):49–53
Newman DK, Willson MM (2011) Review of intermittent catheterization and current best practices. Urol Nurs 31(1):12–28 (48, 29)
Klausner AP (2014) The Lapides legacy: 42 years and cathing. Can J Urol 21(2):7194
Siroky MB (2002) Pathogenesis of bacteriuria and infection in the spinal cord injured patient. Am J Med 113(Suppl 1A):67S-79S
Klausner AP, Steers WD (2011) The neurogenic bladder: an update with management strategies for primary care physicians. Med Clin N Am 95(1):111–120
Subbu S et al (2015) Acta Biomater 26:159–168
Schlager A, Metzger YC, Adler SN (2010) Use of surface acoustic waves to reduce pain and discomfort related to indwelling nasogastric tube. Endoscopy 42:1045–1048
Sterner O, Karageorgaki C, Zürcher M et al (2017) Reducing friction in the eye: a comparative study of lubrication by surface-anchored synthetic and natural ocular mucin analogues. ACS Appl Mater Interfaces 9:20150–20160
Tunney MM, Gorman SP (2002) Evaluation of a poly (vinyl-pyrollidone)-coated biomaterial for urological use. Biomaterials 23:4601–4608
Kazmierska K, Szwast M, Ciach T (2008) Determination of urethral catheter surface lubricity. J Mater Sci Mater Med 19:2301–2306
Naude AM, Heyns CF (2005) What is the place of internal urethrotomy in the treatment of urethral stricture disease? Nat Clin Pract Urol 2:538–545
Funding
This study was funded by Key Research and Development Program from Department of Science and Technology of Sichuan Province (grant number 2021YFS0022) and the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (grant number ZYJC21057).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Xi Liao declares that she has no conflict of interest. Yuwei Liu declares that she has no conflict of interest. Shiqi Liang declares that she has no conflict of interest. Ka Li declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11255_2022_3172_MOESM1_ESM.tif
Gross Hematuria: the proportion of male was found that it did not affect the results of the final forest plot of gross hematuria by meta-regression (TIF 194 kb)
11255_2022_3172_MOESM2_ESM.tif
Gross Hematuria: there was also no evidence of heterogeneity (p=0.060; I-squared =55.8%) or publication bias (t=-1.94, P=0.148) (TIF 127 kb)
11255_2022_3172_MOESM3_ESM.tif
Gross Hematuria: For the results of the sensitivity analysis, all the included studies were within the confidence interval except one study at the lower limit of the 95% CI (TIF 223 kb)
11255_2022_3172_MOESM4_ESM.tif
Microscopic hematuria: There was also no evidence of heterogeneity (p=0.678;I-squared =0.0%) or publication bias (t=-0.65, P=0.633) (TIF 128 kb)
11255_2022_3172_MOESM5_ESM.tif
Microscopic hematuria: For the results of the sensitivity analysis, the included studies were all within the CI (TIF 161 kb)
11255_2022_3172_MOESM6_ESM.tif
Urethral stricture: There was also no evidence of heterogeneity (p=0.983;I-squared =0.0%) or publication bias (t=0.69, P=0.617) (TIF 135 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liao, X., Liu, Y., Liang, S. et al. Effects of hydrophilic coated catheters on urethral trauma, microtrauma and adverse events with intermittent catheterization in patients with bladder dysfunction: a systematic review and meta-analysis. Int Urol Nephrol 54, 1461–1470 (2022). https://doi.org/10.1007/s11255-022-03172-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03172-x