Abstract
Landscape features such as roads, hiking trails, and forest edge are prevalent features of urban forests. These features can negatively influence biodiversity through fragmentation and facilitate the penetration of invasive species, particularly predators such as domestic dogs and cats. This study examined how these landscape features affect the distribution of native and domestic mammals and how domestic species affect the occupancy and activity patterns of native mammals. To this end, we conducted camera trap surveys in forested urban and suburban parks in Indiana and Kentucky, USA, to record the presence of native species (deer, raccoons, coyote, and opossums) and non-native predators (domesticated cats and dogs) in relation to distance to nearest roads, hiking trails, and forest edge. We found negative correlations between proximity to roads, hiking trails, and forest edge and the occupancy of the native species with this effect extending up to 300 m. We also found evidence that the presence of dog and cat was negatively correlated with the occupancy for all native species. We recommend park management consider the impact of roads, hiking trails, and forest edge when designing parks and the enforcement of dog leash regulations for the conservation of large mammals in urban parks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urban forests face many challenges to their ecological integrity because they are usually small, are surrounded by a matrix of urban development, and are isolated from other forests (Collinge 2009). In addition, an intrinsic characteristic of small forests is that their interior is often within the vicinity of forest edges, exposing the interior habitat and wildlife to a variety of threats (Murcia 1995). These threats include pollution of their watersheds, invasive species colonization, and the reduction of native bird and small mammal populations by non-native domestic predators such as cats (Felis catus L.) and dogs (Canis familiaris L.) (Pickett et al. 2001).
Roads and hiking trails are a prevalent feature of urban forests. Roads can negatively impact the biodiversity in urban forests through fragmentation and increased mortality as a result of vehicle collisions (Cole et al. 1997; Fahrig 2003; Melbourne et al. 2004; Coffin 2007; Boston 2016). The noise and disturbance associated with the human activities while using roads and hiking trails put pressure on animals by increasing their energy expenditures and reducing their fecundity (Phillips and Alldredge 2000; Westekemper et al. 2018). Roads and hiking trails have also shown to affect the spatio-temporal habitat use of wildlife (Coppes et al. 2017; Scholten et al. 2018). For instance, some species change their behavior spatially (remaining far from roads and hiking trails) and temporally (being less active during the day time and weekend) (Coppes et al. 2017; Westekemper et al. 2018). Moreover, anthropogenic noise from roads and hiking trails can provoke antipredator behavior of species, which, in turn, negatively affect other ecological and physiological processes such as foraging and reproduction (Frid and Dill 2002; Shannon et al. 2014).
Hiking trails provide opportunities for nature appreciation, passive recreation and education (Farías Torbidoni 2011; Ballantyne et al. 2014; Bötsch et al. 2018). However, some trail systems could potentially have negative ecological effects, especially in small forests, because they fragment the forest by creating significant canopy breaks that act as soft edges and allow access to the interior by invasive or domestic species (Wimpey and Marion 2010; Ballantyne et al. 2014; Hammitt and Cole 2015).
Domestic species represent a major threat to native, urban wildlife because they can potentially affect native species through direct predation, disease transmission, and both interference and exploitative competition (Churcher and Lawton 1987; Young et al. 2011; Hughes and Macdonald 2013; Zapata-Ríos and Branch 2016, 2018). For example, feral or stray predators that reside in or with the vicinity of urban forests often have negative effects on native bird and small mammal populations (Keyser et al. 1998; Crooks and Soulé 1999; Lacerda et al. 2009). Several studies have suggested that hunting by domesticated cats may decrease bird and small mammal populations, affecting both biodiversity and food supply for native predators (Liberg 1984; Churcher and Lawton 1987; Meek 2003; Baker et al. 2003; Woods et al. 2003). Several factors contribute to the negative impact that non-native predators have on native wildlife in urban forests: the amount of fragmentation (Zipkin et al. 2009), the size of habitat fragments (Crooks 2002; Ewers and Didham 2007), and edge effects (Murcia 1995; Lacerda et al. 2009). In addition, the presence of domestic dogs with human hikers impacts the flight initiation distances of mule deer (Odocoileus hemionus) (Miller et al. 2001) and coyote (Canis latrans) abundance has been found to be significantly reduced in recreational areas that allow dogs, regardless of the type of restriction on dog activity (Reed and Merenlender 2011).
The purpose of this study was to examine how the landscape features, particularly roads, hiking trails, and forest edge, affect the occupancy of native and non-native mammals in urban forests. Additionally, we examined if there was a correlation between the occurrences of non-native and native species occupancy and activity.
Method
Data collection
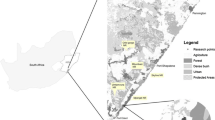
We conducted camera trap surveys from March to April 2018 in eight urban and suburban parks in the states of Indiana and Kentucky, USA. Lapping Park (88.8 ha, 8 sites), Locust Grove (11.2 ha, 8 sites), Cherokee Park (73.94 ha, 7 sites), Beargrass Creek State Nature Preserve (28.6 ha, 10 sites), Parklands (1275.2 ha, 10 sites), Blackacre State Nature Preserve (37.89 ha, 7 sites), Waverly Park (141.1 ha, 8 sites), and Jefferson Memorial Forest (3958 ha, 10 sites) (Fig. 1). All parks are within the boundary of Louisville, Kentucky except Lapping Park, which is located in Clarksville, Indiana. The study areas were mostly old secondary growth, less than 100 years old, dominated by oak species (Quercus rubra and, Q. velutina), maple (Acer saccharum), and other species such as hickory (Carya ovata and C. laciniosa), Kentucky coffee tree (Gymnocladus dioicus), pawpaw (Asimina triloba), and eastern red cedar (Juniperus virginiana). The study sites are surrounded by residential neighborhoods and roads (mean width 3 m/road’s lane). All the study sites encourage hiking on the marked trails. The hiking trails, range in width from 0.5 to 2.0 m, consist of loops through the forest interior and along the forest edge. State law prohibits bringing pets onto the two state nature preserves, but are allowed in the other parks if they are leashed.
Map of study region and camera trap locations (black dots). Numbers refer to the park sites ordered from north to south (1, Lapping Park; 2, Locust Grove; 3, Beargrass Creek State Nature Preserve; 4, Cherokee Park; 5, Parklands; 6, Blackacre State Nature Preserve; 7, Waverly Park; 8, Jefferson Memorial Forest) Sources: Esri, DeLorme, HERE, TomTom, Intermap, increment P Corp., GEBCO, USGS, FAO, NPS, NRCAN, GeoBase, IGN, Kadaster NL, Ordnance Survey, Esri Japan, METI, Esri China (Hong Kong), swisstopo, MapmyIndia, and the GIS User Community
We mapped the forest area for each park using Google Earth and ArcGIS v.10.4.1 (ESRI, Redlands, CA). Because roads and hiking trails are coincident with forest edge, we considered five variables in our study: 1) forest edge that is coincident with road (hereinafter referred to as “Roads”); 2) forest edge that is coincident with hiking trail (hereinafter referred to as “Hiking trails”); 3) forest edge that is not coincident with hiking trail (hereinafter referred to as “Forest edge”); and 4) forest edge that is not coincident with road (hereinafter referred to as “Forest edge 2”); and size of the forest area (hereinafter referred to as “forest area”). We then measured the distance (in meter) from the sampling sites to the nearest road (mean = 288 m, SD = 232.9), hiking trail (mean = 177 m, SD = 411.53), forest edge (mean = 455 m, SD = 936.73), and forest edge 2 (mean = 198 m, SD = 333.93). Previous studies showed that relatively precise occupancy and detection estimates can be obtained by running camera traps for 3–5 weeks across 40–60 locations (Kowalski et al. 2015; Kays et al. 2020). Accordingly, we randomly selected 68 sampling locations, 7–10 sampling locations/park, to set up the camera traps with an average distance of ≈1 km between the cameras.
Cameras were installed in locations with natural funnels, which maximize animal detection by forcing the object in front of cameras (Kelly and Holub 2008). Cameras were attached to trees at a height between 1 to 1.5 m, depending on the topographic feature of a given site, with a locking cable and facing the least obscured view from the tree. Using multi-camera traps per camera-site is recommended to improve species detectability, particularly for rare and/or small species (Pease et al. 2016; O’Connor et al. 2017). However, in practice, this approach is not always possible due to topographic features and logistical constraints (Alonso et al. 2015; Peres et al. 2017). Hence, to minimize the bias that could arise from using single cameras and shorter sampling durations, we used attractants (Webster and Beasley 2019). Use of baits and lures increases species detectability without changing the species movement patterns and temporal activities (Barea-Azcón et al. 2007; Gerber et al. 2012; Zapata-Ríos and Branch 2018). Accordingly, we impaled a can of tuna to the ground, approximately 10 m away from the camera to prevent bait removal. We hypothesized that if a species was more likely to use road, hiking trail, or forest edge, then the species would more likely be recorded closer to the roads, hiking trail, or forest edge (Wampler et al. 2008). Each camera was programmed to record 30-s videos for each event. The cameras were set to record video with a minimum delay of one minute between events. Mammal records were identified to species level and classified into independent events using a criterion of 30 min or more between consecutive records of the same species (O’Brien et al. 2003). Events were viewed as a random sample taken from an underlying continuous temporal distribution, describing the probability of an event occurring at any particular time (Ridout and Linkie 2009). For each event, we recorded time, date, and location. We also recorded the proportion of individual dogs that were leashed and unleashed.
Activity overlap
As dogs are mostly a diurnal species, we examined if there was overlap in the activity patterns of domestic dogs and white tailed deer (Odocoileus virginianus) (the only diurnal mammal) through a two-step procedure. First, the activity pattern of each species was estimated non-parametrically by fitting the capture time, obtained from camera traps for a given species, to a kernel density function (Ridout and Linkie 2009; Linkie and Ridout 2011). Second, we measured the degree of overlap between the estimated activities patterns for dogs and deer using the coefficient of overlap (Ridout and Linkie 2009; Frey et al. 2017). The value of the coefficient of overlap ranges between 0 (no overlap) and 1 (complete overlap) (Linkie and Ridout 2011; Frey et al. 2017). We performed this activity overlap analysis using “activityOverlap” function implemented in “camtrapR” R package (Niedballa et al. 2016).
Occupancy model
A detection history for each species was constructed as following, a value of ‘1’ assigned to those occasions in which the species was detected at least once and a ‘0’ in which the species was not occupied. We modeled these data using single season occupancy models (MacKenzie et al. 2002) as part of the “unmarked” R package (Fiske and Chandler 2011). This package uses the likelihood-based approach to estimate proportion of sites occupied (ψ) and detection probabilities (p), and allows for incorporating covariates into occupancy models in order to reduce variance in parameter estimates (MacKenzie et al. 2017). We evaluated the correlation among the five variables, distance to road, distance to hiking trail, distance forest edge, distance to forest edge 2, and the forest area, and eliminated one of each pair of covariates with high Spearman’s pairwise correlation (rs > 0.7) from the subsequent analysis (Rinehart et al. 2009; Bauder et al. 2017). This resulted in four uncorrelated covariates, distance to road, distance to hiking trail, distance to forest edge, and forest size, to be used in our analysis. To facilitate model convergence, we standardized these correlated covariates using z-score (Bauder et al. 2017).
We did not include covariates such as climate and season because 1) we conducted the survey within one season, 2) the species inhibiting the study area are active year-around and, 3) the bait we used was sufficient enough to attract species occupying the study area regardless of the climatic conditions.
Initially, we created a null model for each species (i.e. both detection probability (p) and occupancy (ψ) were held constant). We then extended this model by including covariates (i.e. distance to road, distance to trail, distance to forest edge, and forest area) and their interactions. We considered models with estimated ψ very close to “1” and broad confidence of interval as not well fitted models and excluded them from the subsequent analysis (MacKenzie et al. 2002). Next, we ranked the remaining candidates models using 1) Akaike’s Information Criterion (AIC) adjusted for small sample sizes (AICc), and 2) weighted AICc (AICcw) (MacKenzie et al. 2002; Rinehart et al. 2009). We used parametric bootstrapping approach proposed by MacKenzie and Bailey (2004) to check for model over-dispersion “c-hat”. If the model was over-dispersed (i.e. “c-hat” > > 1) we used quasi-corrected (QAICc) to select the best approximating models instead of AICc (Burnham and Anderson 2002; MacKenzie and Bailey 2004; Rinehart et al. 2009). A set of best performed models contained all models located within 0.9 AICcw (AIC cumulative weight) and had a ΔAICc/QAICc score less than “4”, and from this set we performed model-averaging to obtain overall estimates of occupancy and detection probability (Burnham and Anderson 2002; MacKenzie et al. 2002; Sewell et al. 2012). We assessed the relative importance of each variable by summing the AIC weights of all the models containing a given variable (Burnham and Anderson 2002), and variables with relative importance >0.2 was considered the most significant variable (Dibner et al. 2017; Oberosler et al. 2017).
Co-occurrence models
We investigated whether the presence of non-native species (domestic cat Felis catus and dog) influences the detection probability and occupancy of the native species by pair-wise comparisons using two-species single-season occupancy models (MacKenzie et al. 2004). The parameters estimated for occupancy were ψA (occupancy of non-native species), ψBA (occupancy of native species when the non-native species is present), and ψBa (occupancy of the native species when the non-native species is absent). We incorporated the best covariates obtained from the single-species models for each species while modeling ψA, ψBA, and ψBa. We assumed native species occupancy is independent of the non-native species if (ψBA = ψBa), and influenced by non-native species, otherwise (i.e. ψBA≠ψBa) (Nagy-Reis et al. 2017).
Results
We recorded 248 trap events with a total of 418 animal photographs including white-tailed deer, raccoon (Procyon lotor), opossum (Didelphis virginiana), coyote (Canis latrans), cat, dog, red fox (Vulpes vulpes), and the eastern cottontail rabbit (Sylvilagus floridanus). The trap nights varied among sites with overall 413 trap nights. The Parklands, Black Acre State Nature Preserve, and Waverly park had the highest number of trap nights (100, 70, and 56 respectively), followed by Beargrass State Nature Preserve, Jefferson Memorial Forest, and Lapping park (each had 40 trap nights), while Cherokee and Locust Grove had the shortest trap nights (35 and 32 respectively). Both red fox and rabbit were excluded from the analysis because of very low detection rates that would fail model convergence and have misleading inference. Most dogs (74%) were observed without a leash.
Activity overlap
Kernel density estimation showed a decrease in deer activity as dogs’ activity increased, coefficient of overlap = 0.46 (Fig. 2), suggesting that deer could be avoiding dogs.
Occupancy model
We successfully fitted single-season occupancy models for our focal species. Goodness-of-fit analysis suggested slight to moderate over-dispersion (“c-hat” between 1.16 and 2.3), accordingly model selection was carried out using QAICc. Model selection showed that no single best model could be selected to draw inference from species data (Table 1). For instance, five of 15 coyote’s candidate models contained about 95% of the QAICcw, while for raccoon and opossum, four models contained about 90% of the QAICcw (Table 1).
Therefore, the overall occupancy estimate (ψ) and detection probability (p) for each species was obtained from the model averaging approach. The results showed that deer and raccoon occupied almost two-thirds of the study area (0.77 and 0.64 respectively) (Table 2). Whereas, cat, and dog were estimated to occupy almost one-third of the study area (0.30 and 0.32 respectively). For all species, the estimated occupancy was higher than the naïve one (i.e. the proportion of sites where a species was photographed at least once). Our analysis showed that both deer and raccoon have the highest detection probabilities (0.33 and 0.38 respectively), followed by opossum and dog (0.12 and 0.16 respectively), whereas, coyote and cat have the lowest detection probabilities (0.11 and 0.08 respectively) (Table 2).
Our analysis showed that the occupancy of the native mammals, deer, opossum, coyote, and raccoon, increased the further a site was away from a road, a hiking trail, and a forest edge, while non-native mammal (cats and dogs) occupancy increased the closer a site was to a road, hiking trail, or a forest edge (Fig. 3). For all species, the models containing distance to road, hiking trail, and forest edge had the best support (i.e., contained >80% of the QAICcw). For deer occupancy, a model containing the interaction between the distance to road and the forest edge was among the best. While the interaction between hiking trail and forest edge was among the best fitted occupancy model for raccoon (Table 3). For opossum, one model out of the best four model included the forest area. For coyote, none of the best model included forest edge. For all species, except coyote, the detection probability was influenced mainly by the size of the forest area, whereas coyote detection probability was influenced by the size of the forest area and the distance to road, hiking trail, and forest edge. The relative importance analysis showed that roads and hiking trails had more importance than forest edge in determining occupancy of most of our focal species, whereas the size of forest area had no significant importance (relative variable importance <0.2) (Table 3).
According to the co-occurrence model, we found evidence that the presence of cat and dog negatively affect the occupancy for all native species (Table 4).
Discussion
Habitat fragmentation and forest degradation are major causes of the changing biodiversity patterns (Fahrig 2003; Zapata-Ríos and Branch 2018) and we expected these to be the key factors in explaining occupancy patterns of our focal species. The occupancy for most native species was negatively affected by roads, hiking trials and forest edge. Whereas, the domestic species occupancy had a positive association with roads, hiking trails, and forest edge. For most species, roads had higher importance in determining occupancy; however, the magnitude of importance of roads, hiking trails, and forest edge differed among species. We believe that roads had more importance in predicting the occupancy of most species because roads exert stronger negative effects than hiking trails and forest edge. Roads have both direct and indirect impacts on wildlife (Boston 2016; Bötsch et al. 2018). Direct impacts include vehicle caused mortality, habitat fragmentations, and limiting species movement (Coffin 2007; Shepard et al. 2008; Boston 2016). Indirect impacts include habitat near roads not being ideal functional sites for wildlife to rest and rear young because of hard edge habitats, noise, and human disturbance (Shepard et al. 2008; Boston 2016). In contrast, hiking trails and forest edge may have lower importance in predicting wildlife occupancy because they are located in suitable habitat, may be used by wildlife to facilitate movement, have lower noise and levels of human disturbance and can be considered a soft edge habitat (Marzano and Dandy 2012; Ballantyne et al. 2014).
White-tailed deer is habitat generalist that avoids dense forest habitats and is most often recorded in fragmented forests (Zeller et al. 2011; Petracca et al. 2014). Deer had a relatively high occupancy near roads and hiking trails (roughly 50%), which was expected as deer are known as fairly adaptable species with high behavioral plasticity and tolerance to human activities (Faas and Weckerly 2010). However, despite their high tolerance of human activities, deer occupancy was still negatively influenced by roads, hiking trails, and forest edge as deer occupancy increased as the distance from roads, hiking trails, and forest edge increased (Fig. 3).
Raccoon and opossum are resource generalist species with high behavioral plasticity who utilize human structures and food sources (Ng et al. 2004; Ordeñana et al. 2010; Fidino et al. 2016), which suggests that forest area, fragmentation, and forest access may not be the only predictors associated with the presence of these two species. Our results together with previous findings imply that raccoon and opossum occupancy may vary according to study sites and could be influenced by other factors such as food availability and intensity of anthropogenic activities (Ordeñana et al. 2010; Reed 2011; Cove et al. 2012; Kowalski et al. 2015).
Coyote were the top predator in our study area and is a known predator of domestic cats and dogs (Grubbs and Krausman 2009; Gehrt et al. 2013; Wang et al. 2015). Coyotes responded negatively to the human activities (i.e. roads and hiking trails), however, the magnitude of the response differed (Fig. 3). For instance, the estimated occupancy for coyote was almost 75% at the sites close to hiking trails, and gradually increased the further a site was away from hiking trails (Fig. 3). In contrast, coyotes had low occupancy near roads but occupancy probability increased sharply as the distance to roads increased. The relatively high occupancy probability near hiking trails could result from coyote being relatively tolerant of human activities that occur mostly during the day and visiting the periphery of hiking trails to forage for domestic species (Markovchick-Nicholls et al. 2008; Ordeñana et al. 2010; Kowalski et al. 2015).
The detectability and occupancy of domestic species is often higher at the forest edges than forest interiors (Reed and Merenlender 2008; Kowalski et al. 2015; Zapata-Ríos and Branch 2018). As expected, the distribution of cat and dog was positively associated with roads, hiking trails and forest edge. About 86 % of dog and 71 % of cat occurrences were located within a distance of a hundred meters from roads, hiking trails, and forest edges, suggesting that these features provide forest access to domestic species. Our models showed a sharper decline in dog occupancy than the decline in cat occupancy as distance from roads increased implying that free-ranging cats are more likely to occur in forest interiors and therefore could be a greater threat to smaller wildlife compared with dogs. Domesticated species rarely venture more than 100 m into the forest, as they are often fed by their owners and therefore may be indulging in “recreational hunting” and therefore not motivated to expend the effort needed to travel through forest undergrowth (Oehler and Litvaitis 1996; Kays and DeWan 2004; Lenth et al. 2008; Young et al. 2011; Hughes and Macdonald 2013; Morin et al. 2018).
The negative association between cats and dogs with native species is likely to have fitness consequences (Creel and Christianson 2008; Zapata-Ríos and Branch 2016). Domestic species often harass or chase native species, which is an energetically costly behavior and a source of stress (Manor and Saltz 2004; Lenth et al. 2008; Young et al. 2011). Most dogs in our study were unleashed, either walking within the vicinity of their owner or free ranging from their owner’s home, which allowed the dogs to potentially harass or chase native species. Deer are known to alter their activity patterns by becoming more nocturnal to avoid dogs, while deer become more diurnal when dogs are rarer (Hughes and Macdonald 2013; Zapata-Ríos and Branch 2016, 2018). Ungulates can be tolerant of human presence, however, they change their behavior if humans are accompanied by dogs (Miller et al. 2001; Lenth et al. 2008). Although cats cannot prey or do not chase the native species in our study, they may have negative interactions with native species or compete for food (Gehrt et al. 2013; McDonald et al. 2015). Domestic cat is thought to be a major predator of smaller wildlife in urban habitats (Bonnington et al. 2013), and their actual impact depends on many factors such as habitat type, geographic location and cat classification (i.e. feral or free ranging) (Medina et al. 2011; Loss et al. 2013; Kitts-Morgan 2015).
A possible source of bias in this study is the sampling duration, which likely affect species detectability and introduces bias to the occupancy estimate (Nichols et al. 2019). We acknowledge that longer sampling duration may yield more precise occupancy estimates (Kays et al. 2020), however, our sampling duration was relatively short due to this experimental approach and logistical constraints. We addressed this issue by using bait to maximize species detection and minimize the bias in occupancy estimate. Using bait to lure species has been successfully applied in several studies, especially for rare or species with low detection rate, to maximize detection rate (Kowalski et al. 2015; Pacifici et al. 2016; Ferreira-Rodríguez and Pombal 2019; Kays et al. 2020). Further, our sampling duration, eight weeks, was longer than the sampling duration of other studies. For instance, in our study the shortest trap nights was 32, which is longer than the commonly used sampling duration, 30 trap nights per location, (Ahumada et al. 2011). Another study had successfully fitted a single season occupancy model with data obtained from short sampling duration of 3 weeks (Kowalski et al. 2015). A recent study showed that a precise occupancy estimate could be produced from a sampling duration of four weeks or more (Kays et al. 2020). As such, we believe that the sampling duration in our study was sufficient to obtain a reliable occupancy estimate with minimum bias. Of course, increasing the sampling duration would, indeed, improve the estimate but we believe it would not significantly change the interpretation of our results. Another source of bias is camera trap height. It is recommended to keep the camera’s height at the level of the target species shoulder (Meek et al. 2012). However, a previous study showed that this recommended height is not always the best option as the camera trap height should be adjusted according to the type of camera, species size, and the objective of the study (Apps and McNutt 2018). In our study, following the recommended height was not possible due to the variation in body size of the target species. Therefore, we adjusted the camera trap height according to the habitat features of a given site. Further, the cameras were deployed in locations with natural funnels to ensure better detection through forcing the animal in front of the cameras. Moreover, we made sure that the bait was visible in our videos to avoid false absence due to camera trap height, which allowed us to detect smaller bodied species such as raccoon and opossum with higher frequency than larger bodied species such as coyote and red fox (Table 2). As such, we believe that any meso-sized species walking near the camera would have been detected (Barea-Azcón et al. 2007; Gerber et al. 2012; Zapata-Ríos and Branch 2018).
Conclusion
Our study suggests that both fragmentation and domestic species are associated with the distribution and activity patterns of native species in urban forests. From a management perspective, our study suggests that roads have a greater impact on native species occupancy than hiking trails and forest edge and their disturbance effect extends roughly 300 m. We suggest that future hiking trail development in urban or small forests should occur on the periphery of the forest instead of passing through the forest interior. A trail system along the perimeter of small preserves would allow the conservation of an interior forest and the protection of native wildlife while still allowing passive human recreation. Therefore, park management should consider the impact of roads, hiking trails, and forest edge when designing parks and the enforcement of dog leash regulations for the conservation of large mammals in urban parks.
References
Ahumada JA, Silva CEF, Gajapersad K, Hallam C, Hurtado J, Martin E, McWilliam A, Mugerwa B, O'Brien T, Rovero F, Sheil D, Spironello WR, Winarni N, Andelman SJ (2011) Community structure and diversity of tropical forest mammals: data from a global camera trap network. Philos Trans R Soc B Biol Sci 366:2703–2711. https://doi.org/10.1098/rstb.2011.0115
Alonso RS, McClintock BT, Lyren LM et al (2015) Mark-recapture and mark-Resight methods for estimating abundance with remote cameras: a carnivore case study. PLoS One 10:e0123032. https://doi.org/10.1371/journal.pone.0123032
Apps P, McNutt JW (2018) Are camera traps fit for purpose? A rigorous, reproducible and realistic test of camera trap performance. Afr J Ecol 56:710–720. https://doi.org/10.1111/aje.12573
Baker PJ, Ansell RJ, Dodds PAA et al (2003) Factors affecting the distribution of small mammals in an urban area. Mamm Rev 33:95–100. https://doi.org/10.1046/j.1365-2907.2003.00003.x
Ballantyne M, Gudes O, Pickering CM (2014) Recreational trails are an important cause of fragmentation in endangered urban forests: a case-study from Australia. Landsc Urban Plan 130:112–124. https://doi.org/10.1016/J.LANDURBPLAN.2014.07.004
Barea-Azcón JM, Virgós E, Ballesteros-Duperón E, Moleón M, Chirosa M (2007) Surveying carnivores at large spatial scales: a comparison of four broad-applied methods. Biodivers Conserv 16:1213–1230. https://doi.org/10.1007/s10531-006-9114-x
Bauder JM, Stevenson DJ, Sutherland CS, Jenkins CL (2017) Occupancy of potential overwintering habitat on protected lands by two imperiled Snake species in the coastal plain of the southeastern United States. J Herpetol 51:73–88. https://doi.org/10.1670/15-064
Bonnington C, Gaston KJ, Evans KL (2013) Fearing the feline: domestic cats reduce avian fecundity through trait-mediated indirect effects that increase nest predation by other species. J Appl Ecol 50:15–24. https://doi.org/10.1111/1365-2664.12025
Boston K (2016) The potential effects of Forest roads on the environment and mitigating their impacts. Curr For Reports 2:215–222. https://doi.org/10.1007/s40725-016-0044-x
Bötsch Y, Tablado Z, Scherl D, Kéry M, Graf RF, Jenni L (2018) Effect of recreational trails on Forest birds: human presence matters. Front Ecol Evol 6:175. https://doi.org/10.3389/fevo.2018.00175
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer New York, New York
Churcher PB, Lawton JH (1987) Predation by domestic cats in an English village. J Zool 212:439–455. https://doi.org/10.1111/j.1469-7998.1987.tb02915.x
Coffin AW (2007) From roadkill to road ecology: a review of the ecological effects of roads. J Transp Geogr 15:396–406. https://doi.org/10.1016/J.JTRANGEO.2006.11.006
Cole EK, Pope MD, Anthony RG (1997) Effects of road management on movement and survival of Roosevelt elk. J Wildl Manag 61:1115. https://doi.org/10.2307/3802109
Collinge SK (2009) Ecology of fragmented landscapes. The Johns Hopkins University press, Baltimore, USA
Coppes J, Burghardt F, Hagen R, Suchant R, Braunisch V (2017) Human recreation affects spatio-temporal habitat use patterns in red deer (Cervus elaphus). PLoS One 12:e0175134. https://doi.org/10.1371/journal.pone.0175134
Cove MV, Jones BM, Bossert AJ, Clever DR Jr, Dunwoody RK, White BC, Jackson VL (2012) Use of camera traps to examine the Mesopredator release hypothesis in a fragmented Midwestern landscape. Am Midl Nat 168:456–465
Creel S, Christianson D (2008) Relationships between direct predation and risk effects. Trends Ecol Evol 23:194–201. https://doi.org/10.1016/J.TREE.2007.12.004
Crooks KR (2002) Relative sensitivities of mammalian carnivores to habitat fragmentation. Conserv Biol 16:488–502. https://doi.org/10.1046/j.1523-1739.2002.00386.x
Crooks KR, Soulé ME (1999) Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400:563–566. https://doi.org/10.1038/23028
Dibner RR, Doak DF, Murphy M (2017) Discrepancies in occupancy and abundance approaches to identifying and protecting habitat for an at-risk species. Ecol Evol 7:5692–5702. https://doi.org/10.1002/ece3.3131
Ewers RM, Didham RK (2007) The effect of fragment shape and species’ sensitivity to habitat edges on animal population size: contributed papers. Conserv Biol 21:926–936. https://doi.org/10.1111/j.1523-1739.2007.00720.x
Faas CJ, Weckerly FW (2010) Habitat interference by Axis deer on white-tailed deer. J Wildl Manag 74:698–706. https://doi.org/10.2193/2009-135
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515. https://doi.org/10.1146/annurev.ecolsys.34.011802.132419
Farías Torbidoni EI (2011) Managing for recreational experience opportunities: the case of hikers in protected areas in Catalonia, Spain. Environ Manag 47:482–496. https://doi.org/10.1007/s00267-010-9606-z
Ferreira-Rodríguez N, Pombal MA (2019) Bait effectiveness in camera trap studies in the Iberian Peninsula. Mammal Res 64:155–164. https://doi.org/10.1007/s13364-018-00414-1
Fidino MA, Lehrer EW, Magle SB (2016) Habitat dynamics of the Virginia Opossum in a highly urban landscape. Am Midl Nat 175:155–167. https://doi.org/10.1674/0003-0031-175.2.155
Fiske I, Chandler R (2011) Unmarked : an R package for fitting hierarchical models of wildlife occurrence and abundance. J Stat Softw 43:1–23. https://doi.org/10.18637/jss.v043.i10
Frey S, Fisher JT, Burton AC, Volpe JP (2017) Investigating animal activity patterns and temporal niche partitioning using camera-trap data: challenges and opportunities. Remote Sens Ecol Conserv 3:123–132. https://doi.org/10.1002/rse2.60
Frid A, Dill LM (2002) Human-caused disturbance stimuli as a form of predation risk. Conserv Ecol 6:11
Gehrt SD, Wilson EC, Brown JL, Anchor C (2013) Population ecology of free-roaming cats and interference competition by coyotes in urban parks. PLoS One 8:e75718. https://doi.org/10.1371/journal.pone.0075718
Gerber BD, Karpanty SM, Kelly MJ (2012) Evaluating the potential biases in carnivore capture–recapture studies associated with the use of lure and varying density estimation techniques using photographic-sampling data of the Malagasy civet. Popul Ecol 54:43–54. https://doi.org/10.1007/s10144-011-0276-3
Grubbs SE, Krausman PR (2009) Observations of coyote–cat interactions. J Wildl Manag 73:683–685. https://doi.org/10.2193/2008-033
Hammitt WE, Cole DN (2015) Wildland recreation : ecology and management, 3rd edn. Wiley-Blackwell
Hughes J, Macdonald DW (2013) A review of the interactions between free-roaming domestic dogs and wildlife. Biol Conserv 157:341–351. https://doi.org/10.1016/J.BIOCON.2012.07.005
Kays R, Arbogast BS, Baker-Whatton M, Beirne C, Boone HM, Bowler M, Burneo SF, Cove MV, Ding P, Espinosa S, Gonçalves ALS, Hansen CP, Jansen PA, Kolowski JM, Knowles TW, Lima MGM, Millspaugh J, McShea WJ, Pacifici K, Parsons AW, Pease BS, Rovero F, Santos F, Schuttler SG, Sheil D, Si X, Snider M, Spironello WR (2020) An empirical evaluation of camera trap study design: how many, how long and when? Methods Ecol Evol 11:700–713. https://doi.org/10.1111/2041-210X.13370
Kays RW, DeWan AA (2004) Ecological impact of inside/outside house cats around a suburban nature preserve. Anim Conserv 7:273–283. https://doi.org/10.1017/S1367943004001489
Kelly MJ, Holub EL (2008) Camera trapping of carnivores: trap success among camera types and across species, and habitat selection by species, on salt Pond Mountain, Giles County, Virginia. Northeast Nat 15:249–262. https://doi.org/10.1656/1092-6194(2008)15[249:CTOCTS]2.0.CO;2
Keyser AJ, Hill GE, Soehren EC (1998) Effects of Forest fragment size, Nest density, and proximity to edge on the risk of predation to ground-nesting passerine birds. Conserv Biol 12:986–994. https://doi.org/10.1046/j.1523-1739.1998.97177.x
Kitts-Morgan SE (2015) COMPANION ANIMALS SYMPOSIUM: sustainable ecosystems: domestic cats and their effect on wildlife populations. J Anim Sci 93:848–859. https://doi.org/10.2527/jas.2014-8557
Kowalski B, Watson F, Garza C, Delgado B (2015) Effects of landscape covariates on the distribution and detection probabilities of mammalian carnivores. J Mammal 96:511–521. https://doi.org/10.1093/jmammal/gyv056
Lacerda ACR, Tomas WM, Marinho-Filho J (2009) Domestic dogs as an edge effect in the Brasília National Park, Brazil: interactions with native mammals. Anim Conserv 12:477–487. https://doi.org/10.1111/j.1469-1795.2009.00277.x
Lenth BE, Knight RL, Brennan ME (2008) The effects of dogs on wildlife communities. https://doi.org/103375/0885-8608(2008)28[218:TEODOW]20CO;2 28:218–227. https://doi.org/10.3375/0885-8608(2008)28[218:TEODOW]2.0.CO;2
Liberg O (1984) Food habits and prey impact by feral and house-based domestic cats in a rural area in southern Sweden. J Mammal 65:424–432. https://doi.org/10.2307/1381089
Linkie M, Ridout MS (2011) Assessing tiger-prey interactions in Sumatran rainforests. J Zool 284:224–229. https://doi.org/10.1111/j.1469-7998.2011.00801.x
Loss SR, Will T, Marra PP (2013) The impact of free-ranging domestic cats on wildlife of the United States. Nat Commun 4:1396. https://doi.org/10.1038/ncomms2380
MacKenzie DI, Bailey LL (2004) Assessing the fit of site-occupancy models. J Agric Biol Environ Stat 9:300–318. https://doi.org/10.1198/108571104X3361
MacKenzie DI, Bailey LL, Nichols JD (2004) Investigating species co-occurrence patterns when species are detected imperfectly. J Anim Ecol 73:546–555. https://doi.org/10.1111/j.0021-8790.2004.00828.x
MacKenzie DI, James Nichols, J. Royle, et al (2017) Occupancy estimation and modeling : inferring patterns and dynamics of species occurrence, Second edi. Academic Press, ebook
MacKenzie DI, Nichols JD, Lachman GB et al (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83:2248–2255. https://doi.org/10.1890/0012-9658(2002)083[2248:ESORWD]2.0.CO;2
Manor R, Saltz D (2004) The impact of free-roaming dogs on gazelle kid/female ratio in a fragmented area. Biol Conserv 119:231–236. https://doi.org/10.1016/J.BIOCON.2003.11.005
Markovchick-Nicholls L, Regan HM, Deutschman DH et al (2008) Relationships between human disturbance and wildlife land use in urban habitat fragments. Conserv Biol 22:99–109. https://doi.org/10.1111/j.1523-1739.2007.00846.x
Marzano M, Dandy N (2012) Recreationist behaviour in forests and the disturbance of wildlife. Biodivers Conserv 21:2967–2986. https://doi.org/10.1007/s10531-012-0350-y
McDonald JL, Maclean M, Evans MR, Hodgson DJ (2015) Reconciling actual and perceived rates of predation by domestic cats. Ecol Evol 5:2745–2753. https://doi.org/10.1002/ece3.1553
Medina FM, Bonnaud E, Vidal E, Tershy BR, Zavaleta ES, Josh Donlan C, Keitt BS, Corre M, Horwath SV, Nogales M (2011) A global review of the impacts of invasive cats on island endangered vertebrates. Glob Chang Biol 17:3503–3510. https://doi.org/10.1111/j.1365-2486.2011.02464.x
Meek PD (2003) Home range of house cats Felis Catus living within a national park. Aust Mammal 25:51–60. https://doi.org/10.1071/am03051
Meek PD, Ballard G-A, Fleming PJS (2012) An introduction to camera trapping for wildlife surveys in Australia. PestSmart Toolkit publication, Invasive Animals Cooperative Research Centre, Canberra, Australia
Melbourne BA, Davies KF, Margules CR, Lindenmayer DB, Saunders DA, Wissel C, Henle K (2004) Species survival in fragmented landscapes: where to from here? Biodivers Conserv 13:275–284
Miller SG, Knight RL, Miller CK (2001) Wildlife responses to pedestrians and dogs. Wildl Soc Bull 29:124–132
Morin DJ, Lesmeister DB, Nielsen CK, Schauber EM (2018) The truth about cats and dogs: landscape composition and human occupation mediate the distribution and potential impact of non-native carnivores. Glob Ecol Conserv 15:e00413. https://doi.org/10.1016/J.GECCO.2018.E00413
Murcia C (1995) Edge effects in fragmented forests: implications for conservation. Trends Ecol Evol 10:58–62
Nagy-Reis MB, Nichols JD, Chiarello AG, Ribeiro MC, Setz EZF (2017) Landscape use and co-occurrence patterns of Neotropical spotted cats. PLoS One 12:e0168441. https://doi.org/10.1371/journal.pone.0168441
Ng SJ, Dole JW, Sauvajot RM, Riley SPD, Valone TJ (2004) Use of highway undercrossings by wildlife in southern California. Biol Conserv 115:499–507. https://doi.org/10.1016/S0006-3207(03)00166-6
Nichols M, Ross J, Glen AS, Paterson AM (2019) An evaluation of systematic versus strategically-placed camera traps for monitoring feral cats in New Zealand. Animals 9:687. https://doi.org/10.3390/ani9090687
Niedballa J, Sollmann R, Courtiol A, Wilting A (2016) camtrapR : an R package for efficient camera trap data management. Methods Ecol Evol 7:1457–1462. https://doi.org/10.1111/2041-210X.12600
O’Brien TG, Kinnaird MF, Wibisono HT (2003) Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Anim Conserv 6:131–139. https://doi.org/10.1017/S1367943003003172
O’Connor KM, Nathan LR, Liberati MR, Tingley MW, Vokoun JC, Rittenhouse TAG (2017) Camera trap arrays improve detection probability of wildlife: investigating study design considerations using an empirical dataset. PLoS One 12:e0175684. https://doi.org/10.1371/journal.pone.0175684
Oberosler V, Groff C, Iemma A, Pedrini P, Rovero F (2017) The influence of human disturbance on occupancy and activity patterns of mammals in the Italian Alps from systematic camera trapping. Mamm Biol 87:50–61. https://doi.org/10.1016/J.MAMBIO.2017.05.005
Oehler JD, Litvaitis JA (1996) The role of spatial scale in understanding responses of medium-sized carnivores to forest fragmentation. Can J Zool 74:2070–2079. https://doi.org/10.1139/z96-235
Ordeñana MA, Crooks KR, Boydston EE, Fisher RN, Lyren LM, Siudyla S, Haas CD, Harris S, Hathaway SA, Turschak GM, Miles AK, van Vuren DH (2010) Effects of urbanization on carnivore species distribution and richness. J Mammal 91:1322–1331. https://doi.org/10.1644/09-MAMM-A-312.1
Pacifici K, Reich BJ, Dorazio RM, Conroy MJ (2016) Occupancy estimation for rare species using a spatially-adaptive sampling design. Methods Ecol Evol 7:285–293. https://doi.org/10.1111/2041-210X.12499
Pease BS, Nielsen CK, Holzmueller EJ (2016) Single-camera trap survey designs miss detections: impacts on estimates of occupancy and community metrics. PLoS One 11:e0166689. https://doi.org/10.1371/journal.pone.0166689
Peres PHF, Polverini MS, Oliveira ML, Duarte JMB (2017) Accessing camera trap survey feasibility for estimating Blastocerus dichotomus (Cetartiodactyla, Cervidae) demographic parameters. Iheringia Série Zool 107. https://doi.org/10.1590/1678-4766e2017041
Petracca LS, Ramírez-Bravo OE, Hernández-Santín L (2014) Occupancy estimation of jaguar Panthera onca to assess the value of east-Central Mexico as a jaguar corridor. Oryx 48:133–140. https://doi.org/10.1017/S0030605313000069
Phillips GE, Alldredge AW (2000) Reproductive success of elk following disturbance by humans during calving season. J Wildl Manag 64:521. https://doi.org/10.2307/3803250
Pickett STA, Cadenasso ML, Grove JM, Nilon CH, Pouyat RV, Zipperer WC, Costanza R (2001) Urban ecological systems: linking terrestrial ecological, physical, and socioeconomic components of metropolitan areas. Annu Rev Ecol Syst 32:127–157. https://doi.org/10.1146/annurev.ecolsys.32.081501.114012
Reed SE (2011) Non-invasive methods to assess co-occurrence of mammalian carnivores. Southwest Nat 56:231–240. https://doi.org/10.1894/F13-JKF-14.1
Reed SE, Merenlender AM (2011) Effects of management of domestic dogs and recreation on carnivores in protected areas in northern California. Conserv Biol 25:504–513. https://doi.org/10.1111/j.1523-1739.2010.01641.x
Reed SE, Merenlender AM (2008) Quiet, nonconsumptive recreation reduces protected area effectiveness. Conserv Lett 1:146–154. https://doi.org/10.1111/j.1755-263X.2008.00019.x
Ridout MS, Linkie M (2009) Estimating overlap of daily activity patterns from camera trap data. J Agric Biol Environ Stat 14:322–337. https://doi.org/10.1198/jabes.2009.08038
Rinehart KA, Donovan TM, Mitchell BR, Long RA (2009) Factors influencing occupancy patterns of eastern newts across Vermont. J Herpetol 43:521–531. https://doi.org/10.1670/08-063R1.1
Scholten J, Moe SR, Hegland SJ (2018) Red deer (Cervus elaphus) avoid mountain biking trails. Eur J Wildl Res 64:1–9. https://doi.org/10.1007/s10344-018-1169-y
Sewell D, Guillera-Arroita G, Griffiths RA, Beebee TJC (2012) When is a species declining? Optimizing survey effort to detect population changes in reptiles. Reptil PLoS ONE 7:43387. https://doi.org/10.1371/journal.pone.0043387
Shannon G, Angeloni LM, Wittemyer G, Fristrup KM, Crooks KR (2014) Road traffic noise modifies behaviour of a keystone species. Anim Behav 94:135–141. https://doi.org/10.1016/j.anbehav.2014.06.004
Shepard DB, Kuhns AR, Dreslik MJ, Phillips CA (2008) Roads as barriers to animal movement in fragmented landscapes. Anim Conserv 11:288–296. https://doi.org/10.1111/j.1469-1795.2008.00183.x
Wampler CR, Frey JK, VanLeeuwen DM et al (2008) Mammals in mechanically thinned and non-thinned mixed-coniferous Forest in the Sacramento Mountains, New Mexico. Southwest Nat 53:431–443. https://doi.org/10.1894/clg-10.1
Wang Y, Allen ML, Wilmers CC (2015) Mesopredator spatial and temporal responses to large predators and human development in the Santa Cruz Mountains of California. Biol Conserv 190:23–33. https://doi.org/10.1016/J.BIOCON.2015.05.007
Webster SC, Beasley JC (2019) Influence of lure choice and survey duration on scent stations for carnivore surveys. Wildl Soc Bull 43:661–668. https://doi.org/10.1002/wsb.1011
Westekemper K, Reinecke H, Signer J, Meißner M, Herzog S, Balkenhol N (2018) Stay on trails – effects of human recreation on the spatiotemporal behavior of red deer Cervus elaphus in a German national park Wildlife Biol 2018:. https://doi.org/10.2981/wlb.00403
Wimpey JF, Marion JL (2010) The influence of use, environmental and managerial factors on the width of recreational trails. J Environ Manag 91:2028–2037. https://doi.org/10.1016/j.jenvman.2010.05.017
Woods M, McDonald RA, Harris S (2003) Predation of wildlife by domestic cats Felis catus in Great Britain. Mamm Rev 33:174–188. https://doi.org/10.1046/j.1365-2907.2003.00017.x
Young JK, Olson KA, Reading RP, Amgalanbaatar S, Berger J (2011) Is wildlife going to the dogs? Impacts of feral and free-roaming dogs on wildlife populations. Bioscience 61:125–132. https://doi.org/10.1525/bio.2011.61.2.7
Zapata-Ríos G, Branch LC (2016) Altered activity patterns and reduced abundance of native mammals in sites with feral dogs in the high Andes. Biol Conserv 193:9–16. https://doi.org/10.1016/J.BIOCON.2015.10.016
Zapata-Ríos G, Branch LC (2018) Mammalian carnivore occupancy is inversely related to presence of domestic dogs in the high Andes of Ecuador. PLoS One 13:e0192346. https://doi.org/10.1371/journal.pone.0192346
Zeller KA, Nijhawan S, Salom-Pérez R, Potosme SH, Hines JE (2011) Integrating occupancy modeling and interview data for corridor identification: a case study for jaguars in Nicaragua. Biol Conserv 144:892–901. https://doi.org/10.1016/J.BIOCON.2010.12.003
Zipkin EF, DeWan A, Andrew Royle J (2009) Impacts of forest fragmentation on species richness: a hierarchical approach to community modelling. J Appl Ecol 46:815–822. https://doi.org/10.1111/j.1365-2664.2009.01664.x
Acknowledgments
We would like to thank IU Southeast for their support of this research. We would also like to thank Adam Haus and the Applied Conservation Biology class for their assistance in the field. In addition, we would like to thank the administration of the different parks for providing logistical support.
Funding
Open Access funding provided by Swedish University of Agricultural Sciences. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. This research was supported by IU Southeast.
Author information
Authors and Affiliations
Contributions
O. A. and W. L. designed the research and conducted the field work. A. S. and O. A. conducted the analyses and contributed to writing the main manuscript text. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soultan, A., Attum, O. & Lahue, W. The relationship between landscape features and domestic species on the occupancy of native mammals in urban forests. Urban Ecosyst 24, 1117–1128 (2021). https://doi.org/10.1007/s11252-021-01100-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-021-01100-y