Abstract
Urban wildlife faces unique physiological and behavioral challenges compared to conspecifics which live in less altered natural habitats. Animals in urban habitats are also exposed to urban stressors and commonly make use of inappropriate food sources from trash bins and dumpsters, which may affect their heath status. The goals of this study were to evaluate overall health of an urban population of eastern gray squirrels (Sciurus carolinensis) and to assess seasonal changes in several health parameters. Squirrels (N = 45) were trapped in Durham, North Carolina, USA and briefly anesthetized with inhalant anesthetic for physical examination, body mass collection, and venipuncture via the femoral vein. Collected blood was analyzed for several health parameters via hematology and biochemistry analyses including assessment of lipids and kidney and liver values. Physical examinations were clinically unremarkable and similar across seasons. Females had higher cholesterol values and lower creatinine values compared to males. Seasonal differences occurred in few parameters and were detected only between summer and either fall or spring. Potassium and monocytes were higher in summer compared to spring, and creatinine, BUN and monocytes were higher in summer compared to fall. Overall, health parameters of urban eastern gray squirrels fell within published reference ranges for the species. Together, these results suggest that urban squirrels in this study population can maintain good health in an anthropogenic habitat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urbanization is one of the fastest-occurring human-induced environmental changes and urban environments are growing around the world (McKinney 2002; Grimm et al. 2008). Wildlife must either adjust to these rapid environmental changes and human-modified habitats, or experience severe population declines and, ultimately, local extinction. In urban areas, high local extinction rates of native plant and animal species have been observed (McKinney 2002; Marzluff 2005). While some animal species are absent from urban environments, other species reproduce successfully and occur at high population densities in anthropogenically modified habitats (Ives et al. 2016; Parsons et al. 2018). Despite the apparent success of urban wildlife, living in close proximity to humans and in intensely modified urban habitats often results in behavioral changes, altered activity patterns and abnormal diets (Ditchkoff et al. 2006; Tuomainen and Candolin 2011; Lowry et al. 2013; Sol et al. 2013; Gaynor et al. 2018). These alterations can have both positive and negative effects on reproduction and survival of animal species (Bowers and Breland 1996; Ditchkoff et al. 2006; Strasser and Heath 2013; Capilla-Lasheras et al. 2017; Gaynor et al. 2018).
Urban habitats differ in many aspects from natural and/or undeveloped rural habitats, exposing urban populations to habitat-specific challenges, also termed ‘urban stressors’. Urban stressors include air, chemical and noise pollution, artificial light at night, infectious diseases, vehicle traffic and human disturbance (Fernández-Juricic 2002; Dowding et al. 2010; Isaksson 2015; Berger et al. 2020; Chatelain et al. 2021). Additionally, dietary quality of natural food sources in urban areas can be altered and urban populations of many taxa consume anthropogenic food sources, which can have both positive and negative health outcomes (Oro et al. 2013; Isaksson 2015; Plaza and Lambertucci 2017; Coogan et al. 2018; Alberti et al. 2020).
Given these alterations in urban habitats, it is likely that urban populations face different physiological and behavioral challenges compared to their rural conspecifics (Birnie-Gauvin et al. 2016), and exposure to urban stressors may alter the physiology and health of urban populations (Murray et al. 2019; Isaksson and Bonier 2020). For example, a meta-analysis determined that exposure to anthropogenic pollution (such as sulfur-dioxide, organic pollutants, ozone, and heavy metals) increases oxidative stress in terrestrial animals, which can cause tissue damage linked to disease and senescence (Isaksson 2010). Additionally, urban and rural populations of many species differ in regards to their immune response (Moore and Siopes 2000; Bedrosian et al. 2011; Capilla-Lasheras et al. 2017; Neto et al. 2020), gut microbiome (Stothart et al. 2019; Maraci et al. 2022) and stress response (reviewed in: Bonier 2012; Pérez-Ortega and Hendry 2023; but see Iglesias-Carrasco et al. 2020). Birds often have a reduced reproductive success in urban habitats (Strasser and Heath 2013; Sumasgutner et al. 2014; Capilla-Lasheras et al. 2017; Seress et al. 2020), which has been linked to poorer nutrition in these environments (Plummer et al. 2013; Seress et al. 2020). Conversely, access to high-calorie, high-fat anthropogenic food sources can positively affect body condition of birds, which might potentially offset other negative effects of urbanization (Auman et al. 2008; Wilcoxen et al. 2015; Townsend et al. 2019). For example, anthropogenic food supplementation of Florida Scrub-Jays (Aphelocoma coerulescens) leads to earlier onset of breeding and increased reproductive output (Schoech and Bowman 2003; Schoech et al. 2008). That said, to date, nutritional ecology of urban mammals and its influence on health are still poorly understood.
In mammals, access to human food sources and waste has been reported to increase body mass (reviewed in: Plaza and Lambertucci 2017) and to positively affect reproductive success, in the form of increased litter size, offspring survival and higher population densities (Eley et al. 1989; Beck 2002; Beckmann and Berger 2003; Prange et al. 2004; Finkler et al. 2011). Anthropogenic food sources often have an altered fatty acid composition, a higher fat and carbohydrate content than natural food sources, and consumption of anthropogenic food can result in an increased intake of sugar and an altered intake of dietary protein, antioxidants and vitamins (Murray et al. 2015; Andersson et al. 2018; Schulte-Hostedde et al. 2018; Wist et al. 2022). Due to the different dietary quality of anthropogenic food, its consumption can result in (potentially unhealthy) weight gain, increased body fat percentage, reduced cardiometabolic health and increased mortality (Altmann et al. 1993; Cypher and Frost 1999; Banks et al. 2003; Otali and Gilchrist 2004; Soto-Calderón et al. 2016; Hillman et al. 2017; Schulte-Hostedde et al. 2018). To date, data on health parameters are available for only a restricted number of urban mammal populations and results regarding differences in these parameters are ambiguous.
The eastern gray squirrel (Sciurus carolinensis) is a medium-sized (300–710 g) tree squirrel native throughout eastern North America (Koprowski 1994) that has been successfully introduced to Europe and South Africa (Benson 2013). Mating occurs December – February and again May – July, and young are born February – March and June – August (Koprowski 1994). Gray squirrels do not hibernate, but rely on fat stores and food caches while overwintering (Koprowski 1994). They are commonly found in both deciduous forests and anthropogenic environments, such as parks and residential areas (Benson 2013). In urban habitats, gray squirrels are often found in high densities (Hein 1997; Parker and Nilon 2008; Benson 2013; Engel et al. 2020; Rimbach et al. 2023). They frequently descend to the ground to forage and make use of backyard food resources, bird feeders, trash bins, and dumpsters (Hansen et al. 2020; Rimbach et al. 2023). Urban squirrels have lower giving-up densities, the density of resources in a patch at which foraging stops, than squirrels living in more natural areas, suggesting that urban individuals experience higher food limitation and/or lower perceived predation risk (Bowers and Breland 1996).
As an altered diet may impact the overall health of squirrels, investigation of the impact of urbanization and access to anthropogenic food sources on health parameters is warranted. Commonly assessed health parameters include physical examination, body composition assessments, and blood and fecal sample analyses (Kophamel et al. 2022). Hematological, serum protein parameters and biochemical analysis are useful tools to assess and monitor health and to detect disease in a wide range of species (Thrall et al. 2012). For example, in water voles (Arvicola terrestris) certain hematological parameters (e.g., low hemoglobin and erythrocyte concentrations) are associated with poor survival (Mathews et al. 2006). Similarly, multiple health parameters, particularly markers of inflammation, are predictive of bottlenose dolphin (Tursiops truncatus) survival (Schwacke et al. 2024). At least one study has evaluated the health status of urban versus rural (forest) gray squirrels in North America (Canada). In that study, body mass and a variety of blood analytes were compared between the two populations. Results indicated that urban squirrels did not differ in body mass from rural squirrels, but compared to rural squirrels (N = 9), urban individuals (N = 6) had lower concentrations of electrolytes (i.e., potassium, phosphorus, chloride) and higher blood glucose concentrations (Schmidt et al. 2022). These results suggest that urban squirrels are exposed to greater exogenous stressors or that they ingest an increased amount of dietary sugar in urban habitats. These population differences (albeit small sample sizes) suggest that urban squirrels may be in a poorer physiological condition and, thus, investigation of this phenomenon in other urban squirrel populations is warranted.
The goals of the current study were to evaluate the overall health of an urban population of gray squirrels and to assess seasonal changes in health parameters. In this study, we assessed squirrel health via several health parameters: full physical examination, body mass, and biochemical and hematologic parameters including assessment of blood lipids and kidney and liver values. Gray squirrels typically show seasonal fattening in preparation for winter (Koprowski 1994; Koprowski et al. 2016). Thus, we predicted that body mass would increase from summer to fall and then decrease from fall until spring. We expected squirrels to follow the winter-summer hematic cycle reported from other rodents, with lowest levels in spring, recovering values during summer and fall and highest values in winter (Sealander 1962; Mira and Mathias 1994; Beldomenico et al. 2008; Katiukhin et al. 2023). Squirrels at the study site consume anthropogenic food sources from trash bins (Rimbach et al. 2023). Thus, we hypothesized that serum biochemistry, blood cell counts, blood lipids and liver values of these urban squirrels would differ from values reported from rural squirrels.
Materials and methods
Study sites
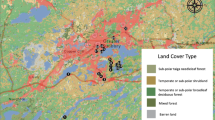
We trapped squirrels in June – July 2020 (summer), October 2020 (fall), January 2021 (winter; no blood collection) and March 2021 (spring; Table 1). We trapped squirrels at an urban site on Duke University West campus (36.0026 N, 78.9408 W), Durham, North Carolina, USA (Fig. 1). Duke West campus (2.9 km2) contains a heterogenous mixture of buildings, sparsely treed grass lawns and several small forested areas interspersed with walking trails. The forested areas contain several species of oak, maple, hickory, and pine, offering natural food sources to the squirrel population. Duke West campus contains the majority of student housing and the campus population comprised ~ 16,780 students in 2021. Thus, Duke West campus is an ideal place to study urban squirrels because these animals will likely encounter humans on a daily basis and they have constant access to anthropogenic food sources from trash bins and dumpsters (Rimbach et al. 2023).
Blood sample collection
We captured squirrels using Tomahawk Model 102 traps (Tomahawk Live Trap Co., WI, USA) baited with peanut butter and unshelled peanuts. We trapped squirrels between 7:00 and 13:00 and checked traps at 45–60 min intervals. We transferred squirrels from traps into an induction chamber and anesthetized them using inhaled isoflurane (Isoflurane USP, Piramal Enterprises Limited, Telangana, India) in 100% oxygen. Anesthesia was maintained with isoflurane via facemask and administered to effect throughout the duration of the procedure. Squirrels were monitored (heart rate, respiratory rate, anesthetic depth) throughout the duration of the anesthetic episode. Individuals were weighed using a spring scale (Pesola, Switzerland), and age (juvenile or adult) and sex were recorded. We determined age class by using a combination of body mass, tooth wear and fur condition. We included both adult and juvenile squirrels of both sexes in this study. Visibly pregnant females were not anesthetized to avoid potential negative effects of anesthesia to the female and her fetuses. For males, we recorded whether they were scrotal (testes descended) or not (testes in the abdominal cavity), and for females, we recorded whether nipples were visible or not. A single suspected pregnant female was included in the study because it was not possible to visually determine her suspected pregnancy prior to anesthesia. Every anesthetized squirrel received a full physical examination by a board-certified zoo veterinarian (OAP).
Venipuncture was performed from the femoral vein using a 25 gauge needle and 1 mL syringe or a 31 gauge needle and a 0.5 mL insulin syringe, and 0.5–0.7 mL (max. of 1 mL) of blood was collected. An abbreviated biochemical panel was performed in the field within 10 min of venipuncture using a VetScan i-STAT 1 point-of-care analyzer (Abbott Point of Care, Princeton, New Jersey, USA) with approximately 100 µl of heparinized whole blood loaded into a CHEM8 + cartridge (Abaxis North America, Union City, NJ, USA). This cartridge measures electrolytes (chloride, sodium, potassium), blood chemistry (ionized calcium, blood urea nitrogen [BUN], creatinine, glucose), acid base (total carbon dioxide [TCO2], anion gap) and certain hematologic parameters (hematocrit and hemoglobin). This method has previously been used on juvenile gray squirrels (Ratliff et al. 2019) to establish reference intervals. We also prepared two fresh whole blood smears immediately before placing the whole blood into heparinized microtainers (BD, Franklin Lakes, NJ, USA). These blood smears were stained at the College of Veterinary Medicine using a Wright-Giemsa stain for subsequent analysis which included a manual estimated white blood cell (WBC) count and a manual differential (% of each WBC type) for each animal using a 100x oil immersion lens. For the estimated WBC count, we counted the number of white blood cells in 10 high power fields in different areas of the slide. We used the mean number of WBCs/field and multiplied this mean by 1,000 to calculate the number of cells 109/L. Average (± SD) PCV in adult squirrels was 44.3 ± 4.1 (range = 37–55) and in juvenile squirrels 39.8 ± 2.9 (range = 35–44). Thus, none of the individuals was considered anemic and we did not correct for PCV during the calculation of the estimated WBC count. For the differential WBC counts, we examined smears in a systematic pattern, scanning from side to side within the monolayer area of the slide and counting until a cumulative number of 100 cells was reached. White blood cells were classified according to morphological and staining characteristics as lymphocyte, neutrophil, eosinophil, basophil or monocyte. Percentages of individual white blood cells were then converted into absolute numbers by reference to the total WBC count using the following formula: absolute number WBC (109 cells/L) = (% of white blood cells counted x total WBC)/ 100. All leukocyte count determinations were performed by one observer (Jörg Jäger) without knowledge of sex, age or date that the samples were collected. All smears were inspected once, and then a random sample, which represented one quarter of the number of smears inspected, was selected to repeat the procedure.
In addition, we obtained blood lactate concentrations using a point-of-care lactate meter (Nova Biomedical, Waltham, MA 02454, USA) immediately following blood collection. Packed cell volume (PCV) and total solids, the latter measured using a refractometer (REC-200ATC, Gain Express Holdings Ltd, Hong Kong), were determined from heparinized whole blood samples within 3–5 h of sample collection. Animals were part of a population that had been previously trapped and tagged as part of a prior study (body mass measurements, but no anesthesia or blood collection; Rimbach et al. 2022), and the prior body mass measurements of these individuals were included in this study for the analysis of seasonal variation of body mass. All squirrels were scanned for the presence of a passive integrated transponder (Avid2024, Avid Identification Systems Inc., Norco, CA, USA), which would signify recapture (only 5 out of the 45 study squirrels had been trapped and tagged previously). If undetected, a transponder was placed subcutaneously in the dorsal interscapular region after blood collection. Following all procedures, squirrels were removed from inhalant anesthetic exposure, placed in an enclosed carrier, and monitored closely until they were standing, coordinated, and exhibiting normal behaviors following anesthetic recovery; at this point, they were released close to their capture site. The remaining heparinized whole blood samples were stored on ice-packs and centrifuged for 10 min at 1500 x g to separate the plasma within 3–5 h after sample acquisition. Following centrifugation, we immediately transferred the plasma samples into polypropylene tubes using a Pasteur pipette. Plasma samples were then frozen (-80 °C) until analysis of blood lipids (cholesterol and triglycerides) and a marker of liver health (aspartate aminotransferase [AST]) using a Beckman DxC 600 clinical analyzer (Beckman Coulter, Brea, California) at the Duke Metabolic Phenotyping Institute, Durham, NC, USA.
Statistical analysis
We analyzed all data in R v.4.2.3 (R Core Team 2023). We used linear mixed models (LMMs) or linear models (in cases when LMMs could not be used due to a singular fit of the model or non-convergence) to assess differences in body mass and all blood analytes between seasons, sex, and age categories. For the body mass analysis, we included body mass as the response variable and season (summer, fall, winter or spring), age category and sex as explanatory variables, and date of measurement as random factor. We separated adults and juveniles for the body mass analysis for two reasons: the model returned a singular fit when using the entire dataset (adults and juveniles) and because adult, but no juveniles, were trapped in spring.
For all other analytes (chloride, sodium, potassium, ionized calcium, TCO2, BUN, creatinine, glucose, anion gap, cholesterol, triglycerides, AST, lactate, PCV, hemoglobin, WBC count, neutrophils, eosinophils, basophils, lymphocytes, monocytes, neutrophil: lymphocyte [N: L] ratio), we included the analyte as response variable and season (summer, fall or spring), sex, and age category as explanatory variables, and body mass as control variable. We used time of day (at removal from trap) as a random factor in LMMs. We tested for one-way interactions between the fixed effect season and sex, age, and body mass, and determined the contribution of interaction terms using likelihood ratio tests (LRT) by comparing the model with the interaction term to the model without. We excluded interaction terms if the explanatory power of the model was not improved by the inclusion of the interaction, based on the LRT (Pinheiro and Bates 2000). For all models, we checked that model assumptions were met using the ‘performance’ package (Lüdecke et al. 2021). Prior to analysis, we log-transformed variables or used the square-root (as appropriate) to fulfill model assumptions of normally distributed residuals. We used the ‘emmeans’ package (Lenth et al. 2023) for pairwise contrast between seasons with P-value adjustment via the Tukey method. We plotted variation in parameters using the ‘ggplot2’ package (Wickham 2016) and a color palette from the ‘ggsci’ package (Xiao 2023).
We used two principal component analyses (PCA) to assess and visualize seasonal groupings. We standardized (centered and scaled) all variables. We included biochemical and blood lipid variables in one PCA: glucose, cholesterol, triglycerides, sodium, potassium and BUN (N = 40 individuals). In the second PCA, we included variables related to hematology: PCV, estimated WBC count, neutrophils, eosinophils, basophils, lymphocytes, monocytes and N: L ratio (N = 43 individuals). We performed PCAs using the function ‘prcomp’ from the ‘stats’ package (R Core Team 2023). We retained two PCA components from each PCA based on the Kaiser–Guttman criterion (eigenvalues > 1) (Jackson 1993) and scree plots. Subsequently, to assess if seasonal differences occurred along these components, we used one LM per component and included season, age category and sex as explanatory variables. We visualized seasonal difference using the ‘factoextra’ package (Kassambara and Mundt 2020).
Results
In total, we collected 45 blood samples and 87 body mass measurements between July 2020 and March 2021 (Table 1). No anesthetic complications were noted, and all squirrels recovered uneventfully from anesthesia. Physical examination in all animals was within clinically acceptable limits with no major abnormalities detected.
Adults (mean ± SD = 539 ± 51 g) were heavier than juveniles (338 ± 70 g; Fig. 2a). Neither adults nor juveniles showed sex differences in body mass (Table 2). There were no seasonal differences in adult body mass (Fig. 2a; Table 2). In juveniles, there was a trend that body mass was higher in winter (410 ± 20 g) compared to fall (308 ± 63 g; Fig. 2a), but there were no other seasonal differences (Fig. 2a; Table 2). Few parameters varied between seasons. Potassium levels were higher in summer compared to spring, and creatinine levels and BUN were higher in summer compared to fall (Fig. 2; Table 2). Few parameters varied between sexes; females had higher cholesterol and lower creatinine values than males (Fig. 2; Table 2). Adults had higher values of potassium, chloride, creatinine and lactate compared to juveniles (Fig. 2; Table 2). There were no sex, age or seasonal differences in glucose, triglycerides, ionized calcium, sodium, AST, anion gap or TCO2 (Fig. 2; Table 2; Table S1).
Violin plots of body mass, glucose, lipids (cholesterol and triglycerides), electrolytes, creatinine, blood urea nitrogen (BUN), aspartate aminotransferase (AST), and anion gap of urban eastern gray squirrels (Sciurus carolinensis) sampled in Durham, NC, USA. Values for adults and juveniles are combined for each plot, except for body mass (a), potassium (g), chloride (h) and creatinine (i), where adults had higher values than juveniles. The shaded area represents the kernel density plot, filled black circles show median values, error bars show the interquartile range, and points represent individual values. Note the truncated y-axis in (f + h). Published minimum and maximum ranges for gray squirrels are displayed as green horizontal lines (Guthrie et al. 1966; Hoff et al. 1976a, b; Ratliff et al. 2019; Stothart et al. 2019; Schmidt et al. 2022)
Number of monocytes was higher in summer compared to fall and spring (Fig. 3f; Table 3). Other parameters did not vary between seasons. Hemoglobin and PCV were higher in adults compared to juveniles (Fig. 3a + b). There was a trend towards adults having more eosinophils compared to juveniles (Table 3). There were no sex, age or seasonal differences in N: L ratio, estimated WBC count, number of basophils, eosinophils, neutrophils, or lymphocytes (Fig. 3; Table 3).
Violin plots of hematologic analytes of urban eastern gray squirrels (Sciurus carolinensis) sampled in Durham, NC, USA. Shaded areas represent kernel density plot, filled black circles show median values, error bars show the interquartile range, and points represent individual values. Values for adults and juveniles are combined for each plot, except for hemoglobin (a), packed cell volume (b) and eosinophils (e). Published minimum and maximum ranges for gray squirrels are displayed as horizontal lines (Guthrie et al. 1966; Hoff et al. 1976a, b; Ratliff et al. 2019; Stothart et al. 2019; Schmidt et al. 2022)
Principal components analysis (PCA)
Together, PC1 and PC2 of the PCA using biochemical and blood lipid parameters explained 51.4% of the variation in variables, and PC1 and PC2 of the PCA using hematological parameters explained 63.4% of variation (Table S3, Table S4). In the first PCA, PC1 represented blood lipids and BUN (driven by a positive contribution of cholesterol and BUN and a negative contribution of triglycerides) and PC2 loaded positively on sodium and negatively on glucose. PC1 values differed between summer and fall (Estimate = -1.53, CI = -2.74 – -0.33, P = 0.010; Fig. 4a), and there were no other seasonal differences (summer vs. spring: Estimate = -1.20, CI = -2.53–0.13, P = 0.085; fall vs. spring: Estimate = -0.33, CI = -1.79–1.12, P = 0.841). There were no seasonal differences in PC2 (all P ≥ 0.36; Fig. 4a). In the second PCA, PC1 loaded positively on estimated WBC count, lymphocytes and basophils, and PC2 loaded positively on N: L ratio and neutrophils. There were no seasonal differences in PC1 (all P ≥ 0.14; Fig. 4b) and PC2 (all P ≥ 0.61; Fig. 4b).
Individual factor maps of the principal components retained from two principal component analyses (PCAs): (a) PCA using biochemical and blood lipid parameters (N = 40), and (b) PCA using hematological parameters (N = 43) of urban eastern gray squirrels (Sciurus carolinensis). Filled circles represent individual squirrels sampled in different seasons and the three large filled circles represent seasonal means
Discussion
The general patterns of our results suggest that the urban habitat in which the current study was conducted is relatively benign for gray squirrels. Overall, health parameters measured in this study fell within published reference ranges for the species (Figs. 2 and 3, Table S5). We detected seasonal differences in few health parameters, i.e., body mass (juveniles only), potassium, BUN, creatinine and monocytes. Apart from body mass changes, all observed seasonal differences occurred between summer and other study periods. These results, together with largely unremarkable physical examinations, suggest that urban squirrels in this study population can maintain good health in an anthropogenic habitat with exposure to human activities and access to anthropogenic food.
Seasonal changes in health parameters
Overall, we observed seasonal differences in few health parameters. During late summer and fall, gray squirrels typically show a period of fattening in preparation for winter (Koprowski 1994; Koprowski et al. 2016). Juveniles in our study were heavier in winter compared to fall, likely due to continued growth, maturation and fattening. In contrast, adults did not show seasonal changes in body mass and thus, we found no evidence of a body mass gain prior to winter. It is possible that seasonal fluctuations of natural food availability are buffered by supplementary (anthropogenic) food resources available in urban habitats. Anthropogenic food, in the form of food waste, is readily available at the study site and is consumed most often in winter (Rimbach et al. 2023). Thus, urban gray squirrels may be less energy limited during the cold months compared to squirrels without access to these resources, allowing them to maintain their body mass throughout winter. Constant access to food resources is further indicated by the lack of seasonal variation in blood glucose levels because food shortages would lead to decreased blood glucose levels (Schradin et al. 2015; Rimbach et al. 2017). Higher air temperatures in urban compared to surrounding areas, termed ‘urban heat island effect’ (Imhoff et al. 2010), may further contribute to a relaxation of energetic constraints. This is because warmer ambient temperatures caused by impervious surfaces can reduce some of the metabolic costs associated with activity at low ambient temperatures. Squirrels had higher potassium and BUN levels in summer compared to spring or fall, respectively, but both parameters fell near the lower limit of published values for gray squirrels. These seasonal changes likely reflect seasonal changes in ingested food sources. Creatinine levels were higher in summer compared to fall. As elevations in creatinine can indicate hemoconcentration and/or dehydration, these results suggest that squirrels may have been less hydrated in summer. Dehydration may also explain higher BUN levels in summer compared to fall. These results likely stem from high ambient temperatures during the summer sampling period and are unlikely to be clinically significant. We detected seasonal differences along the first component (representing blood lipids and BUN) of the PCA using biochemical and blood lipid parameters. However, the factor maps of the principal components showed large overlap between seasons (Fig. 4). This overlap reiterates the results of the other analysis, further highlighting little seasonal variation in health parameters in the studied squirrel population.
Males had higher hemoglobin levels than females, as has been reported previously in the species (Guthrie et al. 1966). In many rodents, red blood cell indices (hematocrit, hemoglobin) are highest in winter, lowest in spring and recover during summer and fall (Mira and Mathias 1994; Beldomenico et al. 2008; Katiukhin et al. 2023). Elevated hematocrit and hemoglobin levels in winter likely reflect higher metabolic activity (Sealander 1962; Rosenmann and Ruiz 1993). However, hematic cycles of other rodents differ from this general pattern (Pérez-Suárez et al. 1990) or are absent altogether (Puchalski and Heldmaier 1986). We did not observe seasonal changes in red blood cell indices. Total energy expenditure of gray squirrels does not vary seasonally (Bryce et al. 2001), indicating that energetic requirements may not vary between seasons. This may be especially true in Durham (NC), where winters are cold-to mild with little snowfall. In addition, the ‘urban heat island effect’ may be buffering urban squirrels from cold temperatures in winter and thus, further eliminate the need for seasonal hematological adjustments to ambient temperature.
The number of monocytes was higher in summer compared to both fall and spring. Monocytes are part of the innate immune system and elevated numbers in summer may indicate a higher occurrence of infection or inflammation. We observed a total of three wounds on trapped squirrels and two were identified on squirrels trapped in summer (i.e., one male with a partially amputated tail, presumably due to previous trauma and a female with an acute wound on her head). Several individuals sampled in summer had monocyte numbers that were higher than reported in reference values (Fig. 3). While possible that these individuals had an underlying source of inflammation or infection, no other health parameter or the finding of the physical examination lends support to this explanation. Alternatively, increased number of monocytes (monocytosis) is often seen with a stress response and thus, may have resulted from capture, brief manual restraint, and anesthesia of squirrels. In addition, manual WBC counts are less accurate and more variable than WBC counts conducted via automated analyzers. Thus, it is possible that these more extreme values are due to misidentifications of WBCs during manual counts rather than reflecting responses to infection or inflammation.
Squirrels in urban habitats
Urban squirrels examined in this study maintained comparable blood glucose levels across seasons, and they had higher blood glucose levels compared to levels reported from both urban and rural squirrels in other studies (summarized in Table S5). The acute stress response to capture and handling can result in hyperglycemia (Sapolsky et al. 2000; Fletcher and Boonstra 2006; Bosson et al. 2012). Capture methods varied between studies summarized in Table S5 and included both capture with and without anesthesia, anesthesia using different anesthetics (inhalant vs. injected) and with and without the use of premedication (i.e., anxiolytic medications like midazolam). Moreover, glucose levels were determined after blood collection within very variable time windows (e.g., within 10 min after collection, in serum or plasma within 12 h of capture). Squirrels from a forest habitat (Korstian Division of Duke Forest (35.9810 N, 79.0168 W), Chapel Hill, NC, USA), captured (December 2020), handled and anesthetized using the same methodology and by the same researchers had lower blood glucose levels (average ± SD = 7.13 ± 1.50 mmol/L; N = 11; unpubl. data) compared to the urban squirrels at Duke University campus (9.98 ± 1.68 mmol/L; N = 43). Therefore, stress due to capture and/or technique is unlikely to be the single explanation for the high blood glucose levels observed in this study. Elevated blood glucose levels have previously also been reported in wildlife that received food provisioning (Knapp et al. 2013) and in species with access to anthropogenic food (Banks et al. 2003; Schulte-Hostedde et al. 2018; Gadau et al. 2019). Urban Eurasian red squirrels (Sciurus vulgaris) incorporate higher proportions of non-natural food items in their diet and have a higher sugar intake than rural conspecifics (Wist et al. 2022). In humans and some animal species, increased sugar consumption is linked to several diseases and dysfunctions such as diabetes, obesity and cardiovascular diseases (Malik et al. 2010; Johnson et al. 2013). For example, obesity and insulin resistance have been reported in yellow baboons (Papio cynocephalus) feeding regularly on trash dumps (Banks et al. 2003). Similarly, racoons (Procyon lotor) with high access to food waste develop hyperglycemia (Schulte-Hostedde et al. 2018). Reference intervals are variable depending on the animals used for the establishment (e.g., with respect to sex, age, body mass, health status), environment/location, seasonality, methods of sample collection and analyses, and the establishment of reference values is time- and labor-intensive (Friedrichs et al. 2012), and often reference intervals are not readily available for wildlife. Thus, comparisons of blood parameters between different studies, even those conducted on the same study species, should be drawn with caution, especially when different methodologies for blood collection and analysis were used.
Squirrels on Duke West campus consume anthropogenic food items from trash bins, mainly starchy foods such as French fries, pizza and bread, but also cookies (Rimbach et al. 2023). Access to anthropogenic food sources is often linked to increased body mass in many mammals (Cypher and Frost 1999; Banks et al. 2003; Otali and Gilchrist 2004; Hillman et al. 2017; Schulte-Hostedde et al. 2018). In contrast, body mass of urban gray squirrels and Eurasian red squirrels (S. vulgaris) is lower (Beliniak et al. 2022) or comparable to that of rural conspecifics (Shimamoto et al. 2020; Schmidt et al. 2022; Wist et al. 2022). The consumption of high-fat anthropogenic food, either in the form of food waste or unsuitable supplemental food, can result in elevated cholesterol and/or triglyceride levels in wildlife (Gavett and Wakeley 1986; Cypher and Frost 1999; Banks et al. 2003; Soto-Calderón et al. 2016; Monzón-Argüello et al. 2018; Castillo-Contreras et al. 2021). While an adequate supply of these blood lipids is critical to physiological functioning, excess concentrations are associated with decreased cardiometabolic health in humans and laboratory animals (Yuan et al. 1997; Tabas 2002; McMillen and Robinson 2005; Nordestgaard and Varbo 2014). For example, mourning doves (Zenaida macroura) showed impaired cardiovascular function after receiving an urban diet (50% anthropogenic foods) for four weeks, even in the absence of changes in body mass, blood glucose, insulin and triglyceride concentration (Basile et al. 2021). However, few studies have assessed effects of elevated blood lipid concentrations on cardiometabolic health and survival in wildlife. In this study, urban squirrels showed cholesterol and triglyceride concentrations comparable to other studies (Guthrie et al. 1966; Hoff et al. 1976b; Schmidt et al. 2022; Table S5). Although we detected elevated blood glucose levels in urban squirrels, we found no evidence that these elevated blood glucose levels were costly or indicative of other signs of poor cardiometabolic health (for example, in the form of obesity or elevated blood lipids). Gray squirrels rely on fat stores and food caches during winter (Koprowski 1994), and thus may have evolved mechanisms regulating body mass (and body fat) which reduce the risk of developing cardiometabolic diseases. These results suggest that potential dietary differences between urban and rural habitats do not (negatively) affect gray squirrel cardiometabolic health.
An anthropogenic diet can increase protein intake and result in elevated BUN and uric acid (Gavett and Wakeley 1986; Knapp et al. 2013; Monzón-Argüello et al. 2018). However, BUN concentrations reported in this study fell within published reference levels (Table S5). Urban squirrels consume meat and poultry from trash bins (Rimbach et al. 2023), but rural squirrels are also known to ingest protein in the form of small vertebrates, bird eggs and hatchlings (Koprowski 1994). This may indicate that urban gray squirrels and squirrels inhabiting less disturbed natural habitats ingest similar amounts of protein, albeit from different sources, similarly to Eurasian red squirrels (Wist et al. 2022). Electrolyte concentrations and blood chemistry parameters fell within published reference values for the species (Fig. 2; Table S5). Squirrels had potassium and AST levels near or below the lower limit of published reference values (Fig. 2; Table S5). Low potassium concentrations have also been reported in other species that consume anthropogenic food and in other urban squirrels (Knapp et al. 2013; Schmidt et al. 2022). Some squirrels had sodium levels above the maximum reported reference values (Ratliff et al. 2019; Schmidt et al. 2022). High sodium and low potassium levels occur in many humans and these electrolyte alterations have been linked to hypertension (Meneely and Battarbee 1976). It is unclear if changes in electrolyte concentrations in wildlife are reflective of similar health effects.
Low red blood cell counts are indicative of anemia whereas high red blood cell counts can indicate hemoconcentration (dehydration). Red blood cell indices (PCV, hemoglobin) of squirrels in this study fell within published reference values (Guthrie et al. 1966; Hoff et al. 1976a; Ratliff et al. 2019). Physical examinations were clinically unremarkable across seasons. We observed wounds on three squirrels: one male with an abscess on his scrotum, one male with a partially amputated tail (presumably due to previous trauma) and a female with an acute wound on her head. A recent study determined that urban squirrels are 4.5 times more likely to have a healed fracture compared to rural individuals (Moncrief et al. 2022), indicating that injuries are more common in urban squirrels. Our results did not show elevated N: L ratio or elevated estimated or differential white blood cell counts in urban squirrels compared to other studies (Table S5). We would have expected squirrels to have elevated levels in all three parameters if they suffer from infection or are under elevated physiological stress (Davis et al. 2008; Johnstone et al. 2012). In a previous study, we also found no differences in physiological stress, measured via hair cortisol concentration, a long-term indicator of circulating glucocorticoids, between squirrels from urban and rural habitats in the study area (Rimbach et al. 2022). It is conceivable that seriously ill individuals are less likely to enter traps because they suffer a higher predation risk or because they are weaker and less active (Genovart et al. 2010). Conversely, sick animals may not be able to forage normally and thus, may be more likely to enter a baited trap. But to date, it is unknown whether habitat differences exist regarding the ‘trap happiness’ of sick individuals. Taken together, our results suggest that urban habitats are relatively benign and do not cause severe adverse physiological responses in urban gray squirrels.
Data availability
All data are presented in the manuscript and supplementary information. Copies of the dataset are available upon request from the corresponding author.
Code availability
R scripts are available from the corresponding author.
References
Alberti M, Palkovacs EP, Des Roches S et al (2020) The complexity of urban eco-evolutionary dynamics. Bioscience 70:772–793. https://doi.org/10.1093/biosci/biaa079
Altmann J, Schoeller D, Altmann SA et al (1993) Body size and fatness of free-living baboons reflect food availability and activity levels. Am J Primatol 30:149–161. https://doi.org/10.1002/ajp.1350300207
Andersson MN, Nilsson J, Nilsson JÅ, Isaksson C (2018) Diet and ambient temperature interact to shape plasma fatty acid composition, basal metabolic rate and oxidative stress in great tits. J Exp Biol 221:jeb186759. https://doi.org/10.1242/jeb.186759
Auman HJ, Meathrel CE, Richardson A (2008) Supersize me: does anthropogenic food change the body condition of silver gulls? Waterbirds 31:122–126. https://doi.org/10.1675/1524-4695(2008)31[122:SMDAFC]2.0.CO;2. A comparison between urbanized and remote, non-urbanized areas
Banks WA, Altmann J, Sapolsky RM et al (2003) Serum leptin levels as a marker for a syndrome X-like condition in wild baboons. J Clin Endocrinol Metab 88:1234–1240. https://doi.org/10.1210/jc.2002-021695
Basile AJ, Renner MW, Kayata L et al (2021) A four-week urban diet imparis vasodilation but not nutriotional physiology in wild-caught mourning doves (Zenaida macroura). Physiol Biochem Zool 94:241–252
Beck AM (2002) The ecology of stray dogs: a study of free-ranging urban animals. Purdue University, West Lafayette, IN
Beckmann JP, Berger J (2003) Using black bears to test ideal-free distribution models experimentally. J Mammal 84:594–606. https://doi.org/10.1644/1545-1542(2003)084%3C0594:UBBTTI%3E2.0.CO;2
Bedrosian TA, Fonken LK, Walton JC, Nelson RJ (2011) Chronic exposure to dim light at night suppresses immune responses in siberian hamsters. Biol Lett 7:468–471. https://doi.org/10.1098/rsbl.2010.1108
Beldomenico PM, Telfer S, Gebert S et al (2008) The dynamics of health in wild field Vole populations: a haematological perspective. J Anim Ecol 77:984–997. https://doi.org/10.1111/j.1365-2656.2008.01413.x
Beliniak A, Gryz J, Klich D et al (2022) Body condition and breeding of urban red squirrels: comparison of two populations affected by different levels of urbanization. Animals 12:3246. https://doi.org/10.3390/ani12233246
Benson E (2013) The urbanization of the eastern gray squirrel in the United States. J Am Hist 100:691–710. https://doi.org/10.1093/jahist/jat353
Berger A, Lozano B, Barthel LMF, Schubert N (2020) Moving in the dark—evidence for an influence of artificial light at night on the movement behaviour of European hedgehogs (Erinaceus europaeus). Animals 10:1306. https://doi.org/10.3390/ani10081306
Birnie-Gauvin K, Peiman KS, Gallagher AJ et al (2016) Sublethal consequences of urban life for wild vertebrates. Environ Rev 24:416–425. https://doi.org/10.1139/er-2016-0029
Bonier F (2012) Hormones in the city: endocrine ecology of urban birds. Horm Behav 61:763–772. https://doi.org/10.1016/j.yhbeh.2012.03.016
Bosson CO, Islam Z, Boonstra R (2012) The impact of live trapping and trap model on the stress profiles of north American red squirrels. J Zool 288:159–169. https://doi.org/10.1111/j.1469-7998.2012.00941.x
Bowers MA, Breland B (1996) Foraging of gray squirrels on an urban-rural gradient: use of the gud to assess anthropogenic impact. Ecol Appl 6:1135–1142. https://doi.org/10.2307/2269597
Bryce JM, Speakman JR, Johnson PJ, Macdonald DW (2001) Competition between Eurasian red and introduced Eastern grey squirrels: the energetic significance of body-mass differences. Proceeding R Soc B 268:1731–1736. https://doi.org/10.1098/rspb.2001.1700
Capilla-Lasheras P, Dominoni DM, Babayan SA et al (2017) Elevated immune gene expression is associated with poor reproductive success of urban blue tits. Front Ecol Evol 5:1–13. https://doi.org/10.3389/fevo.2017.00064
Castillo-Contreras R, Mentaberre G, Fernandez Aguilar X et al (2021) Wild boar in the city: phenotypic responses to urbanisation. Sci Total Environ 773:145593. https://doi.org/10.1016/j.scitotenv.2021.145593
Chatelain M, Massemin S, Zahn S et al (2021) Urban metal pollution explains variation in reproductive outputs in great tits and blue tits. Sci Total Environ 776:145966. https://doi.org/10.1016/j.scitotenv.2021.145966
Coogan SC, Raubenheimer D, Zantis SP, Machovsky-Capuska GE (2018) Multidimensional nutritional ecology and urban birds. Ecosphere 9:e02177. https://doi.org/10.1002/ecs2.2177
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. In: www.r-project.org/
Cypher BL, Frost N (1999) Condition of San Joaquin kit foxes in urban and exurban habitats. J Wildl Manage 63:930–938
Davis AK, Maney DL, Maerz JC (2008) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct Ecol 22:760–772. https://doi.org/10.1111/j.1365-2435.2008.01467.x
Ditchkoff SS, Saalfeld ST, Gibson CJ (2006) Animal behavior in urban ecosystems: modifications due to human-induced stress. Urban Ecosyst 9:5–12. https://doi.org/10.1007/s11252-006-3262-3
Dowding CV, Harris S, Poulton S, Baker PJ (2010) Nocturnal ranging behaviour of urban hedgehogs, Erinaceus europaeus, in relation to risk and reward. Anim Behav 80:13–21. https://doi.org/10.1016/j.anbehav.2010.04.007
Eley RM, Strum SC, Muchemi G, Reid GDF (1989) Nutrition, body condition, activity patterns, and parasitism of free-ranging troops of olive baboons (Papio anubis) in Kenya. Am J Primatol 18:209–219. https://doi.org/10.1002/ajp.1350180304
Engel LD, Carlen EJ, Losos JB, Winchell KM (2020) Eastern gray squirrels (Sciurus carolinensis) differ in abundance and response to humans across urban habitats of St. Louis. Urban Nat 33:1–16
Fernández-Juricic E (2002) Can human disturbance promote nestedness? A case study with breeding birds in urban habitat fragments. Oecologia 131:269–278. https://doi.org/10.1007/s00442-002-0883-y
Finkler H, Hatna E, Terkel J (2011) The influence of neighbourhood socio-demographic factors on densities of free-roaming cat populations in an urban ecosystem in Israel. Wildl Res 38:235–243. https://doi.org/10.1071/WR10215
Fletcher QE, Boonstra R (2006) Impact of live trapping on the stress response of the meadow Vole (Microtus pennsylvanicus). J Zool 270:473–478. https://doi.org/10.1111/j.1469-7998.2006.00153.x
Friedrichs KR, Harr KE, Freeman KP et al (2012) ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol 41:441–453. https://doi.org/10.1111/vcp.12006
Gadau A, Crawford MS, Mayek R et al (2019) A comparison of the nutritional physiology and gut microbiome of urban and rural house sparrows (Passer domesticus). Comp Biochem Physiol 237:110332. https://doi.org/10.1016/j.cbpb.2019.110332
Gavett AP, Wakeley JS (1986) Blood constituents and their relation to diet in urban and rural house sparrows. Condor 88:279–284. https://doi.org/10.2307/1368873
Gaynor KM, Hojnowski CE, Carter NH, Brashares JS (2018) The influence of human disturbance on wildlife nocturnality. Science 360:1232–1235
Genovart M, Negre N, Tavecchia G et al (2010) The young, the weak and the sick: evidence of natural selection by predation. PLoS ONE 5:e9774. https://doi.org/10.1371/journal.pone.0009774
Grimm NB, Faeth SH, Golubiewski NE et al (2008) Global change and the ecology of cities. Science 319:756–760. https://doi.org/10.1126/science.1150195
Guthrie DR, Mosby HS, Osborne JC (1966) Hematological values for the eastern gray squirrel (Sciurus carolinensis). Can J Zool 44:323–327
Hansen CP, Parsons AW, Kays R, Millspaugh JJ (2020) Does use of backyard resources explain the abundance of urban wildlife? Front Ecol Evol 8:570771. https://doi.org/10.3389/fevo.2020.570771
Hein EW (1997) Demonstration of line transect methodologies to estimate urban gray squirrel density. Environ Manage 21:943–947. https://doi.org/10.1007/s002679900078
Hillman AE, Lymbery AJ, Elliot AD, Thompson RCA (2017) Urban environments alter parasite fauna, weight and reproductive activity in the quenda (Isoodon obesulus). Sci Total Environ 607–608:1466–1478. https://doi.org/10.1016/j.scitotenv.2017.07.086
Hoff GL, Lassing EB, Chan MS et al (1976a) Hematological values for free-ranging urban gray squirrels (Sciurus c carolinensis). Am J Vet Res 37:99–101
Hoff GL, McEldowny LE, Bigler WJ et al (1976b) Blood and urinary values in the gray squirrel. J Wildl Dis 12:349–352
Iglesias-Carrasco M, Aich U, Jennions MD, Head ML (2020) Stress in the city: meta-analysis indicates no overall evidence for stress in urban vertebrates. Proceeding R Soc B 287:20201754
Imhoff ML, Zhang P, Wolfe RE, Bounoua L (2010) Remote sensing of the urban heat island effect across biomes in the continental USA. Remote Sens Environ 114:504–513. https://doi.org/10.1016/j.rse.2009.10.008
Isaksson C (2010) Pollution and its impact on wild animals: a meta-analysis on oxidative stress. EcoHealth 7:342–350. https://doi.org/10.1007/s10393-010-0345-7
Isaksson C (2015) Urbanization, oxidative stress and inflammation: a question of evolving, acclimatizing or coping with urban environmental stress. Funct Ecol 29:913–923. https://doi.org/10.1111/1365-2435.12477
Isaksson C, Bonier F (2020) Urban evolutionary physiology. In: Szulkin M, Munshi-South J, Charmantier A (eds) Urban Evolutionary Biology. Oxford University Press, pp 217–233
Ives CD, Lentini PE, Threlfall CG et al (2016) Cities are hotspots for threatened species. Glob Ecol Biogeogr 25:117–126. https://doi.org/10.1111/geb.12404
Jackson D (1993) Stopping rules in principal components analysis: a com- parison of heuristical and statistical approaches. Ecology 74:2204–2214
Johnson RJ, Nakagawa T, Sanchez-lozada LG et al (2013) Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 62:3307–3315. https://doi.org/10.2337/db12-1814
Johnstone CP, Reina RD, Lill A (2012) Interpreting indices of physiological stress in free-living vertebrates. J Comp Physiol B 182:861–879. https://doi.org/10.1007/s00360-012-0656-9
Kassambara A, Mundt F (2020) factoextra: extract and visualize the results of multivariate data analyses. R package version 1.0.7. In: https://CRAN.R-project.org/package=factoextra
Katiukhin LN, Chalabov SI, Bekshokov KS et al (2023) Seasonal changes in blood rheology in little ground squirrels. J Exp Zool Part A 339:474–486. https://doi.org/10.1002/jez.2693
Knapp CR, Hines KN, Zachariah TT et al (2013) Physiological effects of tourism and associated food provisioning in an endangered iguana. Conserv Physiol 1:1–12. https://doi.org/10.1093/conphys/cot032
Kophamel S, Illing B, Ariel E et al (2022) Importance of health assessments for conservation in noncaptive wildlife. Conserv Biol 36:e13724. https://doi.org/10.1111/cobi.13724
Koprowski J (1994) Sciurus carolinensis. Mamm Species 480:1–9
Koprowski JL, Munroe KE, Edelman AJ (2016) Gray not grey: the ecology of Sciurus carolinensis in their native range in North America. In: Craig S, Lurz P, Gurnell J (eds) Grey Squirrels: Ecology & Management of an invasive species in Europe. European Squirrel Initiative, pp 1–17
Lowry H, Lill A, Wong BBM (2013) Behavioural responses of wildlife to urban environments. Biol Rev 88:537–549. https://doi.org/10.1111/brv.12012
Lüdecke D, Ben-Shachar M, Patil I et al (2021) Performance: an R package for assessment, comparison and testing of statistical models. J Open Source Softw 60:3139. https://doi.org/10.21105/joss.03139
Malik VS, Popkin BM, Bray GA (2010) Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 121:1356–1364. https://doi.org/10.1161/CIRCULATIONAHA.109.876185
Maraci Ö, Corsini M, Antonatou-Papaioannou A et al (2022) Changes to the gut microbiota of a wild juvenile passerine in a multidimensional urban mosaic. Sci Rep 12:6872. https://doi.org/10.1038/s41598-022-10734-7
Marzluff JM (2005) Island biogeography for an urbanizing world: how extinction and colonization may determine biological diversity in human-dominated landscapes. Urban Ecosyst 8:157–177. https://doi.org/10.1007/978-0-387-73412-5
Mathews F, Moro D, Strachan R et al (2006) Health surveillance in wildlife reintroductions. Biol Conserv 131:338–347. https://doi.org/10.1016/j.biocon.2006.04.011
McKinney ML (2002) Urbanization, biodiversity, and conservation. Bioscience 52:883–890
McMillen IC, Robinson JS (2005) Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 85:571–633. https://doi.org/10.1152/physrev.00053.2003
Meneely GR, Battarbee HD (1976) High sodium-low potassium environment and hypertension. Am J Cardiol 38:768–785. https://doi.org/10.1016/0002-9149(76)90356-8
Mira A, Mathias M (1994) Seasonal effects on the hematology and blood plasma proteins of two species of mice Mus musculus domesticus and M. spretus (Rodentia: Muridae) from Portugal. Hystrix,. Hystrix 5:63–72
Moncrief ND, Hightower L, Mead AJ, Ivanov K (2022) Prevalence and location of survivable skeletal injuries in two north American tree squirrels (Sciurus). J Mammal 103:663–671
Monzón-Argüello C, Cardona L, Calabuig P et al (2018) Supplemental feeding and other anthropogenic threats to green turtles (Chelonia mydas) in the Canary Islands. Sci Total Environ 621:1000–1011. https://doi.org/10.1016/j.scitotenv.2017.10.126
Moore CB, Siopes TD (2000) Effects of lighting conditions and melatonin supplementation on the cellular and humoral immune responses in Japanese quail Coturnix coturnix japonica. Gen Comp Endocrinol 119:95–104. https://doi.org/10.1006/gcen.2000.7496
Murray M, Cembrowski A, Latham ADM et al (2015) Greater consumption of protein-poor anthropogenic food by urban relative to rural coyotes increases diet breadth and potential for human-wildlife conflict. Ecography 38:1235–1242. https://doi.org/10.1111/ecog.01128
Murray M, Sánchez CA, Becker DJ et al (2019) City sicker? A meta-analysis of wildlife health and urbanization. Front Ecol Environ 17:575–583. https://doi.org/10.1002/fee.2126
Neto PGG, Nowakowski AJ, da Silva a FC et al (2020) Leukocyte profiles of two neotropical anuran species affected by anthropogenic habitat alteration. Anim Conserv 23:524–532. https://doi.org/10.1111/acv.12564
Nordestgaard BG, Varbo A (2014) Triglycerides and cardiovascular disease. Lancet 384:626–635
Oro D, Genovart M, Tavecchia G et al (2013) Ecological and evolutionary implications of food subsidies from humans. Ecol Lett 16:1501–1514. https://doi.org/10.1111/ele.12187
Otali E, Gilchrist JS (2004) The effects of refuse feeding on body condition, reproduction, and survival of banded mongooses. J Mammal 85:491–497. https://doi.org/10.1644/BRG-021
Parker TS, Nilon CH (2008) Gray squirrel density, habitat suitability, and behavior in urban parks. Urban Ecosyst 11:243–255. https://doi.org/10.1007/s11252-008-0060-0
Parsons AW, Forrester T, Baker-Whatton MC et al (2018) Mammal communities are larger and more diverse in moderately developed areas. Elife 7:e38012. https://doi.org/10.7554/eLife.38012
Pérez-Ortega B, Hendry AP (2023) A meta-analysis of human disturbance effects on glucocorticoid hormones in free-ranging wild vertebrates. Biol Rev 98:1459–1471. https://doi.org/10.1111/brv.12962
Pérez-Suárez G, Arévalo F, López-Caballero E, López-Luna P (1990) Seasonal variations in hematological values and heart weight in two small mammals, a mouse: Apodemus sylvaticus, and a Vole: Pitymys Duodecimcostatus. Acta Theriol 35:201–208. https://doi.org/10.4098/at.arch.90-25
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-Plus. Springer, New York, NY
Plaza PI, Lambertucci SA (2017) How are garbage dumps impacting vertebrate demography, health, and conservation? Glob Ecol Conserv 12:9–20. https://doi.org/10.1016/j.gecco.2017.08.002
Plummer KE, Bearhop S, Leech DI et al (2013) Winter food provisioning reduces future breeding performance in a wild bird. Sci Rep 3:2002. https://doi.org/10.1038/srep02002
Prange S, Gehrt SD, Wiggers EP (2004) Influences of anthropogenic resources on raccoon (Procyon lotor) movements and spatial distribution. J Mammal 85:483–490. https://doi.org/10.1644/BOS-121
Puchalski W, Heldmaier G (1986) Seasonal changes of heart weight and erythrocytes in the djungarian hamster, Phodopus sungorus. Comp Biochem Physiol -- Part Physiol 84:259–263. https://doi.org/10.1016/0300-9629(86)90610-9
Ratliff C, Kingsley L, Kusmierczyk J et al (2019) Hematologic and biochemical values of the juvenile eastern gray squirrel (Sciurus carolinensis). J Zoo Wildl Med 50:644. https://doi.org/10.1638/2018-0106
Rimbach R, Pillay N, Schradin C (2017) Both thyroid hormone levels and resting metabolic rate decrease in African striped mice when food availability decreases. J Exp Biol 220:837–843. https://doi.org/10.1242/jeb.151449
Rimbach R, Grant A, Gupte PR et al (2022) Comfortably numb? Regional differences in the relationship between indices of urbanization and a stress indicator in eastern gray squirrels. Urban Nat 9:1–15
Rimbach R, Butler G, Gupte PR et al (2023) Gray squirrels consume anthropogenic food waste most often during winter. Mamm Biol 103:69–81. https://doi.org/10.1007/s42991-022-00326-3
Rosenmann M, Ruiz G (1993) Seasonal changes of blood values in the Andean mouse Abrothrix andinus. Comp Biochem Physiol 105A:119–122
Sapolsky RM, Romero ML, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89
Schmidt C, Treberg JR, Kinnunen RP, Garroway CJ (2022) Serum biochemistry suggests a physiological response to environmental stress in a native urban eastern gray squirrel (Sciurus carolinensis) population. Can Field-Naturalist 136:20–29
Schoech SJ, Bowman R (2003) Does differential access to protein influence differences in timing of breeding of Florida Scrub-jays (Aphelocoma coerulescens) in suburban and wildland habitats? Auk 120:1114–1127. https://doi.org/10.1007/978-0-387-73412-5_26
Schoech SJ, Bridge ES, Boughton RK et al (2008) Food supplementation: a tool to increase reproductive output? A case study in the threatened Florida Scrub-Jay. Biol Conserv 141:162–173. https://doi.org/10.1016/j.biocon.2007.09.009
Schradin C, Pillay N, Kondratyeva A et al (2015) Basal blood glucose concentration in free-living striped mice is influenced by food availability, ambient temperature and social tactic. Biol Lett 11:20150208. https://doi.org/10.1098/rsbl.2015.0208
Schulte-Hostedde AI, Mazal Z, Jardine CM, Gagnon J (2018) Enhanced access to anthropogenic food waste is related to hyperglycemia in raccoons (Procyon lotor). Conserv Physiol 6:coy026. https://doi.org/10.1093/conphys/coy026
Schwacke LH, Thomas L, Wells RS et al (2024) An expert-based system to predict population survival rate from health data. Conserv Biol 38:e14073. https://doi.org/10.1111/cobi.14073
Sealander JA (1962) Seasonal changes in blood values of deer mice and other small mammals. Ecology 43:107–119
Seress G, Sándor K, Evans KL, Liker A (2020) Food availability limits avian reproduction in the city: an experimental study on great tits Parus major. J Anim Ecol 89:1570–1580. https://doi.org/10.1111/1365-2656.13211
Shimamoto T, Uchida K, Koizumi I et al (2020) No evidence of physiological stress in an urban animal: comparison of fecal cortisol metabolites between urban and rural eurasian red squirrels. Ecol Res 35:243–251. https://doi.org/10.1111/1440-1703.12081
Sol D, Lapiedra O, González-Lagos C (2013) Behavioural adjustments for a life in the city. Anim Behav 85:1101–1112. https://doi.org/10.1016/j.anbehav.2013.01.023
Soto-Calderón ID, Acevedo-Garcés YA, Álvarez-Cardona J et al (2016) Physiological and parasitological implications of living in a city: the case of the white-footed tamarin (Saguinus leucopus). Am J Primatol 78:1272–1281. https://doi.org/10.1002/ajp.22581
Stothart MR, Palme R, Newman AEM (2019) It’s what’s on the inside that counts: stress physiology and the bacterial microbiome of a wild urban mammal. Proc R Soc B 286:20192111
Strasser EH, Heath JA (2013) Reproductive failure of a human-tolerant species, the American kestrel, is associated with stress and human disturbance. J Appl Ecol 50:912–919. https://doi.org/10.1111/1365-2664.12103
Sumasgutner P, Nemeth E, Tebb G et al (2014) Hard times in the city-attractive nest sites but insufficient food supply lead to low reproduction rates in a bird of prey. J Wildl Rehabil 34:17–31. https://doi.org/10.1186/1742-9994-11-48
Tabas I (2002) Cholesterol in health and disease. J Clin Invest 110:583–590
Thrall MA, Weiser G, Allison RW, Campbell TW (2012) Veterinary hematology and clinical chemistry, 2nd edn. Wiley-Blackwell
Townsend AK, Staab HA, Barker CM (2019) Urbanization and elevated cholesterol in American crows. Condor 121:1–10. https://doi.org/10.1093/condor/duz040
Tuomainen U, Candolin U (2011) Behavioural responses to human-induced environmental change. Biol Rev 86:640–657. https://doi.org/10.1111/j.1469-185X.2010.00164.x
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York
Wilcoxen TE, Horn DJ, Hogan BM et al (2015) Effects of bird-feeding activities on the health of wild birds. Conserv Physiol 3:1–13. https://doi.org/10.1093/conphys/cov058
Wist B, Stolter C, Dausmann KH (2022) Sugar addicted in the city: impact of urbanisation on food choice and diet composition of the eurasian red squirrel (Sciurus vulgaris). J Urban Ecol 8:juac012. https://doi.org/10.1093/jue/juac012
Xiao N (2023) Ggsci: scientific journal and sci-fi themed color palettes for ggplot2. https://cran.r-project.org/package=ggsci
Yuan YV, Kitts DD, Godin DV (1997) Influence of dietary cholesterol and fat source on atherosclerosis in the Japanese quail (Coturnix japonica). Br J Nutr 78:993–1014. https://doi.org/10.1017/s0007114598001810
Acknowledgements
We thank Charles Veronee, Christi Mela, Claire Parker, Gaby Butler, Hannah Chen, Jörg Jäger, Sophia Rimbach, Lilly Kelemen, Nicholas Amato, William Marcus for help during data collection. We thank Jörg Jäger for blood smear analysis and Daniel Schmitt for lending us his microscope for blood smear analysis. We are also grateful to Michael Muehlbauer and Huaxia Cui for their help with analyzing blood samples for AST and blood lipids. We also thank Andrea Thomson for her help with sample processing and acquisition of supplies. We thank two anonymous reviewers for their constructive comments on an earlier version of the manuscript.
Funding
This work was supported by Duke University and North Carolina State University.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
RR and OAP conceived the idea and designed the methodology; RR and HP coordinated the project; RR, JAB and OAP conducted the field work and collected the data; RR and OAP analyzed the data; RR prepared the figures; RR led the writing of the manuscript. All the authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Ethics approval
We followed all applicable institutional and national guidelines for the care and use of animals, and we complied with the ARRIVE guidelines. All squirrels were trapped and handled with approval by the Duke University Institutional Animal Care and Use Committee (Protocol number: A057-20-03 8 N), North Carolina Wildlife Resources Commission (Permit numbers: 20-SC01363, 21-SC01363), Duke University and Duke Forest (R1920-492).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rimbach, R., Petritz, O.A., Balko, J.A. et al. Urban eastern gray squirrels (sciurus carolinensis) show little seasonal variation in biochemical and hematological parameters. Urban Ecosyst (2024). https://doi.org/10.1007/s11252-024-01566-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s11252-024-01566-6