Abstract
Urbanisation has a strong role in pollinator decline, while properly managed green spaces could promote pollinators – especially bees and butterflies – by providing foraging and nesting habitats in cities. In public spaces, several opportunities exist to help pollinators including rare mowing, flower strip sowing, and bee hotel establishment. However, pollinator-promoting interventions are almost lacking and still understudied in Eastern Europe. We aimed to investigate the impact of rare mowing and annual flower-sowing interventions on pollinators, floral resources, and vegetation attributes in three case studies within Hungary. We compared treatment (extensively mown or sown) and control (intensively mown) site pairs during five sampling occasions within a season. We found positive, but due to the severe drought and management inaccuracies relatively minor effect of both interventions. The extensively mown sites presented higher and greener vegetation with more flowers and pollinators. The sown flower patches provided floral resources and supported the pollinators in the second half of the season, while they were scarcely visited in spring and early summer due to the annual soil scarification and re-sowing. Although the two types of interventions could complement each other during the season, there is room for further improvements. To reach resilient urban ecosystems against climate and environmental changes, we recommend using primarily native and mostly perennial seeds, combining intervention types, planning for the long-term, and avoiding management inaccuracies (e.g. unplanned mowing). Promoting and monitoring plant-pollinators systems may increase the diversity and human well-being in cities, while citizens’ involvement can facilitate these interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human well-being needs well-functioning ecosystems (Bratman et al. 2019). Although pollinators provide essential ecosystem functions e.g. via the reproduction of numerous crops and wild plants (IPBES 2016), their function (i.e. pollination) is threatened by global anthropogenic environmental changes such as intensive agriculture, climate change, and habitat loss (Potts et al. 2010; Vanbergen et al. 2013; IPBES 2016). Land use is one of the key factors in pollinator decline by the deprivation of diverse and natural habitats (Elmqvist et al. 2013; United Nations 2015; Potts et al. 2016), resulting in the loss of rare species and decreased diversity (Bates et al. 2011; Harrison et al. 2019). Urbanisation has a significant role in such land use change and habitat loss (United Nations 2015), driving biodiversity loss even more than agriculture in some parts of the world (Geslin et al. 2013; Rosin et al. 2021). In contrast, green spaces in urban areas can serve biodiversity by providing foraging and nesting habitats as well as refuges for many species, including pollinators (Pereira-Peixoto et al. 2014; Guenat et al. 2019; Baldock 2020; Ayers and Rehan 2021). Therefore, improving habitat conditions in urban areas could increase biodiversity and contribute to pollinator conservation (Aguilera et al. 2019; Persson et al. 2020; Steidle et al. 2022).
Several local- and landscape-scale solutions exist in urban settlements to promote pollinators (Baldock 2020). The proportion of green vegetation is one of the key factors influencing pollinators in an urban landscape (Zanette et al. 2005; Ahrne et al. 2009), thus improving the design and implementation of green infrastructure networks can be remunerative in cities (Nilon et al. 2017; European Commission 2023). Various positive effects can be achieved on pollinators by increasing the amount, size, and quality of urban green spaces (Nilon et al. 2017; Baldock et al. 2019; Daniels et al. 2020). Moreover, green-infrastructure development projects go beyond nature conservation and benefit also humans through clean air as well as psychological and social impacts (Bratman et al. 2019; Barragan-Jason et al. 2023). The public is becoming more and more aware of the relevance of biodiversity in urban areas (Nilon et al. 2017; Hall and Steiner 2019). However, increasing the amount and size of green space is usually extremely hard in densely populated, highly built-up urban environments (Jenks and Jones 2010).
Besides landscape-scale factors, the local management of urban vegetation is one of the main influential elements for pollinator communities (Öckinger et al. 2009; Aguilera et al. 2019; Persson et al. 2020) through the availability of their floral and nesting resources (Lerman et al. 2018; Phillips et al. 2020). Short-term abandonment or reduction of mowing frequency could naturally provide a higher amount of floral resources compared to high-intensity regular cutting, which is the usual management of urban vegetation (Valtonen et al. 2007; Wastian et al. 2016; Del Toro and Ribbons 2020). The achieved higher vegetation can buffer microclimatic conditions, retain water, and create habitat for pollinator offspring (Noordijk et al. 2009; Lange-Kabitz et al. 2021; Wintergerst et al. 2021). Rare mowing may be more cost-effective than the conventional maintenance of urban green areas (i.e. frequently mowed, usually irrigated parks; Garbuzov et al. 2015; O’Sullivan et al. 2017; Mody et al. 2020). Floral availability for pollinators can be also boosted by sowing herbaceous seed mixtures in public spaces (Blackmore and Goulson 2014; Hicks et al. 2016; Norton et al. 2019; Dietzel et al. 2023), which proved to be an efficient and popular approach in agricultural areas (Albrecht et al. 2020; Zamorano et al. 2020; Báldi et al. 2022). In addition, the habitat quality for pollinators in urban areas could also be improved with the diversification of the vegetation structure through planting shrubs and trees, as well as adding nesting places by bare ground surfaces and bee hotels (Baldock 2020), while noting that bee hotels can also host parasites and favour invasive species (Fortel et al. 2016).
In several cities in the USA and Western Europe, these interventions have been widely introduced, and initiatives (e.g. ‘No Mow May’, ‘All-Ireland Pollinator Plan’, ‘UK National Pollinator Strategy’) are helping cities by management guidelines, involving inhabitants in citizen science projects, and revealing the effectiveness by monitoring programmes (DEFRA 2014; Domroese and Johnson 2017; Wilk et al. 2019; Del Toro and Ribbons 2020; Mody et al. 2020; NBDC 2020). In contrast, in Eastern Europe (i.e. in most of the post-Soviet countries), the proportion of urban green spaces decreased until recently (Kabisch and Haase 2013; Kronenberg 2015), although this trend seems to be slowly reversing (Pauleit et al. 2019; Gavrilidis et al. 2020). Pollinator-promoting projects and monitoring studies have been started only in the last few years with a great variation among these countries (Skórka et al. 2013; Dylewski et al. 2019, 2020; Báthoryné et al. 2021), where biodiversity is generally higher than in Western Europe (Batáry et al. 2010; Kovács-Hostyánszki et al. 2016; Török et al. 2020). However, culturally negative attitudes can be observed towards such projects (Kronenberg 2015) compared to Western Europe (Hoyle et al. 2017; Southon et al. 2017). In the Eastern European region, detailed knowledge of the effectiveness of such pollinator promotion projects is still lacking. There are several studies about the effect of urbanisation on pollinators (e.g. Prague, Czech Republic; Konvicka and Kadlec 2011 and Poznan, Poland; Banaszak-Cibicka and Żmihorski 2020; Dylewski et al. 2020), but only a few took the impact of the local management of public green spaces into account (e.g. Poznan, Poland; Dylewski et al. 2019; Pardubice, Czech Republic; Horák et al. 2022, and Prague, Czech Republic; Rada et al. 2023).
In this study, we aimed to investigate the impact of pollinator-promoting interventions (rare mowing and flower sowing) on pollinators and wildflowers through three case studies from two Eastern European cities (Budapest and Veszprém in Hungary). We used an experimental approach, where we sampled pairs of urban green areas. One half of the site pairs were treatment sites (extensively mown or sown areas), while the other half of the pairs were control sites (conventionally managed areas with intensive mowing). We were interested in the (i) differences between the treatment and control sites in vegetation height and cover, the abundance, morphogroup richness and diversity of flowers and pollinators; (ii) temporal changes in the vegetation, pollinators, and flowers within a season; (iii) compositional differences in communities of pollinator guilds between the treatment and control sites during the season. Our aim was to identify the shortcomings and specificities of pollinator-promoting interventions by example studies from an Eastern European country (Hungary) and to make suggestions for the enhancement of such urban pollinator-promoting projects and monitoring studies.
Materials and methods
Studied pollinator-promoting interventions in Hungary
We studied the first attempts at pollinator-promoting management methods in two Hungarian cities. Veszprém city (administrative county centre in the Veszprém Plateau; ~266 m a.s.l.; 126.9 km2; 58,153 inhabitants, Wikipedia 2023b; 10.3 °C annual average and 532 mm annual total precipitation based on 10-year (2012–2021); Vmeteo 2022) started the ‘Wildflower Veszprém’ project in 2017 with reduced mowing frequency (3 mowings/year instead of more than 3) in seven test areas (hereafter: rare mowing in Veszprém).
In Budapest (the capital city within Buda Hills and Pest Alluvial Plain; ~105 m a.s.l.; 525.1 km2; 1,706,851 inhabitants, Wikipedia 2023a; 13.2 °C annual average and 538 mm annual total precipitation based on 10-year (2012–2021); KSH 2022), an extensive grassland management program started in 2021 within the ‘Wildflower Budapest’ project on twenty-two insect-friendly (in total ~ 28 ha) areas by the Horticultural Division of Budapest Public Utilities (Kovács et al. 2021). The mowing frequency in the mown grasslands was reduced to 1–2 mowing/year instead of more than two to enable wild herbaceous plants to develop flowers and seeds, and to disperse (hereafter: rare mowing in Budapest).
Independently from the Wildflower Budapest project, the 12th District of Budapest started the ‘Bee-friendly District’ program in 2018 (Hegyvidéki Önkormányzat 2020). They have been creating an increasing number of annually sown bee pastures (hereafter: flower sowing in Budapest). In this study, we sampled six sites sown with a herbaceous seed mixture of 44 native and adventive plant species originating from a Hungarian distributor (Mix1; Sipos 2020, see Table S1), and another six sites sown with an ornamental seed mixture of 40 different flower varieties originated from a Dutch distributor (Mix2; flower your place 2023; without detailed species list, containing at least 10 North American annual species). The sown sites were scarified and re-sown in spring every year, and regularly watered and weeded during the season.
Study sites
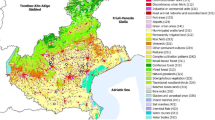
In 2022, we selected 5 site pairs of treatment (3 mowings/year) and control (4–7 mowings/year; conventionally mown parks) for rare mowing in Veszprém, 10 site pairs of treatment (1–2 mowings) and control (3–4 mowings/year; conventionally mown parks and road verges) for rare mowing in Budapest, and 12 site pairs of treatment (sown bee pastures) and control (conventionally mowed parks and road verges) for flower sowing in Budapest (Fig. 1; for coordinates see Supplement 2). During the site selection, we aimed to ensure that all sites were as similar as possible in size, exposure, vegetation type, and potential human impact while keeping paired sites close to each other. However, note that the appropriate controls for flower sowing in Budapest probably would be areas that were watered (and weeded) in the same way as the sown sites. However, such a comparison was not possible because most of the public road verges and parks are not watered in the same way as the sown sites.
The sampling sites within Hungary: rare mowing in Veszprém (A), rare mowing in Budapest (B) and flower sowing in Budapest (C). Numbers and letters represent site pairs. Black letters and numbers are the treatment sites, while grey letters and numbers are the control sites. The uncircled numbers represent the sites sown with Mix1, while the circled numbers are the sites sown with Mix2 in the C part of the Figure. Map data 2022 © OpenStreetMap
Sampling
We sampled the whole season from spring to autumn with five sampling occasions (end of April, mid-May, mid-June, mid-July, and end of August) in 2022. The control-treatment pairs were sampled haphazardly after each other on the same day to have as similar weather and daytime conditions as possible, decreasing the influence of the daily rhythm of flowers and pollinators. We sampled the vegetation, floral resources, and pollinators in 5 circular 160 cm diameter (2 m2) plots per site on each sampling occasion (using a similar, but further developed sampling method than the ‘Fit Count’ of UKCEH 2021). We placed the plots haphazardly paying attention to avoid shade within the sites. Within these circular plots, we measured the average vegetation height [cm] using a tape measure and estimated the green vegetation cover [%]. We recorded the number of flower units per morphogroups of all currently flowering, insect-pollinated herbaceous species (Szigeti et al. 2016; UKCEH 2021). Morphogroups refer to individuals (i.e. flowers and pollinators) that can be distinguished visually by phenotypic characteristics such as colour, size, body type, hairiness, patterns, etc. Furthermore, we recorded visually the number of pollinators at morphogroup levels during continuous 5 min per plot without catching the animals and stopping the stopwatch while writing information. Pollinators were also grouped into major guilds such as honey bees, bumblebees, solitary bees, hoverflies, butterflies, and moths. We conducted the samplings only in suitable weather conditions: rain-free hours; dry vegetation; temperature: 20–30 °C (except in April, when we sampled > 13 °C in full sunshine, and > 16 °C in partly cloudy weather); wind: <15 km/h (verified by an Extech 45,158 mini thermo-anemometer (Teledyne FLIR LLC; Wilsonville, Oregon, USA)), in daytime (April: 9.30 AM – 4.30 PM; May: 9.00 AM – 5.00 PM; June and July: 8.30 AM – 6.00 PM; August: 9.00 AM – 5.00 PM).
A year of challenges due to drought and imperfect management
The year 2022 was extreme in terms of weather conditions combined (and probably interrelated) with some inaccuracies in the site management of treatments. At the drought-monitoring station (Pusztaszabolcs) between Budapest and Veszprém, the drought index was above 3 (extreme drought) on 24 days (Fiala et al. 2018; ODWMS 2023) between 1 April and 1 September 2022. In contrast, there was not a single day in the previous 5 years when the drought index was above 3 (ODWMS 2023). Some management inaccuracies have occurred in both kinds of management methods. Part of these management inaccuracies are related to the drought, e.g. watering might favour the more resistant weeds in the sown areas, while intensive mowing was not needed. Because without precipitation, the vegetation did not grow well, so frequent mowing was not necessary. In addition, the vegetation in the rarely mowed sites did not develop much better and might not produce much more flowers than the control sites. Off-schedule mowings occurred in the program of rare mowing in Veszprém and rare mowing in Budapest, while sometimes un-removed hay also occurred in the latter. In the case of sowing in Budapest, probably a too strong watering in the initial period and a lack of proper weeding caused worse conditions than expected in some sites compared to previous years (pers. obs. of the local authority maintaining the intervention). Due to the drought and management inaccuracies, the following treatment sites of sowing in Budapest program have been withdrawn by the local authority from the pollinator-promoting project (i.e. vegetation has been cut and not watered) after mid-July: 4, 6, 8, Y (Fig. 1). However, we continued the sampling on these sites despite the cessation of the original management thus they were sampled as many times as the other sites.
Statistical analyses
For analyses, we applied the original plot-level data. We tested differences between treatment and control sites in height and green cover of vegetation; total abundance, morphogroup richness and Shannon diversity of floral resources and pollinators during the five sampling occasions. For this, we applied generalized linear mixed models (GLMMs) using Gaussian distribution on the ten-based logarithm of the vegetation height (adding one to all values before transformation due to zeros), Beta distribution on the vegetation green cover, zero-inflated Poisson distribution on abundances and morphogroup richness, and zero-inflated Gaussian distribution on Shannon diversities as the best-fitted distributions (Zuur et al. 2009). We added a small value (0.000001) to all zero and subtracted a small value (0.000001) from all values one of vegetation green cover to be able to analyse ratios because Beta distribution does not accept exact 0 and 1 values. The response variables were vegetation height and green cover; total abundance, morphogroup richness and Shannon diversity of floral resources and pollinators. The explanatory variables were the sampling occasions (i.e. April, May, June, July, and August as factors), the treatment, and their interactions. We included the site code within the site pair code into the model as nested random factors to treat the potential spatial autocorrelation by the sampling structure (i.e. control-treatment pairs of sites were close in space and sampling time) and the pseudoreplication (i.e. five replications during one sampling occasion) within the site. We analysed separately the three case studies. We extracted the model estimates, standard errors and p-values for controls and treatments for all the sampling occasions. Furthermore, we tested the spatial autocorrelations both on our raw data and also on the residuals of our models by Moran I tests (Li et al. 2007) to ascertain that the applied nested random factors are treated the potential autocorrelations (Table S2).
In addition to the previous model for the sowing in Budapest case study, we run models including also the effect of the two types of seed mixture to test their influences. For this, we applied a model selection based on the AIC values by a comparison between the models including (1) the sampling occasions, treatment and their interaction (i.e. the original model presented previously), (2) adding mixture type as an additive explanatory variable, (3) adding mixture type and treatment interaction. In the case of smaller differences than two between the AIC values of compared models, we averaged the models and reported coefficients.
Besides models, we evaluated the seasonal changes graphically. For this, all sampling occasions of each variable were averaged for the treatments, controls, and all sites separately, for each case study, and presented on a figure by these descriptive statistics.
Furthermore, we analysed the effects of treatment on the community composition of main pollinator guilds. For this, we calculated the abundance of pollinator guilds per site and sampling occasion by pooling data from the plots. We used zero-adjusted Bray–Curtis dissimilarities, adding a dummy guild with abundance = 1 to each site in each sampling occasion (Clarke et al. 2006; Borcard et al. 2018), because we did not record any pollinators on some sampling occasions and sites. We applied Permutational Multivariate Analysis of Variance (PERMANOVA; Borcard et al. 2018) to analyse the effect of treatment and season (i.e. sampling occasion) as explanatory variables, as well as their interactions on the community composition separately for the three case studies. We included the sampled site pairs as ‘blocks’ (i.e. random factor) to handle the non-independencies in the sampling structure. To represent the potential differences within the guild composition between treatment and control sites, as well as along the season by 2-dimensional figures, we performed non-metric multidimensional scaling (NMDS; Borcard et al. 2018), using the same zero-adjusted Bray–Curtis dissimilarities as above. We presented the first two dimensions of NMDS, as well as the pollinator guilds, the treatment, and sampling occasions on our plots. In addition, we evaluated the association of pollinator guilds to the combined variables of the treatment and the sampling occasion by indicator species analysis (Dufrêne and Legendre 1997; Borcard et al. 2018) separately for each case study. The indicator species analysis originally could test how the given species within a community associates with given environmental factors; here we only changed the species by the recorded pollinator guilds. Indicator species analysis uses the IndVal index, defined by the mean abundance of the species within the targeted community compared to its mean abundance across the whole analysed community, and presenting the connection of a species to a community, site or even environmental variable (Borcard et al. 2018). Here, the indicator values of the pollinator guilds were tested via the Monte-Carlo simulation using 100,000 permutations. The accepted significance level was p < 0.05 (Borcard et al. 2018).
The statistical analyses were carried out using the R statistical environment (v.4.2.1; RCoreTeam 2022), packages ‘glmmTMB’ v.1.1.5 for GLMMs (Brooks et al. 2017), spdep v.1.2-8 for Moran I tests (Bivand et al. 2015), MuMIn v.1.47.1 for model averaging (Barton 2023), vegan’ v.2.6-4 for PERMANOVA and NMDS (Oksanen et al. 2022), and ‘labdsv’ v.2.0–1 for indicator species analysis (Dufrêne and Legendre 1997; Roberts 2022).
Results
We sampled 1,350 plots during net 113 h (net 21 h (rare mowing in Veszprém), 42 h (rare mowing in Budapest), and 50 h (sowing in Budapest)), where we counted 36,889 floral units and observed 5,917 pollinators in total (per case studies: 5,052 flowers and 904 pollinators (rare mowing in Veszprém); 12,134 flowers and 1,816 pollinators (rare mowing in Budapest); 19,703 flowers and 3,197 pollinators (sowing in Budapest)). Pollinators belonged to 7.6% honey bees, 6.7% bumblebees, 64.0% solitary bees, 10.5% hoverflies, 9.9% butterflies, and 1.3% moths.
Rare mowing in Veszprém
The vegetation height and green cover were significantly higher in treatment sites during the season (except in May and August) compared to the controls (Fig. 2, Table S3). All variables in June and all variables except flower abundance and morphogroup richness in July showed significant differences in favour of the treatment sites, while just vegetation height and green cover, pollinator abundance and morphogroup richness in April. In contrast, one variable, the flower abundance was higher in the control sites in May and August (Fig. 2, Table S3).
For the seasonal changes, the vegetation height and green cover drastically decreased in the treatment sites for the second half of the season (Fig. 3). In the control sites, the vegetation green cover notably increased from July to August (Fig. 3). The flower and pollinator variables also followed the decreasing trend with a peak in April and June (Fig. 3). At the end of the season, both the control and the treatment sites had similar values for all variables (Fig. 3).
In the case of the community composition of pollinator guilds, we did not find significant differences between treatment and control sites (p = 0.0544; R2 = 0.04), while the community composition changed during the season (p = 0.0007; R2 = 0.21) without significant interaction between treatment and season (p = 0.7122; R2 = 0.05; Fig. S1A). The analysis of indicator pollinator guilds combining the sampling occasion and site pair type showed that from the sixty potential links only the solitary bees (in April), honey bees (in June), and butterflies (in July) were linked to treatment sites for rare mowing in Veszprém (Fig. S2, Table S4).
Rare mowing in Budapest
All significant variables were higher for the treatment sites during the season. The vegetation height was consistently higher in all sampling occasions, but the vegetation green cover was higher only in July in the treatment sites compared to the controls (Fig. 2, Table S3). Some variables of the floral resources also showed significant differences in favour of the treatment sites (e.g. higher flower abundance in April and July; higher morphogroup richness in all sampling occasions except August; and higher diversity in May and June; Fig. 2, Table S3). Pollinator abundance, morphogroup richness, and diversity tended to be higher in the treatment sites in the beginning and middle of the season (April and June), while in May and July, only the pollinator abundance and morphogroup richness were higher in the treatment sites, respectively (Fig. 2, Table S3). We did not find any significant difference between the treatment and control sites in August except the vegetation height (Fig. 2, Table S3).
Regarding the seasonal changes, the control and treatment sites showed similar patterns for all variables throughout the season, except the vegetation height, which was consistently low in the control sites, but was higher and constant from May to July in the treatment sites (Fig. 3). The vegetation green cover drastically decreased in the second half of the season in all sites (Fig. 3). The flower and pollinator variables also followed this decreasing trend with a slight peak in June and also in April, respectively (Fig. 3). At the end of the season, both the control and the treatment sites had similar values for all variables (Fig. 3).
In the case of the community composition of pollinator guilds, we did not find significant differences between treatment and control sites (p = 0.0510; R2 = 0.02), while the community composition changed during the season (p < 0.0001; R2 = 0.17) without significant interaction between treatment and season (p = 0.7140; R2 = 0.03; Fig. S1B). Indicator pollinator guild analysis revealed that hoverflies (in April), solitary bees (in April), and bumblebees (in June) were linked to treatment sites for rare mowing in Budapest (Fig. S2, Table S4).
Sowing in Budapest
In the first half of the season, in the control sites, the vegetation green cover and flower abundance were higher in April, and all the vegetation and floral resource variables were higher in May compared to the treatment sites (Fig. 2, Table S2). In June, the vegetation height, flower abundance, and pollinator abundance were higher in the treatment sites (Fig. 2, Table S2). In the second half of the season (July and August), all variables were significantly higher in the treatment sites, except the vegetation green cover and the flower abundance in August (Figs. 2 and 3, Table S2).
For the seasonal changes, the vegetation height slightly decreased, while the green cover drastically decreased in the control sites for the second half of the season (Fig. 3). In the treatment sites, the vegetation height and green cover increased from May to July, which was suppressed by the withdrawal of some sites from the pollinator-promotion until our sampling in August (Fig. 3). The floral resource and pollinator variables followed the same trend as the vegetation variables in the control and the treatment sites, respectively (Fig. 3). There was a peak in all the flower and pollinator variables in the control sites in April and June, while the treatment sites showed the flower and pollinator peak in the second half of the season (except August; Fig. 3).
The models including the seed mixture type as an explanatory variable were the best for more than half of the response variables (except vegetation green cover, pollinator morphogroup richness and diversity; see Table S5), but note that there were very small differences between the AIC values in all cases, so these results should be treated with caution (Table S6).
In the case of the community composition of pollinator guilds, we did not find significant differences between the treatment and control sites (p = 0.0907; R2 = 0.01), while the community composition changed during the season (p < 0.0001; R2 = 0.13) with significant interaction between treatment and season (p = 0.0359; R2 = 0.05; Fig. S1C). Indicator pollinator guild analysis revealed that hoverflies (in June), bumblebees (in July), butterflies (in July), and solitary bees (in July) were linked to treatment sites in the case of sowing in Budapest (Fig. S2, Table S4).
Differences in vegetation height and green cover, abundance, morphogroup richness and diversity of flowers and pollinators, respectively, between the treatment and control site pairs for the three case studies. For the compact and comparable presentation, we calculated scaled differences between treatment and control site pairs by the pooled sampling plots per site. All values of variables were scaled to 0–1 at all sampling occasions, separately for each occasion of the three case studies, and the scaled value of the control site was subtracted from the scaled value of the treatment site pair. Scaled differences show higher values in the control sites between − 1 and 0, and higher values in the treatment sites between 0 and 1. Boxplots show the median, 25th and 75th percentiles; the whiskers indicate the values within 1.5 times the interquartile range. Significant (p < 0.05) differences according to GLMMs are indicated by stars on the corresponding side of the boxes. Numbers and letters represent site pairs (Fig. 1). The darker grey numbers represent the sites sown with Mix1, while the grey numbers are the sites sown with Mix2 in the case of sowing in Budapest
The seasonal changes in the vegetation height and green cover, flower and pollinator abundance and morphogroup richness for the three case studies. The grey connected dots indicate the average of the control sites, while the black connected dots indicate the average of treatment sites. Dashed lines presented each site separately (grey: controls, black: treatments)
Discussion
Knowledge about the influence of local habitat management on urban biodiversity is crucial for the contribution to pollinator conservation in cities. Reduced mowing and sown flower patches have proven to be adequate pollinator-promoting interventions in the USA and Western Europe (Wastian et al. 2016; Norton et al. 2019). However, little is known so far about the effectiveness of any conservation practices in Eastern European urban areas (Csákvári et al. 2021). In this study, we evaluated the effects of interventions on pollinators during a whole season by five sampling occasions, providing novel insights into the fine-scale temporal dynamics of pollinator-promoting interventions, compared to previous studies mostly presenting only yearly pooled or summer-period results (Banaszak-Cibicka and Żmihorski 2020; Dylewski et al. 2020; Fournier et al. 2020).
Effects of pollinator-promoting interventions
Studying three different pollinator-promoting interventions in two Hungarian cities, we found that the less frequently mown public spaces had higher and greener vegetation with more flowers and pollinators than the frequently mown areas, in parallel with earlier studies (Garbuzov et al. 2015; Dylewski et al. 2019; Del Toro and Ribbons 2020; Horák et al. 2022). However, the found positive effect varies even within a season, e.g. the differences mostly disappeared by August. The sown flower patches provided food for many pollinators in the second half of the season, when food resources are insufficient in urban landscapes, such as dry or mown traditional parks and public spaces (Blackmore and Goulson 2014; Hicks et al. 2016; Norton et al. 2019; Mody et al. 2020; Dietzel et al. 2023). Compared to the rarely mown sites, here, the weed seedlings were eliminated annually in April with soil scarification and re-sowing in May. Hence these seedbeds with bare soil did not provide food, thus until the sown species bloomed, the conventionally managed sites were more beneficial for pollinators. Almost neutral results of our community composition and indicator pollinator guild analyses are suggesting a homogenized urban pollinator community within the cities (Deguines et al. 2016). However, any change in community composition can only be expected in the long term in such a highly artificial urban environment, the community composition changes will probably start in treatment sites close to semi-natural areas near the outskirts of the cities (Piano et al. 2019).
Temporal changes within a season
The revealed temporal changes in floral resources and pollinators within the season are probably explained by the seasonality in plant-pollinator systems (Roulston and Goodell 2011; Thomas and Schultz 2016; Szigeti et al. 2018), the floral resource availability in the landscape (Timberlake et al. 2019), the climate of the year (Colom et al. 2021; ODWMS 2023), and the temporal aspects of the different managements (Wastian et al. 2016; Norton et al. 2019). Although the sown sites provided floral resources only in the second half of the season, Mix1 started to flower earlier (in June), than Mix2, causing seasonal differences in floral resources and pollinator communities also among the sown sites. The indicator analyses on pollinator guilds also suggested a seasonality due to the differences in which intervention type (i.e. rare mowing or sowing) attracts which pollinator guilds and at which part of the season.
The studied two types of pollinator-promoting management methods may also complement each other side by side in cities due to the provided flower resources in the first half of the season by reduced mowing and in the second half of the season by annual flower sowings (Blackmore and Goulson 2014; Del Toro and Ribbons 2020). However, a mosaic mowing system alone would be a suitable solution to provide continuous food resources during the whole season while establishing more resilient vegetation even in drought conditions (Valtonen et al. 2006; O’Sullivan et al. 2017) as well as help to avoid the degradation of non-frequently mown urban green areas (O’Sullivan et al. 2017; Unterweger et al. 2018; Wintergerst et al. 2021).
Experiences from a year of drought and imperfect management
The year 2022 was extreme in terms of weather conditions because of the extreme drought in Hungary (ODWMS 2023; see the details in Materials and methods), providing an insight into a climatic event which is expected to be more frequent due to climate change (Descamps et al. 2021). The severe drought may have influenced negatively both the treatment and control sites, decreasing the diversity of wildflowers and pollinators in the urban landscape (Hamblin et al. 2018; Phillips et al. 2018), but also decreasing the potential differences between treatment and control sites. In the case of both rare mowing projects, the original mowing schedule was changed as the treatment sites were collectively mown at the end of summer, due to drought-induced desiccation and fire risk (Winkler et al. 2021). As well, the control sites needed less mowing within the year and treatments did not grow well due to the heavy drought thus the (management and vegetation of the) controls became slightly similar to the treatments. In contrast, the sown sites were regularly watered during the season. Probably the combined effect of sowing and watering plays an important role in promoting urban wildflowers and pollinators with or without severe drought (Rojas-Botero et al. 2023). Together, the positive effect of the pollinator-promoting interventions in the sampled year probably decreased and the intervention has not achieved its higher aim in some sites (Wastian et al. 2016). In addition, the off-scheduled mowing and non-removed hay could have resulted in inhibited re-flowering, organic matter accumulation and desiccation causing potential long-term degradation (e.g. setting back the vegetation for several months; Noordijk et al. 2009; Manninen et al. 2010). In the case of sowing, too strong watering in the initial period and/or later the extreme drought probably damaged seeds and sprouts of sown species, however, may have favoured the more resistant weeds. In addition, weeding seemed improperly carried out during the whole season, resulting in heavy weed infestation (pers. obs. and comm. with local authority). Both management inaccuracies could have contributed to the insufficient emergence/growth of sown species (Hitchmough et al. 2004).
Compared to some Western European examples (Noordijk et al. 2009; Hicks et al. 2016; Norton et al. 2019; Lange-Kabitz et al. 2021), in the post-Soviet, Eastern Europe countries, the urban pollinator-promoting interventions are still in their early stages. The lack of monitoring and the potential inaccuracies in the management of pollinator-promoting interventions are concerning issues (Noordijk et al. 2009; Hicks et al. 2016; Norton et al. 2019; Lange-Kabitz et al. 2021) and decreasing the potential efficiency of these interventions (Wilk et al. 2019). Therefore, promoting and monitoring such urban projects along with the involvement of the citizens could be appropriate for contributing to pollinator conservation also in Eastern European cities (European Commission 2023). However, future implementations can benefit from a couple of recommendations from our study.
Recommendations on pollinator promotion and monitoring within cities
In the two sampled cities, the local authorities used a broader set of biodiversity-supporting initiatives, such as environmental education programmes (e.g. citizens prepare bee hotels), installing bee hotels and birdhouses, continuously increasing the number of rarely mowed sites, and introducing community composting places (Hegyvidéki Önkormányzat 2020; Báthoryné et al. 2021; Takács 2021). These multiple interventions can support pollinators at several levels (Baldock 2020), but some aspects probably need re-considerations. For example, invasive species (e.g. Erigeron annuus (L.) Pers., Solidago sp.) potentially could spread in the rarely mown sites, which problem must be taken into account during designing the mowing schedule by avoiding the invaded areas’ inclusion in pollinator-promoting projects until appropriate eradications (Jang et al. 2020). We also suggest careful consideration of the type of seed mixture used for sowing (Hicks et al. 2016). In our study, Mix 2 contained a higher proportion of Asterales species, blooming in late summer, limiting the pollinator species that could forage on these plots (Ballantyne et al. 2017). In contrast, Mix 1, the mainly native mixture of annual and perennial species with different floral morphologies provided a longer flowering period from June and probably offered less food while for more pollinator species by their diverse floral traits (Salisbury et al. 2015). Seed mixes containing non-native plants pose a potential risk to urban green spaces, as well as semi-natural and protected areas in the neighbourhood (Tinsley et al. 2006; Kandori et al. 2009; Süle et al. 2023). It would be crucial to use only native seed mixtures (Wilk et al. 2019), which even lack standard regulation in Hungary, the EU or worldwide (Tinsley et al. 2006; Mainz and Wieden 2019). However, we can find nice examples from Germany regarding native seed mixture legalisation (Mainz and Wieden 2019), but its utilisation is not mandatory leading to the use of easily accessible non-native mixtures. The native seed mixture should contain a higher proportion of perennial species, facilitating the long-term establishment of sown areas without annual soil disturbance, which could also reduce the costs (Norton et al. 2019) and increase the benefits for ground-nesting bees, overwintering pollinators, and herbivore larvae using the sown species as hostplants (Unterweger et al. 2018; Lange-Kabitz et al. 2021). To improve the quality of floral resources for years with minimal soil disturbance, the best option would be to combine the two types of interventions such as overseeding the green areas with native seed mixture and maintaining them with a mosaic mowing system (Ferreira et al. 2011; Neumüller et al. 2021). Also, note that the timing, ratio, and location of the mown area should be determined site-specifically according to the local environmental and societal conditions.
Despite the imperfect management and drought, these pollinator-promoting practices still had a significant positive effect on the pollinators. Furthermore, we sampled only two cities (one small and one large capital), they and other cities may differ from each other in several socio-ecological attributes, which should be considered. Hence, we need further studies from more and more cities to explore in detail, whether the pollinator-promoting management methods would be also effective and beneficial on a wider spatio-temporal scale (Keilsohn et al. 2018), for example, how much the effectiveness of interventions depends on the correspondence with landscape-scale factors such as the proportion of green infrastructures (Dylewski et al. 2020; Prendergast et al. 2022). Nevertheless, to make sure that these interventions are successful contributions to pollinator conservation, i.e. no sink habitats were created (Gardiner et al. 2018), long-term monitoring is needed on vegetation attributes, floral resources, pollinators, nesting sites and even on co-benefits of these projects such as microclimatic conditions, soil regeneration, and aesthetic values (Norton et al. 2019). As well, to reveal the details in pollinator communities, i.e. which pollinator species prefer or avoid the treatment sites, there is a need for low-impact urban trapping and capturing approaches. However, non-lethal recording at pollinator guild-level can be investigated by our sampling method.
Besides ecological impact, the societal impression of pollinator-promoting intervention should also be taken into account (Southon et al. 2017). Although the reduced mowing benefits pollinators, it may lead to some undesirable negative consequences such as the increase of garbage in tall grass because litter probably will be disposed more likely by citizens here, while noticed less by city workers, degrading these sites in long-term (Turo and Gardiner 2019). Unfortunately, negative attitudes from citizens in addition to some political repercussions toward the rare mowing intervention could be encountered in Hungary (Balázsi 2021). Part of the citizens could be averse to higher vegetation due to untidy appearance, a presumed infestation of ticks, spikelets of Hordeum murinum L. (causing inflammation in dogs), and alien species triggering allergies (e.g. Ambrosia artemisiifolia L.), besides fearing of stings of wasp and bees. All of these factors in local circumstances should be revealed and studied for new types of green space interventions.
Conclusions
Pollinator-promoting interventions, that have been applied previously in Western cities require specified modifications according to the local conditions of other regions, due to the contrasting socioeconomic and ecological contexts (Batáry et al. 2010; Kronenberg 2015; Southon et al. 2017). Monitoring programmes should be established, improved, and their funding has to be secured in the long term, especially in less-developed more biodiverse locations. Our simple sampling method could be suitable for use in a citizen-science project that could be developed into a mobile sampling application offering personalised ecological experience for citizens (sensu Soga and Gaston 2022). Meanwhile, there is considerable demand by stakeholders and local authorities for detailed recommendations (Wilk et al. 2019; NBDC 2020), as we provided above. However, broader datasets are needed to strengthen the general, as well as region-specific measures by evidence. During the maintenance of urban pollinator-promoting interventions, it is essential to pay attention to education and present up-to-date, transparent, interactive information for citizens about management while also gathering their opinions and feedback (Hall and Martins 2020). Interventions should be designed in a resilient way, e.g. using native, drought-resistant plant species and mosaic mowing regime, to be prepared for increasingly frequent extreme weather events such as droughts, due to climate change (Jentsch et al. 2007).
References
Aguilera G, Ekroos J, Persson AS et al (2019) Intensive management reduces butterfly diversity over time in urban green spaces. Urban Ecosyst 22:335–344. https://doi.org/10.1007/s11252-018-0818-y
Ahrne K, Bengtsson J, Elmqvist T (2009) Bumble Bees (Bombus spp) along a gradient of increasing urbanization. PLoS ONE 4:e5574. https://doi.org/10.1371/journal.pone.0005574
Albrecht M, Kleijn D, Williams NM et al (2020) The effectiveness of flower strips and hedgerows on pest control, pollination services and crop yield: a quantitative synthesis. Ecol Lett 23:1488–1498. https://doi.org/10.1111/ele.13576
Ayers AC, Rehan SM (2021) Supporting bees in cities: how bees are influenced by local and landscape features. Insects 12:1–18. https://doi.org/10.3390/insects12020128
Balázsi L (2021) Index: Lekaszálta a méhlegelőt a Fidelitas. https://index.hu/belfold/2021/06/28/mehelegelo-fidelitas-kormany-fovarosi-onkormanyzat-lekaszalas-karacsony-gergely-bardoczi-sandor/. Accessed 1 Apr 2023
Báldi A, Pellaton R, Bihaly ÁD et al (2022) Improving ecosystem services in farmlands: beginning of a long-term ecological study with restored flower-rich grasslands. Ecosyst Heal Sustain 8:1–7. https://doi.org/10.1080/20964129.2022.2090449
Baldock KCR (2020) Opportunities and threats for pollinator conservation in global towns and cities. Curr Opin Insect Sci 38:63–71. https://doi.org/10.1016/j.cois.2020.01.006
Baldock KCR, Goddard MA, Hicks DM et al (2019) A systems approach reveals urban pollinator hotspots and conservation opportunities. Nat Ecol Evol 3:363–373. https://doi.org/10.1038/s41559-018-0769-y
Ballantyne G, Baldock KCR, Rendell L, Willmer PG (2017) Pollinator importance networks illustrate the crucial value of bees in a highly speciose plant community. Sci Rep 7:1–13. https://doi.org/10.1038/s41598-017-08798-x
Banaszak-Cibicka W, Żmihorski M (2020) Are cities hotspots for bees? Local and regional diversity patterns lead to different conclusions. Urban Ecosyst 23:713–722. https://doi.org/10.1007/s11252-020-00972-w
Barragan-Jason G, Loreau M, de Mazancourt C et al (2023) Psychological and physical connections with nature improve both human well-being and nature conservation: a systematic review of meta-analyses. Biol Conserv 277:109842. https://doi.org/10.1016/j.biocon.2022.109842
Barton K (2023) Package ‘ MuMIn ’. Multi-Model Interface
Batáry P, Báldi A, Sárospataki M et al (2010) Effect of conservation management on bees and insect-pollinated grassland plant communities in three european countries. Agric Ecosyst Environ 136:35–39. https://doi.org/10.1016/j.agee.2009.11.004
Bates AJ, Sadler JP, Fairbrass AJ et al (2011) Changing bee and hoverfly pollinator assemblages along an urban-rural gradient. PLoS ONE 6. https://doi.org/10.1371/journal.pone.0023459
Báthoryné NIR, Gergely A, Pernesz K et al (2021) Klímaadaptív gyepgazdálkodás a városban. VKSZ Veszprémi Közüzemi Szolgáltató Zrt., Szent István Egyetem, Veszprém
Bivand R, Altman M, Anselin L et al (2015) Package ‘spdep’. Weighting Schemes, Statistics and Models, Spatial Dependence
Blackmore LM, Goulson D (2014) Evaluating the effectiveness of wildflower seed mixes for boosting floral diversity and bumblebee and hoverfly abundance in urban areas. Insect Conserv Divers 7:480–484. https://doi.org/10.1111/icad.12071
Borcard D, Gillet F, Legendre P (2018) Numerical Ecology with R, 2nd edn. Springer, New York
Bratman GN, Anderson CB, Berman MG et al (2019) Nature and mental health: an ecosystem service perspective. Sci Adv 5. https://doi.org/10.1126/sciadv.aax0903
Brooks ME, Kristensen K, van Benthem KJ et al (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400. https://doi.org/10.32614/rj-2017-066
Clarke KR, Somerfield PJ, Chapman MG (2006) On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray-Curtis coefficient for denuded assemblages. J Exp Mar Bio Ecol 330:55–80. https://doi.org/10.1016/j.jembe.2005.12.017
Colom P, Traveset A, Carreras D, Stefanescu C (2021) Spatio-temporal responses of butterflies to global warming on a Mediterranean island over two decades. Ecol Entomol 262–272. https://doi.org/10.1111/een.12958
Csákvári E, Fabók V, Bartha S et al (2021) Conservation biology research priorities for 2050: a Central-Eastern European perspective. Biol Conserv 264. https://doi.org/10.1016/j.biocon.2021.109396
Daniels B, Jedamski J, Ottermanns R, Ross-Nickoll M (2020) A “plan bee” for cities: Pollinator diversity and plant-pollinator interactions in urban green spaces. PLoS ONE 15:1–29. https://doi.org/10.1371/journal.pone.0235492
DEFRA (2014) The National Pollinator Strategy: for bees and other pollinators in England
Deguines N, Julliard R, de Flores M, Fontaine C (2016) Functional homogenization of flower visitor communities with urbanization. Ecol Evol 6:1967–1976. https://doi.org/10.1002/ece3.2009
Del Toro I, Ribbons RR (2020) No mow May lawns have higher pollinator richness and abundances: an engaged community provides floral resources for pollinators. PeerJ 8:1–16. https://doi.org/10.7717/peerj.10021
Descamps C, Quinet M, Jacquemart AL (2021) The effects of drought on plant–pollinator interactions: what to expect? Environ Exp Bot 182:104297. https://doi.org/10.1016/j.envexpbot.2020.104297
Dietzel S, Rojas-Botero S, Kollmann J, Fischer C (2023) Enhanced urban roadside vegetation increases pollinator abundance whereas landscape characteristics drive pollination. Ecol Indic 147:109980. https://doi.org/10.1016/j.ecolind.2023.109980
Domroese MC, Johnson EA (2017) Why watch bees? Motivations of citizen science volunteers in the great Pollinator Project. Biol Conserv 208:40–47. https://doi.org/10.1016/j.biocon.2016.08.020
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366. https://doi.org/10.2307/2963459
Dylewski Ł, Maćkowiak Ł, Banaszak-Cibicka W (2019) Are all urban green spaces a favourable habitat for pollinator communities? Bees, butterflies and hoverflies in different urban green areas. Ecol Entomol 44:678–689. https://doi.org/10.1111/een.12744
Dylewski Ł, Maćkowiak Ł, Banaszak-Cibicka W (2020) Linking pollinators and city flora: how vegetation composition and environmental features shapes pollinators composition in urban environment. Urban For Urban Green 56:126795. https://doi.org/10.1016/j.ufug.2020.126795
Elmqvist T, Fragkias M, Goodness J et al (2013) Urbanization, Biodiversity and Ecosystem Services: Challenges and Opportunities: A Global Assessment. Springer
European Commission (2023) Revision of the EU Pollinators Initiative
Ferreira ET, Nabinger C, Adelaide D et al (2011) Fertilization and oversowing on natural grassland: effects on pasture characteristics and yearling steers performance. Rev Bras Zootec 40:2039–2047. https://doi.org/10.1590/S1516-35982011000900028
Fiala K, Barta K, Benyhe B et al (2018) Operatív aszály- és vízhiánykezelő monitoring rendszer. Hidrológiai Közlöny 98:14–24
flower your place (2023) Aurora. https://floweryourplace.com/product/aurora/. Accessed 1 Apr 2023
Fortel L, Henry M, Guilbaud L et al (2016) Use of human-made nesting structures by wild bees in an urban environment. J Insect Conserv 20:239–253. https://doi.org/10.1007/s10841-016-9857-y
Fournier B, Frey D, Moretti M (2020) The origin of urban communities: from the regional species pool to community assemblages in city. J Biogeogr 47:615–629. https://doi.org/10.1111/jbi.13772
Garbuzov M, Fensome KA, Ratnieks FLW (2015) Public approval plus more wildlife: twin benefits of reduced mowing of amenity grass in a suburban public park in Saltdean, UK. Insect Conserv Divers 8:107–119. https://doi.org/10.1111/icad.12085
Gardiner MM, Riley CB, Bommarco R, Öckinger E (2018) Rights-of-way: a potential conservation resource. Front Ecol Environ 16:149–158. https://doi.org/10.1002/fee.1778
Gavrilidis AA, Popa AM, Nita MR et al (2020) Planning the “unknown”: perception of urban green infrastructure concept in Romania. Urban For Urban Green 51:126649. https://doi.org/10.1016/j.ufug.2020.126649
Geslin B, Gauzens B, Thébault E, Dajoz I (2013) Plant Pollinator Networks along a gradient of Urbanisation. PLoS ONE 8:e63421. https://doi.org/10.1371/journal.pone.0063421
Guenat S, Kunin WE, Dougill AJ, Dallimer M (2019) Effects of urbanisation and management practices on pollinators in tropical Africa. J Appl Ecol 56:214–224. https://doi.org/10.1111/1365-2664.13270
Hall DM, Martins DJ (2020) Human dimensions of insect pollinator conservation. Curr Opin Insect Sci 38:107–114. https://doi.org/10.1016/j.cois.2020.04.001
Hall DM, Steiner R (2019) Insect pollinator conservation policy innovations: Lessons for lawmakers. Environ Sci Policy 93:118–128. https://doi.org/10.1016/j.envsci.2018.12.026
Hamblin AL, Youngsteadt E, Frank SD (2018) Wild bee abundance declines with urban warming, regardless of floral density. Urban Ecosyst 21:419–428. https://doi.org/10.1136/bmj.1.5645.714-d
Harrison T, Gibbs J, Winfree R (2019) Anthropogenic landscapes support fewer rare bee species. Landsc Ecol 34:967–978. https://doi.org/10.1007/s10980-017-0592-x
Hegyvidéki Önkormányzat (2020) VADVIRÁGOS LEGELŐK A HEGYVIDÉKI MÉHEKNEK. https://www.hegyvidekujsag.hu/archivum/archivum-2020-januar-28/vadviragos-legelok. Accessed 1 Apr 2022
Hicks DM, Ouvrard P, Baldock KCR et al (2016) Food for pollinators: quantifying the nectar and pollen resources of urban flower meadows. PLoS ONE 11:1–37. https://doi.org/10.1371/journal.pone.0158117
Hitchmough J, De La Fleur M, Findlay C (2004) Establishing north american prairie vegetation in urban parks in northern England: part 1. Effect of sowing season, sowing rate and soil type. Landsc Urban Plan 66:75–90. https://doi.org/10.1016/S0169-2046(03)00096-3
Horák J, Šafářová L, Trombik J, Menéndez R (2022) Patterns and determinants of plant, butterfly and beetle diversity reveal optimal city grassland management and green urban planning. Urban For Urban Green 73:0–3. https://doi.org/10.1016/j.ufug.2022.127609
Hoyle H, Jorgensen A, Warren P et al (2017) Not in their front yard” the opportunities and challenges of introducing perennial urban meadows: a local authority stakeholder perspective. Urban For Urban Green 25:139–149. https://doi.org/10.1016/j.ufug.2017.05.009
IPBES (2016) The assessment report of the Intergovernmental Science-Policy platform on Biodiversity and Ecosystem Services on pollinators, pollination and food production. Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services, Bonn, Germany
Jang W, Eskelson BNI, Murray T et al (2020) Relationships between invasive plant species occurrence and socio-economic variables in urban green spaces of southwestern British Columbia, Canada. Urban For Urban Green 47:126527. https://doi.org/10.1016/j.ufug.2019.126527
Jenks M, Jones C (2010) Dimensions of the Sustainable City. Speinger
Jentsch A, Kreyling J, Beierkuhnlein C (2007) A new generation of climate-change experiments: events, not trends. Front Ecol Environ 5:365–374. https://doi.org/10.1890/1540-9295(2007)5[365:ANGOCE]2.0.CO;2
Kabisch N, Haase D (2013) Green spaces of european cities revisited for 1990–2006. Landsc Urban Plan 110:113–122. https://doi.org/10.1016/j.landurbplan.2012.10.017
Kandori I, Hirao T, Matsunaga S, Kurosaki T (2009) An invasive dandelion unilaterally reduces the reproduction of a native congener through competition for pollination. Oecologia 159:559–569. https://doi.org/10.1007/s00442-008-1250-4
Keilsohn W, Narango DL, Tallamy DW (2018) Roadside habitat impacts insect traffic mortality. J Insect Conserv 22:183–188. https://doi.org/10.1007/s10841-018-0051-2
Konvicka M, Kadlec T (2011) How to increase the value of urban areas for butterfly conservation? A lesson from Prague nature reserves and parks. Eur J Entomol 108:219–229. https://doi.org/10.14411/eje.2011.030
Kovács O, Bajor Z, Szigeti V et al (2021) A budapesti “méhlegelők” monitoringja - az első év tapasztalatai. In: 2. Urbanizációs Ökológia Konferencia. 2021. 10. 14–15. Győr, p 48
Kovács-Hostyánszki A, Földesi R, Mózes E et al (2016) Conservation of pollinators in Traditional Agricultural Landscapes - New Challenges in Transylvania (Romania) posed by EU Accession and Recommendations for Future Research. PLoS ONE 11:1–20. https://doi.org/10.1371/journal.pone.0151650
Kronenberg J (2015) Why not to green a city? Institutional barriers to preserving urban ecosystem services. Ecosyst Serv 12:218–227. https://doi.org/10.1016/j.ecoser.2014.07.002
KSH (2022) 15.1.1.37. Magyarország és Budapest időjárásának adatai. https://www.ksh.hu/stadat_files/kor/hu/kor0037.html. Accessed 1 Apr 2023
Lange-Kabitz C, Reich M, Zoch A (2021) Extensively managed or abandoned urban green spaces and their habitat potential for butterflies. Basic Appl Ecol 54:85–97. https://doi.org/10.1016/j.baae.2021.04.012
Lerman SB, Contosta AR, Milam J, Bang C (2018) To mow or to mow less: lawn mowing frequency affects bee abundance and diversity in suburban yards. Biol Conserv 221:160–174. https://doi.org/10.1016/j.biocon.2018.01.025
Li H, Calder CA, Cressie N (2007) Beyond Moran’s I: testing for spatial dependence based on the spatial autoregressive model. Geogr Anal 39:357–375. https://doi.org/10.1111/j.1538-4632.2007.00708.x
Mainz AK, Wieden M (2019) Ten years of native seed certification in Germany – a summary. Plant Biol 21:383–388. https://doi.org/10.1111/plb.12866
Manninen S, Forss S, Venn S (2010) Management mitigates the impact of urbanization on meadow vegetation. Urban Ecosyst 13:461–481. https://doi.org/10.1007/s11252-010-0129-4
Mody K, Lerch D, Müller AK et al (2020) Flower power in the city: replacing roadside shrubs by wildflower meadows increases insect numbers and reduces maintenance costs. PLoS ONE 15:1–29. https://doi.org/10.1371/journal.pone.0234327
NBDC (2020) All-Ireland Pollinator Plan 2021–2025. Waterford
Neumüller U, Burger H, Schwenninger HR et al (2021) Prolonged blooming season of flower plantings increases wild bee abundance and richness in agricultural landscapes. Biodivers Conserv 30:3003–3021. https://doi.org/10.1007/s10531-021-02233-4
Nilon CH, Aronson MFJ, Cilliers SS et al (2017) Planning for the future of urban biodiversity: a global review of city-scale initiatives. Bioscience 67:332–342. https://doi.org/10.1093/biosci/bix012
Noordijk J, Delille K, Schaffers AP, Sýkora KV (2009) Optimizing grassland management for flower-visiting insects in roadside verges. Biol Conserv 142:2097–2103. https://doi.org/10.1016/j.biocon.2009.04.009
Norton BA, Bending GD, Clark R et al (2019) Urban meadows as an alternative to short mown grassland: effects of composition and height on biodiversity. Ecol Appl 29:1095–1115. https://doi.org/10.1002/eap.1946
O’Sullivan OS, Holt AR, Warren PH, Evans KL (2017) Optimising UK urban road verge contributions to biodiversity and ecosystem services with cost-effective management. J Environ Manage 191:162–171. https://doi.org/10.1016/j.jenvman.2016.12.062
Öckinger E, Dannestam Ã, Smith HG (2009) The importance of fragmentation and habitat quality of urban grasslands for butterfly diversity. Landsc Urban Plan 93:31–37. https://doi.org/10.1016/j.landurbplan.2009.05.021
ODWMS (2023) Operational drought and water scarcity management system. https://aszalymonitoring.vizugy.hu/index.php?lang=eng. Accessed 16 Jan 2023
Oksanen J, Simpson GL, Blanchet FG et al (2022) Community Ecology Package. R package version 2.6-4
Pauleit S, Ambrose-Oji B, Andersson E et al (2019) Advancing urban green infrastructure in Europe: outcomes and reflections from the GREEN SURGE project. Urban For Urban Green 40:4–16. https://doi.org/10.1016/j.ufug.2018.10.006
Pereira-Peixoto MH, Pufal G, Martins CF, Klein AM (2014) Spillover of trap-nesting bees and wasps in an urban–rural interface. J Insect Conserv 18:815–826. https://doi.org/10.1007/s10841-014-9688-7
Persson AS, Ekroos J, Olsson P, Smith HG (2020) Wild bees and hoverflies respond differently to urbanisation, human population density and urban form. Landsc Urban Plan 204:103901. https://doi.org/10.1016/j.landurbplan.2020.103901
Phillips BB, Shaw RF, Holland MJ et al (2018) Drought reduces floral resources for pollinators. Glob Chang Biol 24:3226–3235. https://doi.org/10.1111/gcb.14130
Phillips BB, Wallace C, Roberts BR et al (2020) Enhancing road verges to aid pollinator conservation: a review. Biol Conserv 250:108687. https://doi.org/10.1016/j.biocon.2020.108687
Piano E, Souffreau C, Merckx T et al (2019) Urbanization drives cross-taxon declines in abundance and diversity at multiple. Glob Chang Biol 26:1196–1211. https://doi.org/10.1111/gcb.14934
Potts SG, Biesmeijer JC, Kremen C et al (2010) Global pollinator declines: Trends, impacts and drivers. Trends Ecol Evol 25:345–353. https://doi.org/10.1016/j.tree.2010.01.007
Potts SG, Imperatriz-Fonseca V, Ngo HT et al (2016) Safeguarding pollinators and their values to human well-being. Nature 540:220–229. https://doi.org/10.1038/nature20588
Prendergast KS, Dixon KW, Bateman PW (2022) A global review of determinants of native bee assemblages in urbanised landscapes. Insect Conserv Divers 385–405. https://doi.org/10.1111/icad.12569
Rada P, Bogusch P, Pech P et al (2023) Active management of urban fruit orchard meadows is important for insect diversity. Ecol Eng 186. https://doi.org/10.1016/j.ecoleng.2022.106833
RCoreTeam (2022) A language and environment for statistical computing. https://www.r-project.org/
Roberts DW (2022) Ordination and Multivariate Analysis for Ecology
Rojas-Botero S, Teixeira LH, Kollmann J (2023) Low precipitation due to climate change consistently reduces multifunctionality of urban grasslands in mesocosms. PLoS ONE 18:e0275044. https://doi.org/10.1371/journal.pone.0275044
Rosin ZM, Pärt T, Low M et al (2021) Village modernization may contribute more to farmland bird declines than agricultural intensification. Conserv Lett 14:1–10. https://doi.org/10.1111/conl.12843
Roulston TH, Goodell K (2011) The role of resources and risks in regulating wild bee populations. Annu Rev Entomol 56:293–312. https://doi.org/10.1146/annurev-ento-120709-144802
Salisbury A, Armitage J, Bostock H, Salisbury A, Armitage J, Bostock H, Perry J, Tatch et al (2015) https://doi.org/10.1111/1365-2664.12499
Sipos J (2020) Méhlegelő ültetés oktatófilm. https://siposgazda.hu/blog/szaktanacsadas/mehlegelo-ultetes-oktatofilm. Accessed 12 Apr 2023
Skórka P, Lenda M, Moroń D et al (2013) Factors affecting road mortality and the suitability of road verges for butterflies. Biol Conserv 159:148–157. https://doi.org/10.1016/j.biocon.2012.12.028
Soga M, Gaston KJ (2022) Towards a unified understanding of human–nature interactions. Nat Sustain 5:374–383. https://doi.org/10.1038/s41893-021-00818-z
Southon GE, Jorgensen A, Dunnett N et al (2017) Biodiverse perennial meadows have aesthetic value and increase residents’ perceptions of site quality in urban green-space. Landsc Urban Plan 158:105–118. https://doi.org/10.1016/j.landurbplan.2016.08.003
Steidle JLM, Kimmich T, Csader M, Betz O (2022) Negative impact of roadside mowing on arthropod fauna and its reduction with ‘arthropod-friendly’ mowing technique. J Appl Entomol 146:465–472. https://doi.org/10.1111/jen.12976
Süle G, Miholcsa Z, Molnár C et al (2023) Escape from the garden: spreading, effects and traits of a new risky invasive ornamental plant (Gaillardia aristata Pursh). NeoBiota 83:43–69. https://doi.org/10.3897/neobiota.83.97325
Szigeti V, Korösi Á, Harnos A et al (2016) Measuring floral resource availability for insect pollinators in temperate grasslands - a review. Ecol Entomol 41:231–240. https://doi.org/10.1111/een.12298
Szigeti V, Kőrösi Á, Harnos A, Kis J (2018) Temporal changes in floral resource availability and flower visitation in a butterfly. Arthropod Plant Interact 12:177–189. https://doi.org/10.1007/s11829-017-9585-6
Takács N (2021) Extenzíven kezelt gyepek Budapesten. In: Beporzók védelme és települési zöldfelületek. Budapest, pp 1–12
Thomas RC, Schultz CB (2016) Resource selection in an endangered butterfly: females select native nectar species. J Wildl Manage 80:171–180. https://doi.org/10.1002/jwmg.987
Timberlake TP, Vaughan IP, Memmott J (2019) Phenology of farmland floral resources reveals seasonal gaps in nectar availability for bumblebees. J Appl Ecol 56:1585–1596. https://doi.org/10.1111/1365-2664.13403
Tinsley MJ, Simmons MT, Windhager S (2006) The establishment success of native versus non-native herbaceous seed mixes on a revegetated roadside in Central Texas. Ecol Eng 26:231–240. https://doi.org/10.1016/j.ecoleng.2005.10.004
Török P, Dembicz I, Dajic-Stevanovic Z, Kuzemko A (2020) Grasslands of eastern Europe. Encycl World’s Biomes 3–5:703–713. https://doi.org/10.1016/B978-0-12-409548-9.12042-1
Turo KJ, Gardiner MM (2019) From potential to practical: conserving bees in urban public green spaces. Front Ecol Environ 17:167–175. https://doi.org/10.1002/fee.2015
UKCEH (2021) UK Pollinator Monitoring Scheme: Flower-Insect Timed Count guidance. Wallingford
United Nations (2015) World Urbanization Prospects: The 2014 Revision. New York
Unterweger PA, Klammer J, Unger M, Betz O (2018) Insect hibernation on urban green land: a winter-adapted mowing regime as a management tool for insect conservation. BioRisk 2018:1–29. https://doi.org/10.3897/biorisk.13.22316
Valtonen A, Saarinen K, Jantunen J (2006) Effect of different mowing regimes on butterflies and diurnal moths on road verges. Anim Biodivers Conserv 29:133–148
Valtonen A, Saarinen K, Jantunen J (2007) Intersection reservations as habitats for meadow butterflies and diurnal moths: guidelines for planning and management. Landsc Urban Plan 79:201–209. https://doi.org/10.1016/j.landurbplan.2005.09.003
Vanbergen AJ, Baude M, Biesmeijer JC et al (2013) Threats to an ecosystem service: pressures on pollinators. Front Ecol Environ 11:251–259. https://doi.org/10.1890/120126
Vmeteo (2022) 2021. éves összesítő. https://vmeteo.hu/2021-eves-osszesito. Accessed 1 Apr 2023
Wastian L, Unterweger PA, Betz O (2016) Influence of the reduction of urban lawn mowing on wild bee diversity (Hymenoptera, Apoidea). J Hymenopt Res 49:51–63. https://doi.org/10.3897/JHR.49.7929
Wikipedia (2023a) Veszprém. https://hu.wikipedia.org/wiki/Veszprém. Accessed 1 Apr 2023
Wikipedia (2023b) Budapest. https://hu.wikipedia.org/wiki/Budapest. Accessed 1 Apr 2023
Wilk B, Rebollo V, Hanania S (2019) A guide for pollinator-friendly cities. How can spatial planners and landuse managers create favourable urban environments for pollinators?
Winkler J, Malovcová M, Adamcová D et al (2021) Significance of urban vegetation on lawns regarding the risk of fire. Sustainability 13:1–15. https://doi.org/10.3390/su131911027
Wintergerst J, Kästner T, Bartel M et al (2021) Partial mowing of urban lawns supports higher abundances and diversities of insects. J Insect Conserv 25:797–808. https://doi.org/10.1007/s10841-021-00331-w
Zamorano J, Bartomeus I, Grez AA, Garibaldi LA (2020) Field margin floral enhancements increase pollinator diversity at the field edge but show no consistent spillover into the crop field: a meta-analysis. Insect Conserv Divers 13:519–531. https://doi.org/10.1111/icad.12454
Zanette LRS, Martins RP, Ribeiro SP (2005) Effects of urbanization on neotropical wasp and bee assemblages in a brazilian metropolis. Landsc Urban Plan 71:105–121. https://doi.org/10.1016/j.landurbplan.2004.02.003
Zuur AF, Ieno EN, Walker NJ et al (2009) Mixed Effects models and extensions in Ecology with R. Springer, New York
Acknowledgements
We are grateful to Ildikó Báthoryné Nagy (Hungarian University of Agriculture and Life Sciences); the Veszprém Public Utility Services, especially Kata Pernesz; the Green Office of Municipality of the 12th District in Budapest, especially Nóra Laki; the Horticultural Division of Budapest Public Utilities and the Municipality of Budapest, especially Orsolya Kovács managed the setting up of these pollinator-promoting interventions, and enabled and facilitated our samplings. We are grateful to Péter Bakonyi, Borbála Grób, Csaba Hegedűs, Virág Németh, Raul Pellaton, and Flóra Vajna for their help in fieldwork. This project received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 101003476 (www.safeguard.biozentrum.uni-wuerzburg.de).
Funding
Open access funding provided by ELKH Centre for Ecological Research. This study received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 101003476 (www.safeguard.biozentrum.uni-wuerzburg.de).
Author information
Authors and Affiliations
Contributions
Anikó Kovács-Hostyánszki, Miklós Sárospataki, András Báldi, and Viktor Szigeti contributed to the study conception and design. Gabriella Süle, Anikó Kovács-Hostyánszki, Miklós Sárospataki, Tünde Ilona Kelemen, Gabriella Halassy, Anna Horváth, Imre Demeter, and Viktor Szigeti collected data. Viktor Szigeti ran the analyses and prepared manuscript figures. Gabriella Süle and Viktor Szigeti wrote the first version of the manuscript and improved by the thorough help of Anikó Kovács-Hostyánszki, Miklós Sárospataki, and András Báldi. All authors read and approved the manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest/competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Süle, G., Kovács-Hostyánszki, A., Sárospataki, M. et al. First steps of pollinator-promoting interventions in Eastern European urban areas – positive outcomes, challenges, and recommendations. Urban Ecosyst 26, 1783–1797 (2023). https://doi.org/10.1007/s11252-023-01420-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-023-01420-1