Abstract
Urbanization progresses world-wide and the frequency of biological invasions increases. Understanding to what extent urban environments facilitate biological invasions and how this affects ecosystems within and outside urbanized areas thus becomes crucial. We here examine the role of urban environments in the invasion of the butterfly Pieris mannii that expanded across Central Europe within the last two decades. Using standardized butterfly captures at paired urban and (semi)natural field sites within the invaded range in Switzerland, we demonstrate that P. mannii is strongly associated with cities and towns. At least in some urban localities, this species is now the most common butterfly, and densities tend to be particularly high where urban areas are large. Because urban habitats commonly provide non-native host plants and perhaps also the physical structure and microclimate suitable to this butterfly, urbanization has clearly promoted the species’ rapid invasion. Studying phenology over an entire season, we further infer that P. mannii has up to six generations per year, which may allow the species to adjust its life cycle to changing season length during northward expansion. Overall, our study demonstrates how preadaptation to urbanized environments in a relatively specialized insect increases urban biodiversity on a large geographic scale.
Similar content being viewed by others
Introduction
Biological invasions are becoming increasingly frequent (Seebens et al. 2017, 2021). This trend is attributed, on the one hand, to the increased translocation of organisms by humans as a consequence of progressing globalization (Mack et al. 2000; McKinney 2006; Kowarik 2011). On the other hand, human modifications of local environments can generate ecological opportunities for non-native organisms preadapted to the novel conditions, or offer non-native organisms a competitive advantage over natives (Byers 2002; New 2015). A particularly drastic environmental modification is the conversion of (semi)natural habitats into urban environments. Urbanization generally coincides with the establishment of novel ecological conditions such as extensive impervious surfaces, elevated temperatures, a high presence of non-native species, and high habitat heterogeneity on a small spatial scale (Thompson et al. 2003; Menke et al. 2011; Pincebourde et al. 2016; Cadotte et al. 2017; Borden and Flory 2021). Urban environments are therefore often considered hot spots for the establishment of non-native species (McKinney 2006; Kowarik 2011). Much remains to be learned, however, about how urban environments influence the invasion dynamics of particular species (Gaertner et al. 2017; Hui and Richardson 2017), and to what extent urban environments serve as a starting point for the spread of non-native species into natural environments outside urbanized zones (Cadotte et al. 2017; Borden and Flory 2021), potentially with negative effects on native biodiversity and ecosystems (Diamond 1989; Mack et al. 2000; Blackburn et al. 2019).
To address the latter questions, we here investigate the role of urban environments in the invasion of a butterfly, the Southern Small White (Pieris mannii) (Fig. 1, insert photograph). Historically known as native mainly to southern (Mediterranean) Europe and exhibiting highly localized, stable and sedentary populations (Kromer 1963; SBN 1991; Ziegler and Eitschberger 1999; Ziegler 2009), this species has initiated a dramatic range expansion across Central Europe. The first clear signs of an invasion can be traced back to 2005 in south-western Switzerland (Ziegler 2009). By 2008, this country was colonized and range expansion progressed northward into southern Germany (Herrmann 2010; Hensle and Seizmair 2015). By now, the species has reached the northern edge of Germany (Wiemers et al. 2020b). Within less than 20 years, P. mannii has thus expanded by at least 850 km, progressing at a speed of 50–100 km per year. The invasion is particularly well documented for Switzerland and Germany and appears to have initially occurred approximately northward (Ziegler 2009; Herrmann 2010; Wiemers 2016; Wiemers et al. 2020b), but also continues in other directions (Gros 2018; Vantieghem 2018; Wiemers et al. 2020b).
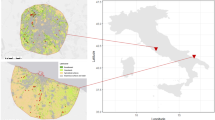
Historical and present distribution of Pieris mannii in Switzerland, and study sites. The black dots in the main map show all localities with historical records of the species, covering the time period 1894–2004. The historical population from the western-most tip of Switzerland likely became extinct historically, as no records were made in this region between 1926 and 2005. The bottom-right insert map shows P. mannii’s post-invasion distribution in Switzerland, based on records from 2005 to 2022. Some regions in central, southern and eastern Switzerland are not occupied by the species because they represent unsuitable high-altitude zones in the Alps. The localities chosen for the present study are located within the newly colonized range and represent urban and natural sites in four regions around larger cities. The locality numbers correspond to those listed in Table 1. Locality 21 (white dot) represents an urban site used to investigate phenology. The top-left insert map shows the situation of Switzerland in Central Europe and the photograph depicts a female specimen of P. mannii
Because the invasion is supposed to have originated from the edge of the native range in south-eastern France and has progressed continuously, it likely did not involve the direct spread of the species by humans but instead occurred via natural dispersal (Ziegler 2009). The causes underlying this sudden dispersal remain to be elucidated; climate change may play a role but is alone considered an unsatisfactory explanation, given the speed of the range expansion, that the expansion started from a geographically localized region (Settele et al. 2008; Vantieghem 2018; Wiemers et al. 2020b), and that climatic conditions within the new range do not seem to have been limiting prior to the expansion (Neu et al. 2021). That the invasion of P. mannii may have been promoted by urban environments is suggested by two observations: first, recent records of this butterfly generally come from urban localities (Ziegler 2009; Herrmann 2010; Vantieghem 2018). Second, although P. mannii qualifies as a host plant specialist within its original native range, with only a handful of Brassicaceae species known to be exploited by the larval stage (caterpillar) (Ziegler and Eitschberger 1999), two of these are planted widely as garden ornament (Iberis sempervirens) or as food (Diplotaxis tenuifolia) in urbanized areas across Central Europe (the latter species is also naturalized outside urban zones) (Fig. S1, Supplementary Information). However, a relatively high observer density in cities may bias the spatial distribution of P. mannii records toward urban areas (see also Gippet et al. 2022), and the butterfly may have broadened its host plant repertoire within the invaded range, allowing the colonization of more natural environments as well (Geier 2016; Köhler 2021; Neu et al. 2021). A formal study is therefore needed to shed light on the role urban environments play in the invasion of P. mannii.
We initiate such an investigation by focusing on P. mannii in its invaded range in Switzerland. Our main goal is to characterize the species’ habitat use through observations in urban and adjacent natural environments. Moreover, since invasion success and particularly the capacity for range expansion is influenced by a given organism’s life cycle and dispersal capacity (Macgregor et al. 2019; Wallingford et al. 2020), we additionally explore P. mannii’s phenology and aspects of its dispersal behavior.
Methods
Study region and butterfly habitat use
In a first observational experiment, we studied P. mannii’s habitat use in the Swiss Plateau, that is, in the northern part of Switzerland situated at low elevation (300—800 m a.s.l.) outside the Alps (Fig. 1). The Swiss Plateau was colonized in the beginning of the butterfly’s documented invasion, between 2005 and 2008 (Ziegler 2009). The study region is thus located outside the edge of P. mannii’s historical range, here formed by sedentary populations known since the late nineteenth century (SBN 1991).
Our observation design involved urban-natural site pairs at five localities within each of four regions around large cities in Switzerland (i.e., 20 localities and 40 observation sites in total; Fig. 1, Table 1). Within each region, the study localities were selected to cover a gradient in the size of the urbanized area, ranging from the main city to small rural towns nearby (resident population sizes for all localities are given in Table 1). All urban sites within the site pairs were chosen based on aerial imagery and were required to represent residential zones with a substantial proportion of vegetated surface (i.e., extensive vegetation-free industrial, commercial or traffic zones were avoided). The corresponding natural sites within the site pairs were selected analogously but had to be situated 1–3 km outside any urban environment. These sites comprised (semi)natural and agricultural open habitat dominated by meadows and pastures, sometimes bordering woodland but never including forest habitat.
Butterfly observations at the study sites were made from August to mid-September 2021. This time window was chosen because it lies well within the species’ flight season when we expected P. mannii to have overlapping generations and hence to be present continuously and at relatively high density (Herrmann 2010; Hensle and Seizmair 2015; Schurian and Siegel 2016; see also our phenology data below). Standardized observations were made by sweep-netting while walking continuously and haphazardly along roads and walks, or also cross-country at the natural sites, for one hour (sectors were allowed to be inspected repeatedly), thus travelling a total distance of around 2 km at each site. Because P. mannii cannot be distinguished reliably from other Pieris species in flight, we captured all representatives from this genus (i.e., P. mannii, P. rapae, P. napi, and P. brassicae). Individuals were then identified to species, recorded, marked with a permanent felt-tip pen to avoid multiple recording, and released immediately, except for some P. mannii that were retained for future genomic work. The latter includes two individuals that could not be identified unambiguously based on wing characters and were confirmed as P. mannii based on genomic sequence data (L. Blattner, unpublished). All observations were made between 10:00 and 17:30 in sunshine and at least 16 °C ambient temperature, with little or no wind. The paired urban and natural sites of a given locality were always visited consecutively in haphazard order on the same day.
Raw individual counts resulting from the above observations were visualized by separating P. mannii individuals from all other Pieris individuals grouped together. As this revealed that P. mannii is associated with urban environments, we further asked whether this butterfly may maintain particularly high population densities where urban areas are relatively large. We explored this idea by performing linear regressions of butterfly counts from all 20 urban sites against the size of these localities quantified by two different metrics (both linearized by log10 scaling): human resident population size obtained from municipality population census data for 2021 (Swiss Federal Statistical Office, https://www.bfs.admin.ch), and site surface area measured by using the online map tool from swisstopo (Swiss Federal Office of Topography, https://map.geo.admin.ch) (Table 1). Surface area was here defined as the perimeter around a given urban site encompassing all contiguous surface characterized by the presence of buildings, allowing this area to exceed a locality’s formal municipal territory. By this definition, the urban site 13 proved distantly connected by urban habitat to site 11, hence these localities share the same surface area value (Table 1). As these two sites are still separated by more than 12 km direct distance, we retained them as independent data points, although excluding site 13 from analysis had a trivial influence on the results and did not affect any conclusion (details not presented). The two regression analyses (i.e., with population size and surface area as predictors) were again done separately for P. mannii and for all other Pieris species combined (or, as supplementary analyses, just for P. rapae). For each butterfly group, we determined the 95% compatibility interval (i.e., the 0.025 and 0.975 distribution quantiles) for the regression slope estimates by bootstrap resampling the urban sites 50,000 times and re-calculating the slope for each iteration (Manly 2006). In these regression analyses, we assume that our metrics of urban area size serve as reasonable proxies for the extent of urban habitat actually suitable to P. mannii. We caution, however, that our focal localities may differ qualitatively with respect to the butterfly’s habitat requirements, and also in their connectivity to other urbanized areas nearby, so we consider these analyses relatively noisy.
Phenology and dispersal
To better understand P. mannii’s phenology and to gain insight into its dispersal behavior, we combined presence-absence data, quantitative captures made in regular time intervals, and information on butterfly age distribution produced during the year 2022 at a single urban site near Basel (locality 21 in Fig. 1 and Table 1, coinciding with the P. mannii sample site from Berner et al. 2023). Unlike the previous experiment on habitat use, observations here did not involve walking but instead were performed within a small perimeter of approximately 10 × 10 m by a single person (DB).
P. mannii overwinters in the pupal stage, and based on observations from previous years, we anticipated the emergence of adult butterflies as early as March (see also Hensle and Seizmair 2015; Schurian and Siegel 2016; Wiemers 2016), and that at least the first generations were well separated temporally (i.e., non-overlapping). We thus started to look out for the species in March and made sporadic sweep net captures until early June; the low density during this early part of the species’ flight season precluded more formal observation schemes. A sudden rise in density in June, however, allowed us to switch to a standardized observation strategy involving the capture of at least ten P. mannii individuals within nine total consecutive time windows of a few days each (2.9 d on average; range 1–8 d). Observations proceeded in this way until early September, when densities dropped and again allowed for sporadic captures only. Irrespective of the species, all Pieris individuals captured (or recaptured) during this observational experiment were provided with a pen marking specific to the corresponding time window, transferred to the fridge, and released only at the end of that day’s observation period. This procedure avoided recaptures of individuals on the same day, and permitted identifying recaptures within and across time windows.
A minimum of ten P. mannii individuals per time window (excluding recaptures) was considered necessary to explore the relative proportion of different butterfly age categories – information we considered helpful for estimating the number of generations over the total flight season. Assigning individuals to age categories was possible because all P. mannii individuals captured (including a haphazard subset of recaptures) were photographed in a standardized way (Fig. S2). Evaluating the condition of the wing fringes from the photographs, individuals were given a condition score from 1–4, with 1 representing fresh butterflies with immaculate fringes, and 4 being individuals with completely worn fringes (a representative individual from each age class is presented in Fig. S2). Wing fringe scoring was performed by a single person (NM) after randomizing the photographs with respect to an individual’s capture date, and hiding the dates. Each individual was scored four times on independent occasions. In 80% of the individuals (total n = 124), all four scores were identical, while the remaining 20% exhibited only one deviant score among the four. We thus used an individual’s median score for analysis. A small subset of individuals (n = 12) were captured and photographed more than once, which allowed directly relating wing fringe condition to approximate butterfly age. This check confirmed that wing fringes permitted reliably separating fresh individuals (i.e., not more than a few days old) from older animals (Fig. S3).
Because the time effort spent observing was recorded for the majority of observation days, the above capture data from study site 21 further allowed an approximate estimation of the density of P. mannii over the flight season – another piece of information we deemed useful for studying phenology. For this, we scaled our observations as the number of P. mannii per hour, hereafter referred to as P. mannii density. Because individuals were occasionally observed but could not be captured and because other Pieris species occurred at the experimental site, our captures (and marking) always targeted every Pieris individual, irrespective of the species. Based on the total number of Pieris individuals observed, and the proportion of P. mannii among all Pieris actually captured on a given day, this allowed estimating P. mannii densities more accurately. For the analysis of phenology, the sporadic observations, age distributions, and density estimates were all graphed together along the seasonal time axis. We here generally included the recaptures, although for roughly half of the recaptures (Fig. S3), photographs were missing so that these observations did not enter the age distributions.
Owing to our marking of all Pieris butterflies, the capture data from site 21 further allowed us to explore P. mannii’s dispersal behavior and life span. The former was performed by using P. rapae for comparison. P. rapae is the sister species of P. mannii (Wiemers et al. 2020a), is an agricultural pest species now spread nearly world-wide due to human introductions (Ryan et al. 2019), and is considered a strong flyer and disperser (Gilbert and Raworth 2005). Based on our observation of an association of P. mannii with urban environments, we asked whether P. mannii shows a less pronounced dispersal tendency relative to P. rapae, which would result in a higher recapture probability at this study site. To examine this idea, we calculated for both Pieris species the probability that an individual was recaptured at least once, with 95% compatibility intervals for the probability estimated by bootstrap resampling individuals 50,000 times. To gain insights into P. mannii’s life span in the wild, we graphed the cumulative time interval between successive captures for all individuals recaptured at least once (n = 28). We emphasize that this approach underestimates individuals’ real life spans, because we were able to track individuals from the first capture to the last recapture only, not from birth to death. For P. rapae, the paucity of recaptures (n = 2) precluded a meaningful exploration of life span.
Finally, we examined the consistency between years in the relative abundance of P. mannii among all Pieris species at the urban study locality 21. We here focused on the period between June 25 and July 11, for which capture data generated as described above were also available from the year 2020 (n = 44 total Pieris individuals). We thus selected from the 2022 data the subset of Pieris captures (n = 46) made during the same period, and compared the relative proportion of P. mannii between the two years, with 95% compatibility intervals estimated by bootstrap resampling of all Pieris individuals.
Results
Habitat use by P. mannii
Our butterfly observations at urban and natural site pairs at 20 localities produced 486 total individual butterfly captures and revealed an unambiguous pattern: the invasive P. mannii was recorded at all but one urban sites, but not a single individual was seen at any of the 20 natural sites nearby (Fig. 2). By contrast, the other Pieris species (essentially P. rapae and P. napi; P. brassicae accounted for only c. 1% of the captures) occurred at both urban and natural sites. This striking difference in P. mannii occurrence between the two site types cannot be explained by differential capture success, as the total number of butterfly captures was very similar between the urban (n = 253) and natural (n = 233) sites. Averaged over all urban sites, P. mannii made up 40% of the butterfly captures. At some urban sites, however, P. mannii was clearly the most common Pieris species (Fig. 2), and captures from two different years at a single urban site (locality 21) suggest that such high abundances are maintained over years (Fig. S4).
Individual counts of P. mannii and other Pieris species at urban (top) and associated natural (bottom) sites. The 20 total localities are ordered consecutively from left to right according to Table 1 (excluding locality 21), hence are ordered by decreasing resident population size within the study regions. The study regions are separated by alternating white and light gray backgrounds
Interestingly, while the other Pieris species were captured in greatest number at those urban study sites situated in relatively small urbanized localities, as expressed by both human resident population size and surface area, P. mannii displayed the opposite trend, with large cities consistently producing large captures (Fig. 3). These contrasting patterns between the butterfly species did not change qualitatively when comparing P. mannii to just P. rapae alone, the most common Pieris across our urban sites (43% of all butterfly captures on average) (Fig. S5).
Individual counts of P. mannii and other Pieris species at urban sites, plotted against human resident population size (top) and the surface area (bottom) of these localities (both log10 scaled). The histograms on the right display the bootstrap density distributions for the linear regression estimates of the two slopes, with the numerical values representing the observed point estimates (also indicated by dark gray triangles on the x-axes). Irrespective of the metric used to quantify locality size, regression slopes best compatible with the data are mostly positive for P. mannii, but negative for the other Pieris species (95% compatibility intervals indicated by light gray triangles on the x-axes)
Phenology
During our study of butterfly phenology over an entire flight season at the experimental site 21, we captured a total of 124 unique individuals of P. mannii. A single first observation of a (fresh) P. mannii occurred in late March, followed by several weeks without any observation (Fig. 4, bottom). New fresh individuals then appeared at the end of April, again followed by several weeks without sightings of (fresh) butterflies. After a remarkable surge in density in early June, the species remained sufficiently frequent until early September to allow standardized captures for estimating age distributions. The last fresh individuals were recorded in mid-September, and the last worn animal in mid-October.
Phenology and density of P. mannii over the 2022 field season at an urban site (locality 21; Table 1, Fig. 1). The bottom part shows observations of P. mannii, color coded by fringe condition. From mid-June to September, densities were high enough to allow systematic captures of at least ten individuals within a few consecutive days. For these capture windows, proportions for the fringe condition categories are presented, with total sample sizes given on top of the bars, and the precise sampling days indicated by gray lines on the bottom of the bars. Outside this period, densities were low and permitted only sporadic captures, shown individually as dots. The upper part displays estimated P. mannii density at each sampling day for which capture effort was recorded, expressed as the number of individual sightings per hour (data not available prior to June)
The age distribution and density data proved of limited utility for distinguishing the generations during the summer months (June–September); although the absence of fresh butterflies among the captures from the end of August and the appearance of such animals in September suggest the emergence of a distinct late-summer generation (Fig. 4, bottom), individuals from all age categories occurred throughout the preceding summer period, indicating broad overlap between generations. Moreover, after a striking peak in June, P. mannii density dropped to consistently low levels without clear peaks during July and early August (Fig. 4, top).
Dispersal and longevity
As hypothesized, our marking of all Pieris butterflies at site 21 revealed distinct recapture probabilities for P. mannii and P. rapae: while every fourth P. mannii was recaptured at least once during the experiment and several individuals were recaptured twice or even three times, only two out of 52 (c. 4%) of P. rapae were recaptured, and only once (Fig. 5a). Furthermore, the maximum time span between successive captures for an individual was 5 days on average in P. mannii (median 3.5 d), whereas the two recaptured P. rapae had both been marked the preceding day. Combined, this strongly suggests more localized dispersal in P. mannii than in its sister species. The mark-recapture data further showed that P. mannii can attain a life span up to at least three weeks under field conditions (Fig. 5b).
a The barplots on the left indicate the proportion of P. mannii and P. rapae individuals captured just once (i.e., not recaptured), or recaptured up to three times, over the summer of 2022 at an urban site (locality 21). Total sample sizes are given on top of the bars. The histograms on the right show for both species the bootstrap density distribution of the probability that an individual was recaptured at least once, and the numbers inside the graphs indicate the observed probabilities (also given as dark gray triangles on the x-axis). Recapture probabilities best compatible with the data range from 0.17–0.34 for P. mannii (95% compatibility interval, indicated by light gray triangles on the x-axis), but only from 0.0–0.10 for P. rapae. b Number of days between consecutive captures for all 28 recaptured P. mannii individuals
Discussion
Our experiment on P. mannii’s habitat use makes clear that the species’ invasion across Central Europe was promoted by urbanization; unlike two congeneric species, we observed this butterfly exclusively in urban environments. This exploitation of ecological opportunity associated with human presence resembles patterns of range expansion documented in other invasive species (Davis et al. 2014; Greig et al. 2017; Marques et al. 2020; Thawley and Kolbe 2020; Rivest and Kharouba 2021). Although we recognize that P. mannii is occasionally reported from (semi)natural habitats within its newly colonized range, the highly consistent association with urban environments in our and other investigations (Ziegler 2009; Herrmann 2010; Geier 2016) and the high density of such habitat across much of invaded Central Europe suggest that most of P. mannii’s global population may now well live synanthropically in towns and cities. Since representatives of the genus Pieris generally dominate urban butterfly communities numerically, P. mannii must qualify as the most common butterfly overall at least in some urban localities within the invaded range (see also Ziegler 2009; Herrmann 2010; and Rivest and Kharouba 2021 for extremely high abundances in another invasive butterfly). This may be true especially for larger cities, given our indication that P. mannii densities tend to scale positively with proxies of city size.
Although our investigation focused on a relatively small region colonized early during P. mannii’s range expansion across Europe (the species has currently progressed c. 700 km further north; Wiemers et al. 2020b), the particular expansion mode, relying strongly on urbanized environments, is likely to be representative of the butterfly’s invasion as a whole. The reason is that records from more recently colonized regions further north also come mostly from urban areas (Herrmann 2010; Hensle and Seizmair 2015; Von Scholley-Pfab and Pfab 2017; Vantieghem 2018). This butterfly thus provides a striking example of an invasive species enriching urban faunas locally, but at the same time making urban biodiversity more homogeneous at a broad spatial scale (Rahel 2002; Sax and Gaines 2003; McKinney 2006; Winter et al. 2009; Piano et al. 2020).
Our work raises the question how urban environments facilitate the establishment of P. mannii. The answer must partly be related to the larval host plants (see also Dexheimer and Despland 2023). This butterfly has indeed been speculated to have broadened its host plant repertoire within the invaded range (Geier 2016; Köhler 2021; Neu et al 2021). However, direct field observations do not support the view that host shifts play a role in this butterfly’s expansion success: at least two plant species known as hosts and wide-spread in the original native range of the species occur commonly, and are widely reported to actually be consumed by P. mannii larvae, in the newly colonized urban environments (Fig. S1) (Ziegler 2009; Herrmann 2010; Hensle and Seizmair 2015; Geier 2016; Pähler 2016; von Scholley-Pfab and Pfab 2017; DB and SR, personal observations). Urban habitats thus generally appear to lie within the species’ original foraging niche. However, equating host plant availability with butterfly habitat suitability may ignore more subtle requirements along the whole life cycle (Dennis et al. 2003; Vanreusel and Van Dyck 2007). Indeed, adult P. mannii appear to prefer sloping, rocky habitats – one reason why this butterfly occurs only locally in its original range (Ziegler and Eitschberger 1999). This structural habitat preference might be coupled with some form of home range fidelity, as indicated by our recapture data revealing a lower dispersal tendency in P. mannii than in its sister species P. rapae. Within our urban study sites, qualitative observations during field work (DB, SR) also suggest that the species frequently flies along vertical structures such as walls, escarpments and hedgerows. Although the latter needs formal examination, we speculate that cities not only offer adequate food resources, but may also structurally resemble natural rocky localities typically inhabited by the species (Ziegler 2009) and provide adequate microclimatic conditions (Pincebourde et al. 2016; Polidori et al. 2021). P. mannii’s establishment in urban environments despite relatively narrow ecological requirements (Ziegler and Eitschberger 1999) emphasizes the importance of fortuitous preadaptation to such environments (McKinney 2006; Kowarik 2011; Cadotte et al. 2017), and highlights that successful urban colonizers need not necessarily be generalists (McIntyre 2000).
What is the ecological impact of P. mannii’s invasion? Since P. mannii is largely synanthropic within its novel range and its larvae here feed on non-native host plant species, direct resource competition with native fauna should not be a concern. However, the butterfly’s expansion may have less obvious consequences. One example might be apparent competition with other Lepidoptera, mediated by shared parasites. Within its invaded range, P. mannii is attacked in the pupal stage by a pteromalid parasitoid wasp (Pteromalus puparum) that also parasitizes a wide range of other lepidopterans (de Graham 1969). The parasitation rate of P. mannii can be extremely high, sometimes approaching 100% (J. Hensle, personal communication; see Tajagi 1987 for similarly high parasitation rates in P. mannii’s sister species), which may be one possible explanation for the dramatic drop in P. mannii densities observed during the summer months at site 21 (Fig. 4, top). (Another possible explanation is that the butterfly undergoes partial heat diapause or quiescence – July and early August 2022 were dry and hot, with daily temperature maxima mostly > 30 °C; DB, SR and NM, personal observations). At least within urban areas, the high abundance of P. mannii might thus expose other Lepidoptera species to elevated parasitation pressure and hence greater mortality.
Another possible consequence of the invasion concerns the loss of ecological and genetic diversity within P. mannii itself. The invasion of this butterfly likely started from a relatively restricted area in south-eastern France (Ziegler 2009), and it is conceivable that the rapid spread of a range-expansive lineage may genetically homogenize native local populations, especially small localized populations at the edge of the original range (Kromer 1963; SBN 1991). This possibility is to be investigated by population genomic analyses including individual samples from the original and newly invaded range. In any event, following to what extent P. mannii remains primarily synanthropic or expands into natural habitats within the newly colonized range is relevant to both conservation and our understanding of invasion dynamics (Cadotte et al. 2017; Borden and Flory 2021).
An important question also concerns to what extent P. mannii’s range expansion may progress northward. Responses of butterflies to global warming suggest that the number of generations may be a crucial determinant of range expansion success, with multiple generation per year allowing the flexible accommodation of the life cycle to changing season length (Macgregor et al. 2019). In our study of P. mannii’s phenology, we identified three well-separated generations from late March to early June. These early generations are likely inferred correctly despite being based partly on sparse observation data. Support comes from the subsequent year (2023) at the same site, where unidentified Pieris first appeared in mid-March (see also Pähler 2016, Schurian and Siegel 2016, and Wiemers 2016 for reports of P. mannii appearing in March within the invaded range), fresh P. mannii were recorded around the April–May transition, and a striking rise in abundance occurred in mid-June (DB, personal observation) – qualitative observations very closely matching those from 2022 (Fig. 4). Between these three initial generations and a relatively well-separated last generation in September, neither our age distribution nor density data resolved the number of summer generations. However, at 23–26 °C constant temperature indoors, P. mannii originating from site 21 complete a full life cycle (larval hatch to oviposition) within around 30 days (DB, personal observation; see Geier 2016 and Pähler 2016 for similar findings). We thus conclude that in the study region, P. mannii can complete six generations per year, consistent with the life cycle inferred from phenology data from further north within the invaded range (Germany; Pähler 2016). The range expansion of this butterfly is thus unlikely to be constrained by season length; provided that other ecological requirements including host plant availability are satisfied, the species may expand further northward by adjusting the number of generations per season.
To summarize, we document that within our study region, range-expansive P. mannii butterflies are strongly synathropic, indicating that the invasion of this species across Central Europe was facilitated by urbanization. Exciting opportunities for future research include genetically resolving the origin of the invasive P. mannii population, exploring potential adaptations to the expansive and/or urban life style, and investigating how expansive P. mannii interact with native conspecific populations and with other organisms within and outside urban environments.
Data availability
All raw data underlying our analyses, and a compilation of all R code used for analysis, are provided on the figshare repository (https://doi.org/10.6084/m9.figshare.24943653).
References
Berner D, Ruffener S, Blattner LA (2023) Chromosome-level assemblies of the Pieris mannii butterfly genome suggest Z-origin and rapid evolution of the W chromosome. Genome Biol Evol 15:evad111. https://doi.org/10.1093/gbe/evad111
Blackburn TM, Bellard C, Ricciardi A (2019) Alien versus native species as drivers of recent extinctions. Front Ecol Environ 17:203–207. https://doi.org/10.1002/fee.2020
Borden JB, Flory SL (2021) Urban evolution of invasive species. Front Ecol Environ 19:184–191. https://doi.org/10.1002/fee.2295
Byers JE (2002) Impact of non-indigenous species on natives enhanced by anthropogenic alteration of selection regimes. Oikos 97:449–458
Cadotte MW, Yasui SLE, Livingstone S, MacIvor JS (2017) Are urban systems beneficial, detrimental, or indifferent for biological invasion? Biol Invasions 19:3489–3503. https://doi.org/10.1007/s10530-017-1586-y
Davis AY, Malas N, Minor ES (2014) Substitutable habitats? The biophysical and anthropogenic drivers of an exotic bird’s distribution. Biol Invasions 16:415–427. https://doi.org/10.1007/s10530-013-0530-z
Diamond JM (1989) The present, past and future of human-caused extinctions. Phil Trans R Soc Lond B 325:469–477. https://doi.org/10.1098/rstb.1989.0100
Dennis RLH, Shreeve TG, Van Dyck H (2003) Towards a functional resource-based concept for habitat: a butterfly biology viewpoint. Oikos 102:417–426
Dexheimer E, Despland E (2023) Newly introduced butterfly species’ urban habitat use driven by shorter vegetation and exotic plants. Biol Invasions 25:1767–1777. https://doi.org/10.1007/s10530-023-03009-3
Gaertner M, Wilson JRU, Cadotte MW et al (2017) Non-native species in urban environments: patterns, processes, impacts and challenges. Biol Invasions 19:3461–3469
Geier T (2016) Observations on the occurrence of the range-expanding butterfly species Pieris mannii (Mayer, 1851) in the lower Nahe area (Rhineland-Palatinate) with proof of three larval host plant species (Lepidoptera: Pieridae). Nachr Entomol Ver Apollo 37:27–40
Gilbert N, Raworth DA (2005) Movement and migration patterns in Pieris rapae (Pieridae). J Lepid Soc 59
Gippet JMW, Rocabert C, Colin T et al (2022) The observed link between urbanization and invasion can depend on how invasion is measured. Divers Distrib 28:1171–1179. https://doi.org/10.1111/ddi.13509
de Graham MWR, V. (1969) The Pteromalidae of north-western Europe (Hymenoptera: Chalcidoidea). Bull Br Mus Nat Hist, Entomol Suppl 16:1–909. https://doi.org/10.5962/p.258046
Greig EI, Wood EM, Bonter DN (2017) Winter range expansion of a hummingbird is associated with urbanization and supplementary feeding. Proc R Soc B 284:20170256. https://doi.org/10.1098/rspb.2017.0256
Gros P (2018) Arealausweitungen thermophiler Arten: Erster Nachweis von Pieris mannii (MAYER, 1851) aus den Bundesländern Salzburg und Oberösterreich (Lepidoptera: Pieridae). Linzer Biol Beitr 50:373–379
Hensle J, Seizmair M (2015) Papilionidae, Pieridae, Nymphalidae, Lycaenidae und Hesperiidae 2014. Atalanta 46:11–81
Herrmann R (2010) Die aktuelle Arealexpansion und Einbürgerung des Karstweißlings, Pieris mannii (Mayer, 1851), in Südwestdeutschland. Atalanta 41:197–206
Hui C, Richardson DM (2017) Invasion Dynamics. Oxford Academic, Oxford
Köhler J (2021) Bemerkenswerte Beobachtungen zu Biologie und Verhalten des Karstweisslings Pieris mannii (MAYER, 1851) an einer Population im Wendland im Nordosten Niedersachsens (Lepidoptera, Pieridae). Atalanta 52:6–9
Kowarik I (2011) Novel urban ecosystems, biodiversity, and conservation. Environ Pollut 159:1974–1983. https://doi.org/10.1016/j.envpol.2011.02.022
Kromer E (1963) Ein Beitrag über die Biologie und Flugstellen von Pieris manni Mayer in Niederösterreich. Z Wiener Entomol Ges 48:55–121
Macgregor CJ, Thomas CD, Roy DB et al (2019) Climate-induced phenology shifts linked to range expansions in species with multiple reproductive cycles per year. Nat Commun 10:4455. https://doi.org/10.1038/s41467-019-12479-w
Mack RN, Simberloff D, Mark Lonsdale W et al (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710. https://doi.org/10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2
Manly BFJ (2006) Randomization, bootstrap and Monte Carlo methods in biology, 3rd edn. Chapman & Hall, Boca Raton
Marques PS, Resende Manna L, Clara Frauendorf T et al (2020) Urbanization can increase the invasive potential of alien species. J Anim Ecol 89:2345–2355. https://doi.org/10.1111/1365-2656.13293
McIntyre NE (2000) Ecology of urban arthropods: a review and a call to action. Ann Entomol Soc Am 93:825–835. https://doi.org/10.1603/0013-8746(2000)093[0825:EOUAAR]2.0.CO;2
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Conserv 127:247–260. https://doi.org/10.1016/j.biocon.2005.09.005
Menke SB, Guénard B, Sexton JO et al (2011) Urban areas may serve as habitat and corridors for dry-adapted, heat tolerant species; an example from ants. Urban Ecosyst 14:135–163. https://doi.org/10.1007/s11252-010-0150-7
Neu A, Lötters S, Nörenberg L, Wiemers M, Fischer K (2021) Reduced host-plant specialization is associated with the rapid range expansion of a Mediterranean butterfly. J Biogeogr 48:3016–3031. https://doi.org/10.1111/jbi.14258
New TR (2015) Alien species in urban environments. In: New TR (ed) Insect conservation and urban environments. Springer International Publishing, Cham, pp 87–101
Pähler R (2016) Ein Blick auf die aktuelle Arealexpansion und Einbürgerung des Karstweißlings Pieris mannii (MAYER, 1851) in Deutschland sowie Anmerkungen zu den Flugzeiten (Lep., Pieridae). Melanargia 28:117–135
Piano E, Souffreau C, Merckx T et al (2020) Urbanization drives cross-taxon declines in abundance and diversity at multiple spatial scales. Glob Chang Biol 26:1196–1211. https://doi.org/10.1111/gcb.14934
Pincebourde S, Murdock CC, Vickers M et al (2016) Fine-Scale microclimatic variation can shape the responses of organisms to global change in both natural and urban environments. Integr Comp Biol 56:45–61. https://doi.org/10.1093/icb/icw016
Polidori C, García-Gila J, Blasco-Aróstegui J et al (2021) Urban areas are favouring the spread of an alien mud-dauber wasp into climatically non-optimal latitudes. Acta Oecol 110:103678. https://doi.org/10.1016/j.actao.2020.103678
Rahel FJ (2002) Homogenization of freshwater faunas. Annu Rev Ecol Syst 33:291–315. https://doi.org/10.1146/annurev.ecolsys.33.010802.150429
Rivest SA, Kharouba HM (2021) Anthropogenic disturbance promotes the abundance of a newly introduced butterfly, the European common blue (Polyommatus icarus; Lepidoptera: Lycaenidae), in Canada. Can J Zool 99:642–652. https://doi.org/10.1139/cjz-2021-0009
Ryan SF, Lombaert E, Espeset A et al (2019) Global invasion history of the agricultural pest butterfly Pieris rapae revealed with genomics and citizen science. Proc Natl Acad Sci USA 116:20015–20024. https://doi.org/10.1073/pnas.1907492116
Sax DF, Gaines SD (2003) Species diversity: from global decreases to local increases. Trends Ecol Evol 18:561–566. https://doi.org/10.1016/S0169-5347(03)00224-6
SBN (1991) Tagfalter und ihre Lebensräume, 3rd edn. Schweizerischer Bund für Naturschutz, Basel
Schurian K, Siegel A (2016) A contribution to the life history and ecology of Pieris mannii (Mayer, 1851) in Hesse, Germany (Lepidoptera: Pieridae). Nachr Entomol Ver Apollo 37:15–21
Seebens H, Blackburn TM, Dyer EE et al (2017) No saturation in the accumulation of alien species worldwide. Nat Commun 8:14435. https://doi.org/10.1038/ncomms14435
Seebens H, Bacher S, Blackburn TM et al (2021) Projecting the continental accumulation of alien species through to 2050. Glob Chang Biol 27:970–982. https://doi.org/10.1111/gcb.15333
Settele J, Kudrna O, Harpke A et al (2008) Climatic risk atlas of European butterflies. BioRisk 1:1–712
Tajagi M (1987) The reproductive strategy of the gregarious parasitoid, Pteromalus puparum (Hymenoptera: Pteromalidae) 3. Superparasitism in a Field Population Oecologia 71:321–324
Thawley CJ, Kolbe JJ (2020) Artificial light at night increases growth and reproductive output in Anolis lizards. Proc R Soc B 287:20191682. https://doi.org/10.1098/rspb.2019.1682
Thompson K, Austin KC, Smith RM et al (2003) Urban domestic gardens (I): Putting small-scale plant diversity in context. J Veget Sci 14:71–78. https://doi.org/10.1111/j.1654-1103.2003.tb02129.x
Vanreusel W, Van Dyck H (2007) When functional habitat does not match vegetation types: A resource-based approach to map butterfly habitat. Biol Conserv 135:202–211. https://doi.org/10.1016/j.biocon.2006.10.035
Vantieghem P (2018) First sightings of the southern small white Pieris mannii (Lepidoptera : Pieridae) in the Low Countries. Phegea 46:2–7
Von Scholley-Pfab A, Pfab F (2017) Beobachtungen zur Einwanderung und Ökologie von Pieris mannii (MAYER, 1851) im Großraum München (Lepidoptera: Pieridae). NachrBl Bayer Ent 66:58–65
Wallingford PD, Morelli TL, Allen JM et al (2020) Adjusting the lens of invasion biology to focus on the impacts of climate-driven range shifts. Nat Clim Chang 10:398–405. https://doi.org/10.1038/s41558-020-0768-2
Wiemers M (2016) Augen auf für neue Arten – zur Bestimmung und weiteren Ausbreitung des Karstweißlings Pieris mannii (Mayer, 1851) in Deutschland. Oedippus 32:34–36
Wiemers M, Chazot N, Wheat CW et al (2020a) A complete time-calibrated multi-gene phylogeny of the european butterflies. Zookeys 938:97–124. https://doi.org/10.3897/zookeys.938.50878
Wiemers M, Schmitz O, Caspari A, Berner D (2020b) Augen auf für neue Arten - Neues zum Karstweissling (Pieris mannii) mit der Bitte um Mitarbeit. Oedippus 38:45–47
Winter M, Schweiger O, Klotz S et al (2009) Plant extinctions and introductions lead to phylogenetic and taxonomic homogenization of the European flora. Proc Natl Acad Sci USA 106:21721–21725. https://doi.org/10.1073/pnas.0907088106
Ziegler H (2009) Zur Neubesiedlung der Nordwestschweiz durch Pieris mannii (Mayer, 1851) im Sommer 2008 (Lepidoptera, Pieridae). Entomo Helv 2:129–144
Ziegler H, Eitschberger U (1999) Der Karstweissling Pieris mannii (Mayer, 1851). Systematik, Verbreitung, Biologie (Lepidoptera, Pieridae). Neue Ent Nachr 45:5–169
Acknowledgements
The Gubler Heinzmann family kindly shared a car for field work, Tino and Rémy Grandchamp aided butterfly sampling at site 21, and Hannes Baur and Jürgen Hensle offered information on Pteromalus parasitoids. Distribution data for P. mannii were obtained from the Swiss topic center on fauna (info fauna), Neuchâtel. Two reviewers offered constructive feedback on the initial manuscript.
Funding
Open access funding provided by University of Basel This study was funded by the Swiss National Science Foundation grant 310030_200374 to DB.
Author information
Authors and Affiliations
Contributions
DB and SR conceived the study; SR and DB conducted field work; SR, NM and DB analyzed and interpreted data; DB wrote the manuscript, with input from SR and NM.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ruffener, S.C., Matthey-de-l’Endroit, N. & Berner, D. Invasion of Pieris mannii butterflies across Central Europe facilitated by urbanization. Urban Ecosyst (2024). https://doi.org/10.1007/s11252-024-01507-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s11252-024-01507-3