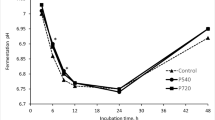
Abstract
In this study, the in vitro apparent rumen degradability of organic matter (ARDOM) and plant secondary metabolites (ARDPSM) of three tropical legumes (Mucuna pruriens, Canavalia ensiformis, and Leucaena leucocephala) were assessed. For this, 3 experiments were set up, i.e., single end-point incubations (24 h) with ruminal inoculum from either Belgian or Cuban sheep, as well as kinetic assessments (0 h, 2 h, 4 h, 6 h, 8 h, 10 h, 12 h, and 24 h) inoculum from Belgian sheep. L-mimosine, L-canavanine, Concanavalin A (Con A), and trypsin inhibitor (TI) were the plant secondary metabolites (PSM) targeted in this study. In all three experiments, both beans, as well as forage/bean meals of M. pruriens and C. ensiformis and their PSM, were extensively degraded during 24 h incubation, irrespective of the inoculum source (0.44 to 0.70 and 0.43 to 0.78 g/g of organic matter (OM) for ARDOM, respectively, and > 0.80 g/g for L-canavanine, > 0.76 TIU/TIU for TI, and > 0.95 g/g for Con A, for both legumes). Forage meal of L. leucocephala was considerably less degraded, with apparent ruminal degradabilities of 0.20 g/g OM and 0.35 g/g OM after 24 h incubation with Belgian or Cuban sheep inoculum, respectively. This could – at least partially – be related to L-mimosine, present in L. leucocephala, which was hardly degraded in the Belgian incubation, while a more extensive ruminal breakdown was observed under the Cuban conditions (0.05 g/g PSM vs. 0.78 g/g PSM, respectively). The negative effect of L-mimosine on OM degradability was supported in an additional in vitro experiment with straw and inoculum from Belgian sheep, as ruminal degradation of straw was 31% lower when pure L-mimosine was supplemented.





Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- ADFom:
-
Acid detergent fiber expressed exclusive of residual ash
- ARDOM:
-
Apparent in vitro rumen degradability of organic matter
- ARDSPM:
-
Apparent rumen degradability of plant secondary metabolites
- Con A:
-
Concanavaline A
- DM:
-
Dry matter
- ha:
-
Hectare
- ME:
-
Metabolizable energy
- NDFom:
-
Neutral detergent fiber not assayed with a heat stable amylase and expressed exclusive of residual ash
- NPAA:
-
Non-protein amino acids
- OM:
-
Organic matter
- CP:
-
Crude protein
- PCA:
-
Proximate chemical analysis
- PSM:
-
Plant secondary metabolites
- SCFA:
-
Short-chain fatty acids
- TI:
-
Trypsin inhibitor
References
A.O.A.C., 2005. Official methods of analysis of AOAC International, (AOAC International, Gaithersburg, Maryland, USA.)
Agbede, J.O., and Aletor, V.A., 2005. Studies of the chemical composition and protein quality evaluation of differently processed Canavalia ensiformis and Mucuna pruriens seed flours, Journal of Food Composition and Analysis, 18, 89-103
Ahmed, M., Jusoh, S., Alimon, A., Ebrahimi, M., and Samsudin, A., 2018. Nutritive and anti-nutritive evaluation of Kleinhovia hospita, Leucaena leucocephala and Gliricidia sepium with respect to their effects on in vitro rumen fermentation and gas production, Tropical Animal Science Journal, 41, 128-136
Ajayi, F., Akande, S., Adegbite, A., and Idowu, B., 2009. Assessment of seven under-utilized grain legume foliages as feed resources for ruminants, Livestock Research for Rural Development, 21, 149-156
Ajayi, F.T., Akande, S.R., Odejide, J.O., and Idowu, B., 2010. Nutritive evaluation of some tropical under-utilized grain legume seeds for ruminant’s nutrition, Journal of Animal Sciences, 6, 1-7
Allison, M.J., Hammond, A.C., and Jones, R.J., 1990. Detection of ruminal bacteria that degrade toxic dihydroxypyridine compounds produced from mimosine, Applied and Environmental Microbiology, 56, 590-594
Anantasook, N., Wanapat, M., Cherdthong, A., and Gunun, P., 2013. Changes of microbial population in the rumen of dairy steers as influenced by plant containing tannins and saponins and roughage to concentrate ratio, Asian-Australas J Anim Sci, 26, 1583-1591
Anitha, R., Jayavelu, S., and Murugesan, K., 2005. Antidermatophytic and bacterial activity of mimosine, Phytotherapy Research, 19, 992-993
Babayemi, O.J., Bamikole, M.A., and Daodu, M.O., 2009. In vitro gas production and its prediction on metabolizable energy, organic matter digestibility and short chain fatty acids of some tropical seeds, Pakistan Journal of Nutrition, 8, 1078-1082
Belmar, R., Nava-Montero, R., Sandoval-Castro, C., and McNab, J.M., 2007. Jack bean (Canavalia ensifomis L. DC) in poultry diets: antinutritional factors and detoxification studies – a review, World's Poultry Science Journal, 55, 37-59
Castro-Montoya, J., De Campeneere, S., Van Ranst, G., and Fievez, V., 2012. Interactions between methane mitigation additives and basal substrates on in vitro methane and VFA production, Animal Feed Science and Technology, 176, 47-60
Castro-Montoya, J., Peiren, N., Cone, J.W., Zweifel, B., Fievez, V., and De Campeneere, S., 2015. In vivo and in vitro effects of a blend of essential oils on rumen methane mitigation, Livestock Science, 180, 134-142
Castro-Montoya, J.M., and Dickhoefer, U., 2020. The nutritional value of tropical legume forages fed to ruminants as affected by their growth habit and fed form: a systematic review, Animal Feed Science and Technology, 269, 114641
Cherney, D., Siciliano-Jones, J., and Pell, A., 1993. Forage in vitro dry matter digestibility as influenced by fiber source in the donor cow diet, Journal of Animal Science 71, 1335-1338
Chikagwa-Malunga, S.K., Adesogan, A.T., Sollenberger, L.E., Badinga, L.K., Szabo, N.J., and Littell, R.C., 2009. Nutritional characterization of Mucuna pruriens: 1. Effect of maturity on the nutritional quality of botanical fractions and the whole plant, Animal Feed Science and Technology, 148, 34-50
Debevere, S., Cools, A., Baere, S.D., Haesaert, G., Rychlik, M., Croubels, S., and Fievez, V., 2020. In vitro rumen simulations show a reduced disappearance of deoxynivalenol, nivalenol and enniatin b at conditions of rumen acidosis and lower microbial activity, Toxins, 12, 101
Demeyer, D., 1991. Quantitative aspects of microbial metabolism on rumen and hindgut. In: J.P. Jouany (ed), Rumen microbial metabolism and ruminant digestion, 1991, (INRA, Paris, France), 217–237
Derakhshani, H., Corley, S.W., and Al Jassim, R., 2016. Isolation and characterization of mimosine, 3, 4 DHP and 2, 3 DHP degrading bacteria from a commercial rumen inoculum, Journal of Basic Microbiology, 56, 580-585
Díaz, M.F., González, A., Padilla, C., and Curbelo, F., 2003. Comportamiento de la producción de forrajes y granos de Canavalia ensiformis, Lablab purpureus y Stizolobium niveum en siembras de septiembre, Revista Cubana de Ciencia Agrícola, 37, 65-71
Dominguez-Bello, M.G., and Stewart, C.S., 1990a. Degradation of mimosine, 2,3-dihydroxy pyridine and 3-hydroxy-4(1H)-pyridine by bacteria from the rumen of sheep in Venezuela, FEMS Microbiology Ecology, 6, 283-289
Dominguez-Bello, M.G., and Stewart, C.S., 1990b. Effects of feeding Canavalia ensiformis on the rumen flora of sheep, and of the toxic amino acid canavanine on rumen bacteria, Systematic and Applied Microbiology, 13, 388-393
Du, S., Xu, M., and Yao, J., 2016. Relationship between fibre degradation kinetics and chemical composition of forages and by-products in ruminants, Journal of Applied Animal Research, 44, 189-193
E.C., 2009. European Union Official Diary. Reglament (CE) no152/2009., 2009, (European Community Committee, Brusselas, Belgium), 54
Estell, R., 2010. Coping with shrub secondary metabolites by ruminants, Small Ruminant Research, 94, 1-9
Galindo, J., Marrero, Y., Ruiz, T., González, N., Díaz, A., Aldama, A.I., Moreira, O., Hernández, J., Torres, V., and Sarduy, L., 2009. Efecto de una mezcla múltiple de leguminosas herbáceas y Leucaena leucocephala en la población microbiana y productos fermentativos del rumen de añojos mestizos de Cebú, Revista Cubana de Ciencia Agrícola, 43
Gao, W., Chen, A., Zhang, B., Kong, P., Liu, C., and Zhao, J., 2015. Rumen degradability and post-ruminal digestion of dry matter, nitrogen and amino acids of three protein supplements, Asian-Australasian journal of animal sciences, 28, 485-493
García, D., Wencomo, H., Gonzáles, M., Medina, M., and Cova, L., 2008. Caracterización de diez cultivares forrajeros de Leucaena leucocephala basada en la composición química y la degradabilidad ruminal, Revista MVZ Córdoba, 13, 1295-1303
Gaviria, X., Naranjo, J.F., and Barahona, R., 2015. In vitro fermentation kinetics of Leucaena leucocephala and Megathyrsus maximus and their mixtures, with or without energy supplementation, Pastures and Forages, 8, 55-63
Gemeda, B.S., and Hassen, A., 2015. Effect of tannin and species variation on in vitro digestibility, gas, and methane production of tropical browse plants, Asian-Australas J Anim Sci, 28, 188-199
Ghosh, M.K., and Samiran, B., 2007. Mimosine toxicity-a problem of Leucaena feeding in ruminants, Asian Journal of Animal and Veterinary Advances, 2, 63-73
Heuzé, V., Tran, G., Nozière, P., Lessire, M., and Lebas, F., 2017. Soybean seeds. In: a.p.b.I. In: Feedipedia, CIRAD, AFZ and FAO (ed), In: Feedipedia, a programme by INRAE, CIRAD, AFZ and FAO, 2017, In: Feedipedia, a programme by INRAE, CIRAD, AFZ and FAO)
Hoffmann, E.M., Muetzel, S., and Becker, K., 2003. The fermentation of soybean meal by rumen microbes in vitro reveals different kinetic features for the inactivation and the degradation of trypsin inhibitor protein, Animal Feed Science and Technology, 106, 189-197
Honda, M.D., and Borthakur, D., 2019. Mimosine concentration in Leucaena leucocephala under various environmental conditions, Tropical Grasslands-Forrajes Tropicales, 7, 164-172
Hung, L.V., Wanapat, M., and Cherdthong, A., 2013. Effects of Leucaena leaf pellet on bacterial diversity and microbial protein synthesis in swamp buffalo fed on rice straw, Livestock Science, 151, 188-197
Janardhanan, K., Gurumoorthi, P., and Pugalenthi, M., 2003. Nutritional potential of five accessions of a South Indian tribal pulse, Mucuna pruriens var utilis I. The effect of processing methods on the content of l-dopa, phytic acid, and oligosaccharides, Tropical and Subtropical Agroecosystems, 1, 141-152
Jeyanathan, J., Escobar, M., Wallace, R.J., Fievez, V., and Vlaeminck, B., 2016. Biohydrogenation of 22:6n-3 by Butyrivibrio proteoclasticus P18, BMC Microbiology, 16, 104
Jones, R.J., and Megarrity, R.G., 1986. Successful transfer of DHP-degrading bacteria from Hawaiian goats to Australian ruminants to overcome the toxicity of Leucaena, Australian Veterinary Journal, 63, 259-262
Kambashi, B., Picron, P., Boudry, C., Théwis, A., Kiatoko, H., and Bindelle, J., 2014. Nutritive value of tropical forage plants fed to pigs in the Western provinces of the Democratic Republic of the Congo, Animal Feed Science and Technology, 191, 47-56
Lackey, J.A., 1977. A revised classification of the tribe Phaseoleae (Leguminosae: Papilionoideae), and its relation to canavanine distribution, Botanical Journal of the Linnean Society, 74, 163-178
Lima-Orozco, R., Castro-Alegría, A., and Fievez, V., 2013. Ensiled sorghum and soybean as ruminant feed in the tropics, with emphasis on Cuba, Grass and Forage Science, 68, 20-32
Lima-Orozco, R., Van Daele, I., ÁLvarez-Hernández, U., and Fievez, V., 2014. Combined conservation of jack bean and velvet bean with sorghum: evaluation of lab-scale silages and in vitro assessment of their nutritive value, The Journal of Agricultural Science, 152, 967-980
Lima-Orozco, R., Van Daele, I., Álvarez-Hernández, U., and Fievez, V., 2016. Combination of the underutilised legumes Canavalia ensiformis (L.) D.C. and Mucuna pruriens (L.) D.C. with sorghum: integrated assessment of their potential as conserved ruminant feed, Cuban Journal of Agricultural Science, 50,
Lima, R., Díaz, R.F., Castro, A., and Fievez, V., 2011. Digestibility, methane production and nitrogen balance in sheep fed ensiled or fresh mixtures of sorghum–soybean forage, Livestock Science, 141, 36-46
Makkar, H.P.S., Francis, G., and Becker, K., 2007a. Bioactivity of phytochemicals in some lesser-known plants and their effects and potential applications in livestock and aquaculture production systems, Animal, 1, 1371-1391
Makkar, H.P.S., Siddhuraju, P., and Becker, K., 2007b. Plant secondary metabolites, (Humana Press, Totowa, New Jersey, USA.)
Mohamed, Z.Z., Sobhy, M.A.S., and Nader, D.S., 2018. Review article on Leucaena leucocephala as one of the miracle timber trees, International Journal of Pharmacy and Pharmaceutical Sciences, 10, 1-7
Moore, K.J., and Jung, H.-J.G., 2001. Lignin and fiber digestion, Rangeland Ecology & Management/Journal of Range Management Archives, 54, 420-430
Nguyen, B.C.Q., and Tawata, S., 2016. The chemistry and biological activities of mimosine: a review, Phytotherapy Research, 30, 1230-1242
Njidda, A.A., and Nasiru, A., 2010. In vitro gas production and dry matter digestibility of tannin-containing forges of semi-arid region of North-Eastern Nigeria, Pakistan Journal of Nutrition, 9, 60-66
Rodgers, K.J., Samardzic, K., and Main, B.J., 2017. Toxic nonprotein amino acids. In: C.R. Carlini and R. Ligabue-Braun (eds), Plant Toxins, 2017, (Springer Netherlands, Dordrecht), 263-285
Rosenthal, G.A., 1991. Chapter 1 - nonprotein amino acids as protective allelochemicals. In: G.A. Rosenthal and M.R. Berenbaum (eds), Herbivores: their Interactions with secondary plant metabolites (Second edition), 1991, (Academic Press, San Diego), 1-34
Schofield, P., 2000. Gas production methods. In: J.P.F. D’Mello (ed), Farm animal metabolism and nutrition, 2000, (CABI Publishing, Wallingford, Oxon OX10 8DE, UK), 209–232
Shelton, H.M., Kerven, G., and Dalzell, S.A., 2019. An update on leucaena toxicity: is inoculation with Synergistes jonesii necessary?, Tropical Grasslands-Forrajes Tropicales, 7, 146-153
Siddhuraju, P., Vijayakumari, K., and Janardhanan, K., 1996. Chemical composition and protein quality of the little-known legume, velvet bean (Mucuna pruriens (L.) DC.), Journal of Agricultural and Food Chemistry, 44, 2636-2641
Soltan, Y.A., Morsy, A.S., Lucas, R.C., and Abdalla, A.L., 2017. Potential of mimosine of Leucaena leucocephala for modulating ruminal nutrient degradability and methanogenesis, Animal Feed Science and Technology, 223, 30-41
Soltan, Y.A., Morsy, A.S., Sallam, S.M.A., Lucas, R.C., Louvandini, H., Kreuzer, M., and Abdalla, A.L., 2013. Contribution of condensed tannins and mimosine to the methane mitigation caused by feeding Leucaena leucocephala, Archives of Animal Nutrition, 67, 169-184
SPSS., 2012. Software for windows, release 21.0, Inc., 2012, (Property of IBM Corporation. , Chicago, Illinois. USA),
Supapong, C., Cherdthong, A., Seankamsorn, A., Khonkhaeng, B., Wanapat, M., Gunun, N., Gunun, P., Chanjula, P., and Polyorach, S., 2017. Effect of Delonix regia seed meal supplementation in Thai native beef cattle on feed intake, rumen fermentation characteristics and methane production, Animal Feed Science and Technology, 232, 40-48
Tan, H.Y., Sieo, C.C., Abdullah, N., Liang, J.B., Huang, X.D., and Ho, Y.W., 2011. Effects of condensed tannins from Leucaena on methane production, rumen fermentation and populations of methanogens and protozoa in vitro, Animal Feed Science and Technology, 169, 185-193
Trotta, R.J., Klotz, J.L., and Harmon, D.L., 2018. Effects of source and level of dietary energy supplementation on in vitro digestibility and methane production from tall fescue-based diets, Animal Feed Science and Technology, 242, 41-47
Udoh, P., and Ekpenyong, J., 2001. Effect of Mucuna urens (horse eye bean) on the gonads of male guinea-pigs, Phytotherapy Research, 15, 99-102
Unnawong, N., Cherdthong, A., and So, S., 2021a. Crude saponin extract from Sesbania grandiflora (L.) Pers pod meal could modulate ruminal fermentation, and protein utilization, as well as mitigate methane production, Tropical Animal Health and Production, 53, 196
Unnawong, N., Cherdthong, A., and So, S., 2021b. Influence of supplementing Sesbania grandiflora pod meal at two dietary crude protein levels on feed intake, fermentation characteristics, and methane mitigation in Thai purebred beef cattle, Veterinary Sciences, 8, 35
Vaithiyanathan, S., Sheikh, Q., and Kumar, R., 2005. Effect of transinoculation of goat rumen liquor on degradation and metabolism of mimosine in sheep fed with Leucaena leucocephala leaves, Asian-Australasian Journal of Animal Sciences, 18, 332-339
Van Soest, P.J., Robertson, J.B., and Lewis, B.A., 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition, Journal of Dairy Science, 74, 3583-3597
Vlaeminck, B., Braeckman, T., and Fievez, V., 2014. Rumen metabolism of 22:6n-3 in vitro is dependent on its concentration and inoculum size, but less dependent on substrate carbohydrate composition, Lipids, 49, 517-525
Weimer, P.J., 2015. Redundancy, resilience, and host specificity of the ruminal microbiota: implications for engineering improved ruminal fermentations, Frontiers in Microbiology, 6,
Weimer, P.J., Stevenson, D.M., Mertens, D.R., and Hall, M.B., 2011. Fiber digestion, VFA production, and microbial population changes during in vitro ruminal fermentations of mixed rations by monensin-adapted and unadapted microbes, Animal Feed Science and Technology, 169, 68-78
Acknowledgements
This research was supported by the special research fund for developing countries (BOF scholarship: BOF.DCV.2014.0009.01) of Ghent University, Belgium, and Universidad Central “Marta Abreu” de Las Villas (UCLV), Cuba (Project No. 10042), and National Program of Basic Sciences (Project No: P223LH001-025), and the VLIR-UOS partner program (ZEIN2015RIP29) for Institutional University Cooperation between UCLV, Cuba, and Flemish universities. Special acknowledgments to the staff of the Laboratory for Animal Nutrition and Animal Product Quality of Ghent University and the Laboratory for Animal Nutrition and the Clinical Laboratory at Facultad de Ciencias Agropecuarias (UCLV), Cuba, for the technical assistance during this research.
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, and supervision: V. F., R. L-O., and E. A-O.; formal analysis: E. A-O.; investigation: E. A-O., P.Y. F-R., R. L-O., and B. R-B.; writing – original draft: E. A-O.; writing – review and editing: V. F., R. L-O.; project administration and funding acquisition: V. F., R. L-O.
All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Animal welfare
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to. All experimental procedures with fistulated sheep were conducted and approved by the ethical commission of the Institute for Agricultural and Fisheries Research (ILVO) [approval number EC2014_222], in accordance with the European Directive (EU) No 241/2014.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Artiles-Ortega, E., de la Fé – Rodríguez, P.Y., Reguera-Barreto, B. et al. In vitro rumen degradability of tropical legumes and their secondary metabolites depends on inoculum source. Trop Anim Health Prod 54, 330 (2022). https://doi.org/10.1007/s11250-022-03327-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-022-03327-z