Abstract
Purpose
Renal inflammatory response is involved in the development and progression of diabetic kidney disease (DKD). We sought to evaluate pentraxin-3 (PTX3) and adropin variability as inflammatory markers among type 2 diabetes mellitus (T2DM) patients with different urinary albumin, and to examine if these factors assist in the early diagnosis of diabetic kidney disease.
Methods
We enrolled 447 T2DM patients and 100 healthy non-diabetic control subjects in this study. The patients with T2DM were divided into three groups based on their urinary albumin/creatinine ratio (UACR): the normoalbuminuric group (DM group, UACR < 30 mg/g); the microalbuminuric group (DKD1 group, 30 ≤ UACR ≤ 300 mg/g); the macroalbuminuric group (DKD2 group, UACR > 300 mg/g). The levels of PTX3 and adropin were determined by enzyme-linked immunosorbent assay (ELISA). Spearman correlation and multiple linear regression analysis were performed to determine the correlations among these inflammatory markers and other clinical parameters. Receiver operating characteristic (ROC) curves analysis was used to assess the diagnostic potential of PTX3 and adropin for DKD.
Results
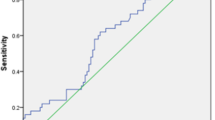
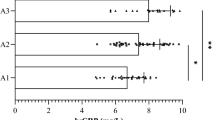
Compared to non-diabetes, serum levels of PTX3 were distinctly elevated, whereas the adropin were significantly declined in diabetic patients (p < 0.05). Significantly higher levels of PTX3 and lower levels of adropin were seen in the macroalbuminuric patients compared with the microalbuminuric patients (p < 0.05). Multiple stepwise linear regression analysis showed that the control of hemoglobin A1c (HbA1c) and UACR were independent factors associated with PTX3 and adropin. In addition, ROC curves analysis showed PTX3 and adropin could be used to evaluate the early detect of DKD, further adropin might be a better marker than PTX3 in compliance with their veracity.
Conclusion
As inflammatory markers, the diverse changes of pentraxin-3 and adropin showed that they may forecast the renal damage in diabetic patients in varying degrees and link with the pathogenesis of diabetic kidney disease.

Similar content being viewed by others
References
Hayder ZS, Kareem ZS (2020) Resistin hormone in diabetic kidney disease and its relation to iron status and hepcidin. Int Urol Nephrol 52:749–756. https://doi.org/10.1007/s11255-020-02434-w
Techatanawat S, Surarit R, Chairatvit K, Roytrakul S, Khovidhunkit W, Thanakun S, Izumi Y, Khovidhunkit SP (2019) Salivary and serum cystatin SA levels in patients with type 2 diabetes mellitus or diabetic nephropathy. Arch Oral Biol 104:67–75. https://doi.org/10.1016/j.archoralbio.2019.05.020
Galsgaard J, Persson F, Hansen TW, Jorsal A, Tarnow L, Parving HH, Rossing P (2017) Plasma high-sensitivity troponin T predicts end-stage renal disease and cardiovascular and all-cause mortality in patients with type 1 diabetes and diabetic nephropathy. Kidney Int 92(5):1242–1248. https://doi.org/10.1016/j.kint.2017.04.018
Xiao Y, Chen L, Fan Y, Yan P, Li S, Zhou X (2019) The effect of boletus polysaccharides on diabetic hepatopathy in rats. Chem Biol Interact 308:61–69. https://doi.org/10.1016/j.cbi.2019.05.013
Matoba K, Takeda Y, Nagai Y, Kawanami D, Utsunomiya K, Nishimura R (2019) Unraveling the role of inflammation in the pathogenesis of diabetic kidney disease. Int J Mol Sci 20:1–15. https://doi.org/10.3390/ijms20143393
Zhu H, Yu W, Xie Y, Zhang H, Bi Y, Zhu D (2017) Association of Pentraxin 3 Gene Polymorphisms with Susceptibility to Diabetic Nephropathy. Med Sci Monit 23: 428–436. https://doi.org/10.12659/msm.902783
Liu Y, Yu C, Ji K, Wang X, Li X, Xie H, Wang Y, Huang Y, Qi D, Fan H (2019) Quercetin reduces TNF-α-induced mesangial cell proliferation and inhibits PTX3 production: involvement of NF-κB signaling pathway. Phytother Res 33:2401–2408. https://doi.org/10.1002/ptr.6430
Rathore M, Girard C, Ohanna M, Tichet M, Ben Jouira R, Garcia E, Larbret F, Gesson M, Audebert S, Lacour JP, Montaudié H, Prod'Homme V, Tartare-Deckert S, Deckert M (2019) Cancer cell-derived long pentraxin 3 (PTX3) promotes melanoma migration through a toll-like receptor 4 (TLR4)/NF-κB signaling pathway. Oncogene 38:5873–5889. https://doi.org/10.1038/s41388-019-0848-9
Bako HY, Ibrahim MA, Isah MS, Ibrahim S (2019) Inhibition of JAK-STAT and NF-κB signalling systems could be a novel therapeutic target against insulin resistance and type 2 diabetes. Life Sci 239:117–125. https://doi.org/10.1016/j.lfs.2019.117045
Maekawa M, Tadaki H, Tomimoto D, Okuma C, Sano R, Ishii Y, Katsuda Y, Yoshiuchi H, Kakefuda R, Ohta T, Sasase T (2019) A Novel TNF-α converting enzyme (TACE) selective inhibitor JTP-96193 prevents insulin resistance in KK-Ay type 2 diabetic mice and diabetic peripheral neuropathy in type 1 diabetic mice. Biol Pharm Bull 42:1906–1912. https://doi.org/10.1248/bpb.b19-00526
Akcılar R, Emel Koçak F, Şimşek H, Akcılar A, Bayat Z, Ece E, Kökdaşgil H (2016) The effect of adropin on lipid and glucose metabolism in rats with hyperlipidemia. Iran J Basic Med Sci 19:245–251
Yuan X, Chen R, Ouyang Q, Lin X, Ai Z, Zhang Y, Yang X (2020) Novel associations of serum adropin and lipopolysaccharide-binding protein versus lipid profiles in childhood obesity. J Pediatr Endocrinol Metab 33:265–270. https://doi.org/10.1515/jpem-2019-0329
Xie H, Li C, Wen Y, Ye W, Cai J, Li H, Li X, Li X (2020) Association of diabetes with failure to achieve complete remission of idiopathic membranous nephropathy. Int Urol Nephrol 52(2):337–342. https://doi.org/10.1007/s11255-019-02348-2
Siddiqui K, Joy SS, Al-Rubeaan K (2019) Association of urinary monocyte chemoattractant protein-1 (MCP-1) and kidney injury molecule-1 (KIM-1) with risk factors of diabetic kidney disease in type 2 diabetes patients. Int Urol Nephrol 51(8):1379–1386. https://doi.org/10.1007/s11255-019-02201-6
Yamaguchi Y, Itabashi M, Yumura W, Takei T (2020) Geriatric assessment of estimated glomerular filtration rate: a cross-sectional study. Clin Exp Nephrol 24(3):216–224. https://doi.org/10.1007/s10157-019-01797-4
Zhong Y, Lee K, Deng Y, Ma Y, Chen Y, Li X, Wei C, Yang S, Wang T, Wong NJ, Muwonge AN, Azeloglu EU, Zhang W, Das B, He JC, Liu R (2019) Arctigenin attenuates diabetic kidney disease through the activation of PP2A in podocytes. Nat Commun 10:1–15. https://doi.org/10.1038/s41467-019-12433-w
Milas O, Gadalean F, Vlad A et al (2019) Pro-inflammatory cytokines are associated with podocyte damage and proximal tubular dysfunction in the early stage of diabetic kidney disease in type 2 diabetes mellitus patients. J Diabetes Complications 34:107–114. https://doi.org/10.1016/j.jdiacomp.2019.107479
Li XQ, Chang DY, Chen M, Zhao MH (2019) Deficiency of C3a receptor attenuates the development of diabetic nephropathy. BMJ Open Diabetes Res Care 7:1–9. https://doi.org/10.1136/bmjdrc-2019-000817
Xiao H, Sun X, Liu R, Chen Z, Lin Z, Yang Y, Zhang M, Liu P, Quan S, Huang H (2019) Gentiopicroside activates the bile acid receptor Gpbar1 (TGR5) to repress NF-kappaB pathway and ameliorate diabetic nephropathy. Pharmacol Res 151:104559. https://doi.org/10.1016/j.phrs.2019.104559
Qiu L, Xu R, Wang S, Li S, Sheng H, Wu J, Qu Y (2015) Honokiol ameliorates endothelial dysfunction through suppression of PTX3 expression, a key mediator of IKK/IkB/NF-kB, in atherosclerotic cell model. Exp Mol Med 47:e171. https://doi.org/10.1038/emm.2015.37
Jaillon S, Bonavita E, Gentile S, Rubino M, Laface I, Garlanda C, Mantovani A (2014) The long pentraxin PTX3 as a key component of humoral innate immunity and a candidate diagnostic for inflammatory diseases. Int Arch Allergy Immunol 165:165–178. https://doi.org/10.1159/000368778
Bala C, Rusu A, Ciobanu DM, Craciun AE, Roman G (2018) The association study of high-sensitivity C-reactive protein, pentraxin 3, nitrotyrosine, and insulin dose in patients with insulin-treated type 2 diabetes mellitus. Ther Clin Risk Manag 28(14):955–963. https://doi.org/10.2147/TCRM.S162086
Pang Y, Tan Y, Li Y, Zhang J, Guo Y, Guo Z, Zhang C, Yu F, Zhao MH (2016) Pentraxin 3 is closely associated with tubulointerstitial injury in lupus nephritis: a large multicenter cross-sectional study. Medicine (Baltimore) 95:e2520. https://doi.org/10.1097/MD.0000000000002520
Hung TW, Tsai JP, Lin SH, Lee CH, Hsieh YH, Chang HR (2016) Pentraxin 3 activates JNK signaling and regulates the epithelial-to-mesenchymal transition in renal ribrosis. Cell Physiol Biochem 40:1029–1038. https://doi.org/10.1159/000453159
Takashi Y, Koga M, Matsuzawa Y, Saito J, Omura M, Nishikawa T (2018) Circulating pentraxin 3 is positively associated with chronic hyperglycemia but negatively associated with plasma aldosterone concentration. PLoS ONE 13:1–10. https://doi.org/10.1371/journal.pone.0196526
Uzun S, Ozari M, Gursu M, Karadag S, Behlul A, Sari S, Koldas M, Demir S, Karaali Z, Ozturk S (2016) Changes in the inflammatory markers with advancing stages of diabetic nephropathy and the role of pentraxin-3. Ren Fai 38:1193–1198. https://doi.org/10.1080/0886022X.2016.1209031
Chen X, Luo J, Wu M, Pan Z, Xie Y, Wang H, Chen B, Zhu H (2018) Study on association of pentraxin 3 and diabetic nephropathy in a rat model. J Diabetes Res 13(2018):8968573. https://doi.org/10.1155/2018/8968573
Xiao Y, Yang N, Zhang Q, Wang Y, Yang S, Liu Z (2014) Pentraxin 3 inhibits acute renal injury-induced interstitial fibrosis through suppression of IL-6/Stat3 pathway. Inflammation 37(5):1895–1901. https://doi.org/10.1007/s10753-014-9921-2
Maciorkowska M, Musiałowska D, Małyszko J (2019) Adropin and irisin in arterial hypertension, diabetes mellitus and chronic kidney disease. Adv Clin Exp Med 28:1571–1575. https://doi.org/10.17219/acem/104551
Celik HT, Bilen M, Kazancı F, Yildirim ME, İncebay İB, Erdamar H (2019) Serum adropin as a predictive biomarker of erectile dysfunction in coronary artery disease patients. Cent Euro J Uro 72:302–306. https://doi.org/10.5173/ceju.2019.1666
Akcilar R, Kocak FE, Simsek H, Akcilar A, Bayat Z, Ece E, Kokdasgil H (2016) Antidiabetic and hypolipidemic effects of adropin in streoptozotocin-induced type 2 diabetic rats. Bratisl Lek Listy 117:100–105. https://doi.org/10.4149/bll_2016_020
Li S, Sun J, Hu W, Liu Y, Lin D, Duan H, Liu F (2019) The association of serum and vitreous adropin concentrations with diabetic retinopathy. Ann Clin Biochem 56:253–258. https://doi.org/10.1177/0004563218820359
Thapa D, Xie B, Zhang M et al (2019) Adropin treatment restores cardiac glucose oxidation in pre-diabetic obese mice. J Mol Cell Cardiol 129:174–178. https://doi.org/10.1016/j.yjmcc.2019.02.012
Acknowledgements
The authors appreciate all members of the Department of Endocrinology, Baoding NO.1 Central Hospital and Department of Ophthalmology, for their sincere advice.
Funding
This work was funded by Project of National Natural Science Foundation of China (81500644), Hebei province medical applicable technology tracking project (G2019012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have declared that no conflict of interest exists.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted (Ethics Committee of Baoding NO.1 Central Hospital approval number #2017010) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, B., Tian, X., Guo, S. et al. Pentraxin-3 and adropin as inflammatory markers of early renal damage in type 2 diabetes patients. Int Urol Nephrol 52, 2145–2152 (2020). https://doi.org/10.1007/s11255-020-02568-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-020-02568-x