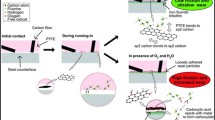
Abstract
Ultralow wear rates and low friction have been observed for carbon fiber reinforced PTFE (CF/PTFE) when sliding against steel or cast iron in dry gas environments. Although the strong environmental sensitivity of this tribosystem is well known, the origin of the outstanding tribological performance in dry gas remains unanswered. Some researchers attribute the low friction and wear to the formation of carbon-rich surfaces in the absence of oxygen and moisture in the environment. However, low friction between carbon surfaces is generally dependent on moisture. In this paper, extensive analyzes are conducted on the tribofilms formed on the CF/PTFE surface and the steel counterface after sliding in a high-purity nitrogen environment. TEM analysis of a cross-section of the tribofilm on the steel surface reveals that the sliding surface consists mainly of iron (II) fluoride and not carbon, even though a significant amount of carbon was observed near the surface. XPS and TEM analysis further revealed that the tribofilm formed on the worn composite surface consisted of nanoparticle agglomerates, anchored to the PTFE matrix and to each other by carbon with turbostratic structure. Turbostratic carbon also formed an ultrathin and surface-oriented superficial layer on top of the agglomerates. Governing mechanisms of the low friction and wear of the CF/PTFE—steel tribosystem were investigated by complementary tribotests with pure graphite samples and MD simulations of the identified surfaces. These indicated that the low friction between the carbon and iron fluoride in the tribofilms is due to poor adhesion between the distinctly different surfaces.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Carbon fibers are commonly used to improve the mechanical, thermal and wear properties of polymers [1]. Typically, carbon fiber reinforcement reduces the wear rate of polymers with several orders of magnitude and provides moderate coefficient of friction (0.25–0.4) when sliding in ambient air [1,2,3]. In fact, many carbon fiber reinforced polymers perform similarly at ambient conditions [1], which could be attributed to the predominant load support of the fibers due to the preferential wear of the polymer [4,5,6].
The properties and microstructure of carbon fibers vary significantly depending on precursor material, production process and heat treatment temperatures [7,8,9]. Generally, carbon fibers are non-graphitic, which means that the carbon atoms are ordered in two-dimensional hexagonal networks (graphene layers), but lack the three-dimensional crystalline long-range order that graphite has [10]. Nonetheless, parallel stacking of the graphene layers in the non-graphitic carbon is present but with rotational and translational misalignment [11]. The dimensions of the graphene stacks are in the nanoscale, where the height and layer extension typically increase and the interplanar distance decrease with higher heat treatment temperature [12, 13]. If the precursor material is graphitizable, the non-graphitic carbon will eventually convert to graphite when heating the material above a certain temperature (typically 2000–3000 K) [8]. The structural arrangement of the graphene layers of non-graphitic carbons is commonly called “turbostratic” as proposed by Biscoe and Warren [14]. It is worth noting that the two terms are often used interchangeably in literature to describe carbonized materials with disordered graphene stacks. Both terms will be used in this paper as well.
Carbon fiber reinforced polymers are especially suitable in tribological dry gas applications, such as dynamic seals in hydrogen gas compressors. Drastic improvements in the tribological performance of carbon fiber reinforced and carbon filled polymers have been observed by several authors when operating in dry gas environments compared to ambient air [15,16,17]. Sawae et al. reported coefficient of frictions down to 0.04 for CF/PTFE in dry hydrogen compared to about 0.3–0.4 in humid air, along with a reduction of the wear rate with about an order of magnitude. The improvement was suggested to be attributed to the self-lubricating properties of the carbon fibers in dry gas environments. The formation of carbon-based tribofilms on both the counterface and worn CF/PTFE surface has been confirmed with Raman after sliding in high-purity hydrogen environment [15]. Contrarily, no such carbon tribofilms were found after sliding in ambient air. It was hypothesized by the authors that oxygen and moisture might cause carbon wear particles to oxidize or agglomerate and thereby prevent them from staying in the sliding interface and form the tribofilms. Similar findings have been reported by Oyamada et al. [16], where a low coefficient of friction (< 0.1) was achieved with CF/PEEK after sliding in a nitrogen environment. They noted an increased carbon content on the CF/PEEK surface after sliding in nitrogen, and fine wear particles from the carbon fibers had been embedded on the surface of the polymer matrix. The correlation between a dry gas environment and low friction and wear is consistent with the reported findings from other studies with polymers reinforced with carbon fibers and other graphitic and non-graphitic carbon fillers [17,18,19,20].
The general theory is that carbon fibers promote low friction and wear of polymers in dry gas environments by decomposition of the fibers in the sliding interface and the formation of carbon-rich tribofilms on both of the sliding surfaces. However, it is not clear why these films should be tribologically beneficial in the first place. Literature generally claims that moisture is needed to promote low friction of graphite and non-graphitic carbons when sliding against itself or steel [21,22,23,24,25]. According to the adsorption theory, moisture or other active gases are needed to passivate dangling bonds on the edge sites of graphene sheets in order to prevent strong adhesion between two graphitic or non-graphitic surfaces [26,27,28].
Carbon-based tribofilms generated in dry gas environments by carbon fiber reinforced polymer composites have been analyzed by several authors. However, there is a clear knowledge gap regarding the nature of these type of tribofilms, as well as mechanisms behind the low friction and wear. Merely suggestions are given in literature due to insufficient analysis methods and difficulties related to the complexity of analyzing fluorine and carbon containing thin films. This paper aims to expand current knowledge through extensive characterization of the tribofilms formed on a CF/PTFE surface and a steel counterface in a high-purity nitrogen environment. Considering the analysis results of the tribofilms, the friction and wear of graphite in the trace moisture environment were also investigated to elucidate the mechanisms behind the low friction and wear of the CF/PTFE—steel tribosystem.
2 Method
2.1 Materials and Material Preparations
Carbon fiber reinforced PTFE (CF/PTFE) samples with 20/80 wt% concentration were prepared at Nanjing Tech University. PTFE was supplied from DuPont (7A-J) and the PAN-based carbon fibers from Nanjing Fiberglass Research and Design Institute. The length and diameter of the carbon fibers were in the range of 15 to 150 µm and 5 to 7 µm, respectively. The CF/PTFE samples had a diameter of 8 mm and a length of 10 mm. The samples were grinded in the sample holder against the counterface with an abrasive paper in between to assure conformity. The specific preparation details have been described previously [17]. In light of the results in this paper, see Sect. 3.2, complementary tests were also conducted with pure graphite samples. A 5 mm graphite rod with 99.997% purity was supplied by Sigma Aldrich. The graphite samples required a gentler approach due to the poor abrasive resistance and were lightly rubbed against the counterface with a 2000p abrasive paper in between to assure conformity.
For the counterface, hardened 34CrNiMo6 steel (460 HV30) was selected, which has shown good receptiveness for transfer film formation from carbon fiber reinforced PTFE composites and low friction [4]. The counterface was grinded with figure-eight motions against abrasive papers to achieve a random texture. The counterface used against the CF/PTFE sample was finished with 400p for a surface roughness of about Sa = 0.1 µm. The counterface used against the graphite sample was grinded to a mirror finish to minimize abrasion. A mirror finished counterface (Sa = 0.01 µm) has already been tested against a similar PTFE composite with no significant difference in friction and wear behavior compared to a roughness around Sa = 0.1 µm [4].
2.2 Experimental Method
Tribotests were conducted in an environmentally controlled 3-pin-on-disc tribometer described in detail in a previous publication [29]. Essentially, the test configuration consists of three CF/PTFE pins mounted in a self-aligning test pin holder with the flat ends in contact with the flat steel counterface disc, see schematic illustration and critical dimensions in Fig. 1. The test configuration is enclosed by a climate chamber. The chamber is connected to a controlled nitrogen supply and the gas is recirculated from the chamber through an oxygen sensor and a dew point sensor. A moisture trap is also connected after the sensors in the recirculation loop to remove excess moisture. For all tribotests, the climate chamber was purged and over-filled with nitrogen to a relative pressure of 0.4 bar. The environment was stabilized before tests were started, where the moisture content was kept at about 1–2 ppm by volume and the initial oxygen concentration for each test was 10 ± 5 ppm. The CF/PTFE samples were tested for 200 km of sliding at a velocity of 0.75 m/s, a nominal contact pressure of 2 MPa and a near-contact temperature of 80 °C. Before initiating sliding, load and heat were applied and maintained for 2 h to stabilize the near-contact temperature and to minimize the effect of creep. Two shorter repetitions were also conducted for 100 km of sliding to ensure good repeatability. The complementary tests with graphite samples were run at mild and severe sliding conditions. The mild condition had a sliding velocity of 0.2 m/s and a contact pressure of 0.5 MPa and the severe condition was the same as for the CF/PTFE. The sliding distance for the graphite tests was 700 m (~ 1 h at mild sliding) or until wear exceeded 200 µm. Wear is measured by an in-situ capacitance displacement sensor, Fig. 1a, which continuously measures the displacement of the test pin holder. The specific wear rate is calculated by linearization of the height loss over a sliding interval and then convert the resulting linear wear rate, \(w\) [µm/km], to specific wear rate, \({w}_{\mathrm{s}}\) [mm3/Nm]. The conversion from linear wear rate to specific wear rate is described by
where P is the nominal contact pressure and the conversion factor 106 is used to convert µm to mm and km to m. Due to the significant thermal expansion of the CF/PTFE material, the wear rate can only be accurately calculated during steady-state sliding where the contact temperature and coefficient of friction are stable. The coefficient of friction µ is calculated by dividing the frictional torque measurement with the applied normal load L and the average sliding radius r,
2.3 Tribofilm Characterization
The tribofilms formed on the steel surface and the CF/PTFE surface were extensively analyzed using different techniques described below. The tribofilm formed on a metal counterface after sliding against a polymer composite is commonly called transfer film due to the transfer of polymeric material from the composite to the metal surface. However, for the specific materials and environment studied in this paper, the authors have reason to believe that the term tribofilm is more appropriate to describe the film generated on the steel counterface. This will be further discussed in Sect. 3.2. To eliminate confusion, the terms polymer-tribofilm and steel-tribofilm will be used hereafter to distinguish between the tribofilm formed on the CF/PTFE surface and the steel counterface, respectively.
Light optical microscopy (LOM) and scanning white light interferometry (SWLI) were used to inspect the tribofilms at lower magnifications to analyze surface features and detect signs of wear. A Zygo NewView 9000 was utilized for the SWLI measurements, with filtration of the data done in MountainsMap 9. With the Zygo instrument, an intensity map is recorded with each topography measurement which provides an optical visualization of the measured area. Surfaces were also analyzed at higher magnification using a Scanning Electron Microscope (SEM), Jeol JSM-IT300.
To facilitate elemental and chemical analysis of the thin polymer-tribofilm on the CF/PTFE surface, X-ray photoelectron spectroscopy (XPS) was used (Physical Electronics Quantera II Scanning XPS Microprobe). The probed depth is typically 1–10 nm depending on the electron density of the material, due to electrons in the sample absorbing the generated photoelectrons. A common drawback of this technique is the large spot size of the analysis, typically larger than 100 µm. However, with the instrument used it is possible to generate analysis spots smaller than 10 µm. To improve the positioning of the analysis, a Scanning X-ray image (SXI) was used, generated by the total photoelectron intensity from each spot as the X-ray is scanned over the surface. Both electrons and Ar+ ions were used to neutralize the sample, enabling an insulating sample to be analyzed. Survey spectra of the polymer-tribofilm were recorded before and after a short sputtering. The X-ray was generated with 200u50W15kV (X-ray beam size: 200 µm, power: 50 W and e-beam energy: 15 kV) and the acquisition was performed with 224 pass energy, 50 ms per step and 0.8 eV step size. After the sputtering, maps were acquired to investigate the variation of carbon and fluorine over the surface. For the mapping to be useful, a smaller spot size of about 10 µm was used [x-ray generation 10u1.25W15kV (x-ray beam size: 10 µm, power: 1.25 W and e-beam energy: 15 kV)], increasing the lateral resolution. The acquisition settings were 112 pass energy and 100 ms per step. Spectra with high energy resolution were acquired from the tribofilm at locations with different total photoelectron density. The X-ray generation was the same as for the mapping, but the acquisition settings were 26 pass energy, 100 ms per step and 0.05 eV step size. Depth profiles were acquired from the same spots, with a low sputter effect. The bond between carbon and fluorine is sensitive to sputter damage with preferential sputtering of fluorine being reported [30,31,32].
To analyze the microstructure and the elemental composition of the cross-section of tribofilms, Transmission Electron Microscopy (TEM)/Scanning Transmission Electron Microscopy (STEM) in combination with Focused Ion Beam (FIB) sample preparation was utilized. Thin lamellas were extracted from representable areas of the polymer-tribofilm and the steel-tribofilm with a Zeiss Crossbeam 550 FIB. Before inserting the CF/PTFE sample in the FIB instrument, the worn surface was covered with an amorphous carbon coating and a gold/palladium sputter coating. The latter to reduce charging during area selection and the former to protect the surface from subsequent platinum deposition. Before extracting a lamella, a thick platinum coating was deposited on the extraction region to protect and support the lamella during further preparation steps. The steel-tribofilm was protected similarly. However, since the steel-tribofilm was more conductive, the gold/palladium coating was not needed. After extracting the lamellas, they were soldered to a grid and carefully milled with FIB.
High-resolution TEM/STEM images were taken with a Thermo Fischer Scientific Titan Themis 200 with an acceleration voltage of 200 kV. In the STEM mode, four images were recorded for each scan using bright-field (BF) imaging, annular bright-field (ABF), annular dark-field (ADF) and high-angle annular dark-field (HAADF). For BF and ABF imaging, crystalline and high-mass elements appear dark, while for ADF and HAADF, they appear bright. The elemental composition of the steel-tribofilm was analyzed with the equipped Super-X Energy Dispersive X-ray Spectroscope (EDS) system.
3 Results and Discussion
3.1 Tribological Test and Surface Analysis
The coefficient of friction and height loss of the primary test with the CF20/PTFE composite during 200 km of sliding are shown in Fig. 2a. The specific wear rate, calculated from 20 km of sliding to 200 km, was \(1.7\times {10}^{-8}\) mm3/Nm and the coefficient of friction was 0.05 after running-in and slowly increased during the test to 0.07. The coefficient of friction and height loss for the two repetitions during 100 km of sliding are shown in Fig. 2b. Although the tribological behavior during running-in slightly varies, the friction and wear during steady-state sliding are very similar for all three tests as shown in Table 1. Note that the positive displacement during the initial sliding for all tests and at around 20 km of sliding for repetition 2 is due to thermal expansion as a result of frictional heating.
Coefficient of friction and height loss of the CF/PTFE composite during sliding against the steel counterface. The primary test (200 km of sliding) is depicted in a and the two shorter repetitions (100 km of sliding) are depicted in b. The green shaded regions (50 to 100 km) indicate the data used to calculate the average coefficient of friction and specific wear rate presented in Table 1 (Color figure online)
Representative LOM and SEM images of the worn surface of CF/PTFE after sliding against the steel counterface in dry nitrogen are shown in Fig. 3a and b, respectively. The optical micrograph, Fig. 3a depicts three distinct features of the surface: carbon fibers in bright gray, tribofilm in medium gray and damaged areas in dark gray. Similar features can be observed in the scanning electron micrograph but in the reverse grayscale order. Note that the damaged regions are not visible in the SEM image, which could indicate that these areas are also covered with similar material as the tribofilm. The tribofilm formed on the steel surface can be seen in Fig. 13.
3.2 Composition and Microstructure of Tribofilms
A survey spectrum with XPS gives an overview of the composition of the polymer-tribofilm, with maximum energy range but low energy resolution. The results before and after 10 min of sputtering (Ar+, 1kV1 × 1 mm) are shown in Fig. 4. The results indicate that the polymer-tribofilm consists of mainly carbon and fluorine, with oxygen only included before sputtering. A small amount of iron seems to be present in the tribofilm as well. The information depth is about 5 nm.
The generated photoelectrons in XPS can be used to acquire an image showing the total intensity in each analysis spot (Scanning X-ray Image, SXI). In a similar manner, elemental analysis can be performed in each analysis spot. To increase the speed of this mapping process, a position-sensitive detector is used to measure different energies centered over the investigated binding energies, instead of stepping the energy. The investigated energies corresponded to fluorine 1s, oxygen 1s and carbon 1s (686, 531 and 285 eV respectively). The investigated area was in the center of the already sputtered area and no oxygen peak appeared. The total area mapped was 92 × 82 analysis spots with 5 µm spacing (460 × 410 µm). The min–max range of fluorine and carbon indicates that high total photoelectron intensity is coupled with a higher concentration of fluorine in the sample, see Fig. 5a–c. The areas with high fluorine concentration can be explained by grooves on the CF/PTFE surface as indicated by the topography and corresponding intensity map of a similar region of the surface, Fig. 5d–e.
Depth profiles were acquired from a similar area of the same sample. The sputter settings were 18 times 10 min using 1kV1 × 1 mm. Two spots were chosen for the depth profiling, from regions with slightly different total energy densities in the SXI. Before starting the depth profiling the surface was cleaned with 1 min of sputtering. Energy ranges over fluorine (692–682 eV), oxygen (536–526 eV) and carbon (293–282 eV) were acquired using high energy resolution settings. Oxygen was only found in the first measurement before sputtering and was excluded from the composition calculations. To compensate for the different tendencies to generate photoelectrons, the results were recalculated using atomic sensitivity factors (fluorine 1 and carbon 0.196 [33]). The depth profiles are shown in Fig. 6.
Due to the C–F bond being quite sensitive to sputtering (preferential sputtering of fluorine) [30,31,32] a low sputter energy was used. At the beginning of the depth profile, the two measured spots of the polymer-tribofilm are very similar with a high carbon content. With sputtering, the carbon content starts to decrease until reaching a turning point (20 min for spot 1 and 40 min for spot 2). After the turning point, there is a slow decrease in fluorine content, which could be accredited to the preferential sputtering of fluorine. Thus, the data after the turning points have limited value. It appears there is a very thin tribofilm covering the surface, which is sputtered through quickly. The tribofilm mainly contains carbon but also a significant amount of fluorine. The time to sputter through the film, and thus the thickness, depends on what is beneath it. Spot 1 is covered by a thinner film and the material beneath has almost a 2:1 relation between carbon and fluorine. Spot 2 is covered by a roughly twice as thick tribofilm and the material beneath has almost a 1:2 relation between carbon and fluorine. The relation between carbon and fluorine at the turning points indicates that at spot 2, the PTFE matrix was underneath the tribofilm and at spot 1, both carbon fiber and PTFE were underneath. However, the absolute atomic concentration values depend on which atomic sensitivity factors are used and literature are not consistent on what factors to use [33,34,35].
Investigating the binding energies closer further indicates that the CF/PTFE surface is covered by a very consistent film. The binding energy peaks of both fluorine 1s and carbon 1s appear at the same energies both before the sputtering and at the respective turning points of the depth profiles, see Fig. 7. The peak positions indicate both the presence of C–C bonds (284.5 eV) and C–F bonds (between 288 and 293 eV depending on the type of bond). The characteristic peaks for the C–F bonds in PTFE are around 292.5 eV for the C1s and about 689.5 eV for the F1s [33, 36], which are both at higher binding energies than the observed C–F related peaks in Fig. 7. The binding energy of ~ 687 eV, as observed in Fig. 7, is commonly reported for the F1s in C–F bonds in fluorinated carbon materials [37,38,39,40]. Generally, lower binding energy in the C1s in the C–F bonds was also reported in the cited papers compared to the C–F bonds in PTFE. A similar trend has been observed in an XPS study of different organofluorines, where the binding energies for C–F bonds increased with a higher F/C ratio of the compound [41]. Hence, the negative energy shift observed in Fig. 7 for the C–F bonds strongly suggests tribochemical bonding between carbon wear particles and heavily defluorinated PTFE. No positive energy shift could be observed throughout the depth profiles, which would indicate undamaged PTFE. However, this is expected due to the preferential sputtering of fluorine.
Scanning transmission electron microscopy (STEM) image of the cross-section of the steel-tribofilm, Fig. 8, shows that the surface of the film is super smooth. The maximum peak-to-valley height (Rmax) is about 10 nm, indicating that the average roughness (Ra) of the steel-tribofilm for the analyzed cross-section may be in the range of a few nanometers or less. This indicates a remarkable reduction in local contact stresses, as a result of the increased real contact area. Surprisingly, the steel-tribofilm, and more importantly, the surface of the film, mainly consists of a polycrystalline material. The spacing of the lattice fringes in Fig. 8d, 0.34 nm and 0.26–0.27 nm, corresponds well with the d-spacing of the (101) and (110) planes of iron (II) fluoride (FeF2) [42, 43]. This is further supported by the overlapping iron and fluorine elements in the transfer film in the EDS map, Fig. 8f. Moreover, the atomic ratio of F/Fe in the steel-tribofilm was roughly 2. The lattice fringe spacing of 0.26–0.27 nm could also correspond to the d-spacing of the (104) plane of iron (III) fluoride (FeF3) [43, 44]. However, due to the lack of lattice fringes corresponding to other planes of the FeF3, such as the d(012) = 0.37 nm, it can be disregarded as a main constituent of the tribofilm. Supplementary SEM–EDS analysis (see Online Resource 1, Fig. S1) also suggests that a small amount of chromium fluoride may be present in the steel-tribofilm.
The finding that the sliding surface of the steel-tribofilm is mainly iron fluoride goes against previous studies [15, 45], where the sliding surface of the steel-tribofilm in a similar environment was believed to consist mainly of carbon. A significant amount of carbon is indeed present in the steel-tribofilm, but mainly below the surface as indicated by the regions with turbostratic structure in Fig. 8b, c and the subsurface carbon regions in the EDS map Fig. 8e. The rather random distribution of carbon in the steel-tribofilm implies that worn carbon fiber particles in the sliding interface have simply filled voids in the tribofilm. Due to the continuous generation of iron fluoride in the sliding interface, the carbon inclusions become covered. Note that the thick layer of carbon at the top of the EDS map is the protective carbon layer applied during FIB sample preparation. The protective layer and the carbon inclusions could be easily distinguished from one another due to the difference in structure.
Other notable observations from the TEM analysis are the subsurface cracks in the steel-tribofilm found in several locations, one shown in Fig. 8b, along with grain deformation of the steel close to the sliding contact. Moreover, the average film thickness was about 150 nm for the extracted region, which is close to what has been estimated with Scanning White-Light Interferometry (SWLI) previously [4]. Nano-indentations at low contact depths indicate that tribofilm is relatively hard, with a measured hardness of roughly 3–5 GPa (see Online Resource 1, Fig. S2).
The analysis of the “transfer film” formed on the steel surface, here called steel-tribofilm, strongly indicates that the film is generated predominantly by tribochemical reactions between PTFE and the steel surface [46]. Furthermore, no signal relating to the original polymer has been found on the surface from FTIR measurement (see Online Resource 1, Fig. S3). Thus, the use of the term “transfer film” to describe this film is quite incorrect from its definition [47]. This type of tribochemically generated film would be better described as a tribofilm, or more specifically an inorganic tribofilm.
Two lamellas were extracted from the worn CF/PTFE surface to analyze, (1) a cross-section of the tribofilm formed on the PTFE matrix, Fig. 9a–d, and (2) a cross-section of carbon fibers on the surface, Fig. 9e–g. Figure 9a shows one of the polished windows of the lamella extracted from the polymer-tribofilm, where the rectangles depict regions that are shown in Fig. 9b–d at higher magnification. It was obvious during the preparation of the lamellas that the PTFE matrix was preferentially milled away by the ion beam. However, a relatively large region of the PTFE matrix, with a height of at least 200 nm, remained below the polymer-tribofilm after the FIB-milling. The tribofilm formed on the PTFE matrix is roughly 50 to 100 nm thick and consists of nanoparticle (NP) agglomerates, Fig. 9b, and turbostratic carbon Fig. 9d. The latter can be determined by comparison of the structure and d-spacing of the carbon fiber shown in Fig. 9e. Even though, slightly higher d-spacings were measured in the carbon on the tribofilm. The turbostratic carbon was found both around the agglomerates and inside them. On top of the agglomerates lies a thin carbon film, about 5 nm thick, that is preferentially oriented parallel with the sliding surface. The preferential orientation of the superficial carbon layer with the sliding surface is commonly associated with low friction in graphite studies, due to the low surface energy of the basal planes in comparison with the edge planes [25, 27, 48, 49]. A closer look at the crystalline NPs, Fig. 9d.1 and d.4, indicates that agglomerates contain iron fluoride NPs, as worn from the steel-tribofilm, and turbostratic carbon. The presence of iron fluoride may also be indicated by the peak around 712 eV in the XPS survey spectrum, Fig. 4 [50]. Although carbon-rich polymer-tribofilms have been reported before in dry gas [15, 16, 45], the presence of a surface-oriented and ultrathin superficial carbon layer has not. Neither has the presence of NP agglomerates been reported, which seems to be important for the formation of the superficial carbon layer. Moreover, the NP agglomerates likely provide structural reinforcement of the polymer-tribofilm, where the carbon seems to bind the NPs together and anchor the agglomerates to the PTFE matrix.
A similar tribofilm as on the PTFE matrix can be observed on the carbon fiber, Fig. 9f, g, where iron fluoride NPs are embedded on the surface and covered by a thin layer of surface-oriented carbon. The fast Fourier transform (FFT) filtered regions in Fig. 9f shows the diagonal orientation of the bulk CF and how the carbon seemingly grows vertically between the NPs and then curves around them to finally orient with the surface. Both the tribofilm between the carbon fibers and the fibers in contact had very smooth surfaces, however not as smooth as the steel-tribofilm.
The polymer-tribofilm is strongly adhered to the PTFE matrix. The low surface energy and the chemical inertness of PTFE make it unrealistic to attribute the strong adhesion to van der Waals forces solely. Instead, chemical reactions need to be considered. As strongly indicated by the vast amount of iron fluoride in the steel-tribofilm, the defluorination of the PTFE molecules must take place to a large extent. Density function theory (DFT) simulations [51] have also shown that defluorination is the most probable degradation mechanism of the PTFE molecule. The high degree of defluorination of PTFE molecules in the sliding interface results in a vast amount of carbon radicals in the defluorinated molecules. These could covalently bond with the turbostratic carbon, illustrated in Fig. 10, which possibly explains the strong adhesion between tribofilm and the PTFE matrix. Chain scission of the PTFE molecule, which is also a likely degradation mechanism of the PTFE [51], could also provide similar anchoring of the polymer-tribofilm. The creation of carbon radicals through degradation of the PTFE may also explain the significant amount of PTFE throughout the polymer-tribofilm as shown by the XPS analysis. Hypothetically, wear particles of PTFE and carbon fiber are mixed and churned in the sliding contact, bonding to each other through covalent bonds and van der Waals forces. In the process, nano-sized wear particles from the transfer film (iron fluoride NPs) get mixed in as well. The nanoscale agglomerates then anchor to the PTFE surface, as described previously.
3.3 Tribological Comparison with Graphite
In an attempt to elucidate the governing mechanism behind the friction and wear of the tribo-generated surfaces, complementary tribotests were conducted with pure graphite pins. The graphite pins were used to simulate the surface-oriented carbon film found on the very top of the polymer-tribofilm, as graphite and non-graphitic carbon have shown similar friction properties [21, 26]. These pins were tested against the iron fluoride steel-tribofilm and a bare steel surface, in the same environment as for the CF/PTFE pins.
The results from the tribotests with the pure graphite tribotests are shown in Fig. 11. The test against the steel counterface showed high friction (0.45) and the wear was extremely severe at the mild sliding conditions. The wear limit was achieved after only 10 min of sliding. The friction and wear against the steel surface correspond well with the literature for graphite sliding in a dry nitrogen environment [21, 25, 52, 53]. Contrarily, the coefficient of friction when sliding against the pre-generated steel-tribofilm was low (~ 0.08) at mild sliding, Fig. 11a and very close to the final coefficient of friction of the CF/PTFE, Fig. 2. Moreover, no wear could be measured during the hour-long test, Fig. 11b. A test was also conducted at severe sliding conditions, same as for the tests with CF/PTFE. Initially, the coefficient of friction, Fig. 11a, was similar to the mild test. However, after about 130 m of sliding the friction started to increase and a coefficient of friction close to that against the steel surface was shortly achieved. Simultaneously, a transition to severe wear occurred.
Coefficient of friction (a) and height loss (b) for graphite samples sliding against pre-generated steel-tribofilm and steel counterface. The sliding velocity and contact pressure were 0.2 m/s and 0.5 MPa, respectively, for “Mild” conditions and 0.75 m/s and 2 MPa for “Severe” conditions (Color figure online)
The graphite surface before and after mild sliding against the pre-generated steel-tribofilm is shown in Fig. 12. As depicted in Fig. 12a, b, the initial surface is very dull and graphite flakes are randomly oriented. After sliding, Fig. 12c, d, the surface became smooth and shiny, similar to the polymer-tribofilm, and it appears as if the graphite flakes have become oriented with the surface. This correlates well with literature, where a smooth graphite surface with a bright appearance has been linked to the preferential orientation of crystallites parallel to the surface after sliding at a low friction state [54, 55]. Contrarily, the graphite surface became extremely rough after sliding against steel. Moreover, Raman measurements (see Online Resource 1, Fig. S4) of the graphite surface after sliding against the iron fluoride tribofilm showed higher relative intensity of the D band compared to before sliding. This indicates that the near-surface became more turbostratic and less graphitic [7], i.e. more similar to the carbon in the polymer-tribofilm.
The typical appearance of the steel-tribofilm formed on steel counterface after sliding against the CF/PTFE can be seen in Fig. 13a. The steel-tribofilm is very thin and evenly covers the steel surface. Sliding of graphite against the steel-tribofilm at mild conditions did not affect the steel-tribofilm significantly. Narrow streaks of thicker graphite transfer are sparsely distributed around the center of the wear track, Fig. 13b. Apart from the streaks of graphite transfer and signs of slight wear, the pre-generated steel-tribofilm is mainly intact. Optical micrographs of the wear track after sliding at the more severe conditions, Fig. 13c, d, indicate that the pre-generated steel-tribofilm had been worn off. Uncovered steel can be seen in several regions where the steel-tribofilm previously covered the counterface. Moreover, the thick graphite transfer seems to have adhered directly to the steel surface, i.e. the pre-generated steel-tribofilm was likely removed prior to the formation of the thick graphite transfer film. A similar thick graphite film can be seen in Fig. 13e, f after graphite sliding directly against the steel surface.
As clearly indicated by the lack of transferred material in Fig. 13b, the adhesion between graphite and the iron fluoride tribofilm is very poor in comparison with graphite against steel, Fig. 13e. Moreover, the friction between the graphite samples and the transferred graphite is evidently high in the tested trace moisture environment. Due to the similar structure of graphite and non-graphitic (turbostratic) carbon [12, 13, 56, 57] and their similar tribological behavior [21, 26, 58], it is highly plausible that the poor adhesion between iron fluoride and carbon is key to the low friction in the analyzed CF/PTFE—steel tribosystem.
3.4 Molecular Dynamics Simulation
To fortify the low adhesion theory and to gain further insights of the interaction between iron (II) fluoride and sp2 carbon, molecular dynamics (MD) simulations were performed. The molecular model and principal simulation results are briefly reported in this section. For more details, the readers are refered to the Online Resource 1, Sect. 5. In the first stage of preparing the molecular model for MD simulation, an iron (II) fluoride model was built by expanding the FeF2 unit cell [59, 60] into a 10 × 10 × 3 supercell, Fig. 14a, using VESTA 3.5.0 [61]. To simulate the carbon surface, a carbon fiber structure was modeled, Fig. 14b, following the method published by Vuković et al. [62]. Note that hydrogen termination technique is employed on the edge carbon atoms (revealed by the red dots in Fig. 14b) to emulate the presence of small quantities of hydrogen, nitrogen, and oxygen in PAN carbon fibers that may persist following the manufacturing processes [63]. Moreover, it helps maintain charge neutrality in the simulation system. This method is commonly used for graphene in nanoscale simulations and is deemed a reasonable approximate [62]. The initial simulation setup was configured according to Fig. 14c, where a normal stress σ was applied in the Z direction on the outer layers of the molecular models and a relative motion was applied in the X direction with a velocity V to simulate the shear deformation.
Simulations were carried out using the LAMMPS package [64] at three distinct steps. Firstly, the temperature was increased from 25 to 80 °C during 0.1 ns followed by an equilibration step for 0.1 ns. Lastly, normal stress and a relative sliding motion was applied and simulated for 1 ns. Four simulations were performed at different normal stresses, σ = 2, 20, 100 and 200 MPa, and with a constant shear velocity of V = 0.75 m/s. A first glance of the recorded simulation at a normal stress of 2 MPa, see Online Resource 2, shows that sliding takes place between the iron fluoride and the carbon surfaces. Moreover, the carbon fiber remains its initial structure to a high degree and no material transfer can be observed, indicating low wear. The resulting shear stress as a function of normal stress are shown in Fig. 15, where the shear stress is derived from the average shear force from the last 0.2 ns of each simulation. The results reveal a linear increase in the average shear stress as the normal stress escalates, albeit with a distinct offset. To determine the friction coefficient of the system, a linear equation proposed by Amontons-Coulomb and expanded by Derjaguin [65] is employed. The linear expression, τxz = μσzz + τ0, incorporates the Derjaguin intercept (τ0), which depends on the adhesion at the sliding surfaces. By a linear fit of the shear stress data, the friction coefficient, μ, can be accurately derived. In this regard, the coefficient of friction between carbon fiber and iron fluoride is determined to be 0.060, which is very close to the experimental values. The Derjaguin intercept of roughly 38 MPa in Fig. 15 can be compared to values between 25 and 120 MPa for PTFE–PTFE (as approximated from results published in [66]) and about 500 MPa for phosphate esters confined between iron surfaces [67]. Moreover, the interaction energy between the carbon fiber and the iron fluoride was compared with the interaction energy between an identical carbon fiber model and a conventional surface, namely magnetite. Results showed that the interaction energy was more than twice as high for the magnetite compared to the iron fluoride. These observations indicate that the adhesion between the iron fluoride and carbon surfaces are low.
Variation in mean shear stress with normal stress for carbon fiber on iron fluoride. The dashed line is the linear fit of the shear data, which equation corresponds to the Amontons-Coulomb friction expression modified by Derjaguin [65]
Overall, the simulation results are in close agreement with the experimental results and fortifies the proposed theory that the low friction in the CF/PTFE—steel system is due to low adhesion between the iron (II) fluoride and turbostratic carbon, which are generated on the steel substrate and the CF/PTFE surface, respectively.
3.5 Mechanistic Discussion
Metal fluorides are known to participate in the formation of transfer films in PTFE—metal and certain PTFE composite—metal tribosystems [46, 68,69,70,71], including the specific material and similar environment as studied in this paper [15, 45]. However, metal fluorides are typically reported to firmly adhere a transfer film to its counterface, not to take part in the actual sliding contact [46, 71, 72]. In recent publications by Sawae et al. [15] and Kojima et al. [45], the low friction and wear of a similar CF/PTFE—metal tribosystem operating in dry hydrogen was attributed to the sliding between a carbon tribofilm and a carbon-rich transfer film, as indicated by XPS and Raman analysis. However, low friction has, to the best of the authors' knowledge, not been reported for carbon–carbon couples in dry nitrogen or hydrogen environments in a flat-on-flat sliding configuration. Generally, moisture is needed in inert gas environments to promote low friction of graphite or other carbons with sp2 configuration, through passivation of dangling bonds [23, 26, 27, 73]. One paper has been found reporting low friction in argon and helium for sliding graphite couple, however, information about the residual moisture content is inadequate [74]. Moreover, the same research group did not observe low friction in dry hydrogen [54]. Opposed to previous beliefs [15], our work shows that the carbon in the steel-tribofilm does not seem to provide any lubricious properties since it is mainly found below the surface. From the results and discussion in this paper, the authors strongly suggest that the sliding takes place between two distinctly different surfaces, namely the iron (II) fluoride on the steel counterface and the surface-oriented superficial carbon layer on top of the polymer-tribofilm and the carbon fibers. From this insight, the authors propose that the principal mechanism of the low friction of the CF/PTFE—steel tribosystem in dry gas is simply low adhesion between iron (II) fluoride and sp2 carbons. This proposition is supported by observation from the graphite tests as well as the MD simulations of carbon fiber sliding against iron (II) fluoride. In the graphite test, no visible graphite transfer on most of the pre-generated iron fluoride tribofilm was detected during low friction sliding. Moreover, the transition from low to high friction after a period of sliding at severe conditions could be attributed to failure of the iron fluoride tribofilm and the subsequent heavy transfer of graphite to the steel surface. The worn graphite surface after sliding in low friction share several similarities as the sliding surface of the polymer-tribofilm, including turbostratic structures and oriented superficial layers. Hence, if the sliding was to take place mainly between two carbon surfaces in the CF/PTFE—steel tribosystem as suggested by others, the graphite should reasonably be able to maintain the low friction and wear state against the pre-generated tribofilm by supplying carbon to the sliding interface. From the MD simulations, it was clear that the interaction between the carbon fiber surface and the iron fluoride surface was weak and the coefficient of friction of the system was very close to experimental values.
Since the friction behavior can be related to the carbon in the polymer-tribofilm, the fluorine in the polymer-tribofilm must have other functions than providing low friction. As depicted in Fig. 13, the iron fluoride tribofilm shows signs of wear even at the mild sliding conditions. This indicate that without the supply of more fluorine, the iron fluoride film cannot be sustained for longer sliding durations. Hence, the presence of fluorine in the polymer-tribofilm may be essential in maintaining the low friction and wear state by continuous replenishment of the iron fluoride film. As previously discussed, the nanoparticle (NP) agglomerates likely provide mechanical reinforcement to the polymer-tribofilm, where the ultrathin surface-oriented carbon layer prevents interaction with the iron fluoride in the steel-tribofilm. Hypothetically, the iron fluoride in the NP agglomerates may promote the formation of a robust and ultrathin carbon layer due to preferential encapsulation of iron fluoride NPs by carbon, as indicated in [75]. However, further studies are needed to determine the exact function of the iron fluoride NPs found in the polymer-tribofilm. A summary of the characterized tribofilms formed on the CF/PTFE and steel surfaces after sliding in dry nitrogen and proposed mechanisms are schematically illustrated in Fig. 16.
The insights provided in this paper of the ultralow wear and low friction of CF/PTFE sliding against steel in high-purity nitrogen provides a stronger foundation for researchers in the field to develop better materials. Finetuning the composition of the counterface material or adding certain metallic nanoparticles to the CF/PTFE composite that are tribochemically compatible with PTFE could likely promote the formation of even more wear resistant tribofilms.
4 Conclusion
-
The two sliding surfaces of a CF/PTFE—steel tribosystem in dry nitrogen are shown to be vastly different. One is essentially a tribochemically generated tribofilm of iron (II) fluoride on the steel counterface. The other is an ultrathin (~ 5 nm) surface-oriented carbon layer with turbostratic structure on the top of the tribofilm on the CF/PTFE surface. This finding contradicts suggestions reported in other CF/PTFE studies in similar environments, where the sliding has been believed to majorly take place between two carbon-rich surfaces.
-
The carbon-based tribofilm on the CF/PTFE surface is about 50–100 nm thick and has three main features, ordered from the surface to the PTFE matrix; (1) the turbostratic carbon layer preferentially oriented with the sliding surface, (2) nano-particle agglomerates of iron fluoride, turbostratic carbon and degraded PTFE, which likely provides mechanical reinforcement of the tribofilm, and (3) turbostratic carbon binding the agglomerates together and anchoring the tribofilm to the PTFE matrix. The surface oriented carbon layer (1) was also observed on top of the carbon fibers.
-
The iron fluoride-based tribofilm on the steel surface is about 150 nm thick and has a super smooth surface which minimizes abrasion of the CF/PTFE surface.
-
Tribotests with graphite samples sliding against the iron fluoride tribofilm, indicate that the low friction of the CF/PTFE—steel tribosystem in dry nitrogen may be attributed to the poor adhesion between sp2 carbon materials and iron fluoride. Unmeasurable wear and similar coefficient of friction as for the CF/PTFE was achieved with graphite until the iron fluoride tribofilm wore off.
-
MD simulations of carbon fiber sliding against iron (II) fluoride showed weak interaction between the surfaces and similar coefficient of friction as the CF/PTFE—steel system. This further supports the proposed mechanism behind the low friction of the CF/PTFE—steel system.
-
The presence of degraded PTFE throughout the cross-section of the carbon-based tribofilm indicates a continuous supply of fluorine to the interface. Thus, the iron fluoride tribofilm can be continuously replenished and the low friction and ultralow wear state maintained.
Data Availability
The data that support the findings of this study are available from the corresponding author, Pontus Johansson, upon reasonable request.
References
Giltrow, J.P., Lancaster, J.K.: Carbon-fibre reinforced polymers as self-lubricating materials. Proc. Inst. Mech. Eng. 182, 147–157 (1967). https://doi.org/10.1243/PIME_CONF_1967_182_417_02
Dong, F., Hou, G., Cao, F., Yan, F., Liu, L., Wang, J.: The lubricity and reinforcement of carbon fibers in polyimide at high temperatures. Tribol. Int. 101, 291–300 (2016). https://doi.org/10.1016/j.triboint.2016.04.035
Flöck, J., Friedrich, K., Yuan, Q.: On the friction and wear behaviour of PAN- and pitch-carbon fiber reinforced PEEK composites. Wear 225–229, 304–311 (1999). https://doi.org/10.1016/S0043-1648(99)00022-8
Johansson, P., Marklund, P., Björling, M., Shi, Y.: Effect of roughness on the running-in behavior and tribofilm formation of carbon fiber reinforced PTFE composite in trace moisture environment. Wear 500–501, 204367 (2022). https://doi.org/10.1016/j.wear.2022.204367
Wang, Y.: Tribological properties of ultrahigh-molecular-weight polyethylene ( UHMWPE ) composites reinforced with different contents of glass and carbon fibers. Ind. Lubr. Tribol. 1, 22–30 (2019). https://doi.org/10.1108/ILT-01-2018-0005
Lancaster, J.K.: The effect of carbon fibre reinforcement on the friction and wear of polymers. J. Phys. D 1, 549 (1968). https://doi.org/10.1088/0022-3727/1/5/303
Schuepfer, D.B., Badaczewski, F., Guerra-Castro, J.M., Hofmann, D.M., Heiliger, C., Smarsly, B., et al.: Assessing the structural properties of graphitic and non-graphitic carbons by Raman spectroscopy. Carbon N Y 161, 359–372 (2020). https://doi.org/10.1016/j.carbon.2019.12.094
Wang, F.: Carbon fibers and their thermal transporting properties. In: Zhang, G. (ed.) Thermal transport in carbon-based nanomaterials, pp. 135–184. Elsevier, Amsterdam (2017)
Newcomb, B.A.: Processing, structure, and properties of carbon fibers. Composites A 91, 262–282 (2016). https://doi.org/10.1016/j.compositesa.2016.10.018
Fitzer, E., Köchling, K.-H., Boehm, H.P., Marsh, H.: Recommended terminology for the description of carbon as a solid (IUPAC recommendations 1995). Pure Appl. Chem. 67, 473–506 (1995)
Ruland, W., Smarsly, B.: X-ray scattering of non-graphitic carbon: an improved method of evaluation. J. Appl. Crystallogr. 35, 624–633 (2002). https://doi.org/10.1107/S0021889802011007
Osswald, O., Loeh, M.O., Badaczewski, F.M., Pfaff, T., Fischer, H.E., Franz, A., et al.: On the highly ordered graphene structure of non-graphitic carbons (NGCs)—a wide-angle neutron scattering (WANS) study. C (Basel) 9, 27 (2023). https://doi.org/10.3390/c9010027
Toth, P.: Nanostructure quantification of turbostratic carbon by HRTEM image analysis: State of the art, biases, sensitivity and best practices. Carbon N Y 178, 688–707 (2021). https://doi.org/10.1016/j.carbon.2021.03.043
Biscoe, J., Warren, B.E.: An x-ray study of carbon black. J. Appl. Phys. 13, 364–371 (1942). https://doi.org/10.1063/1.1714879
Sawae, Y., Morita, T., Takeda, K., Onitsuka, S., Kaneuti, J., Yamaguchi, T., et al.: Friction and wear of PTFE composites with different filler in high purity hydrogen gas. Tribol. Int. (2021). https://doi.org/10.1016/j.triboint.2021.106884
Oyamada, T., Ono, M., Miura, H., Kuwano, T.: Effect of gas environment on friction behavior and tribofilm formation of PEEK/carbon fiber composite. Tribol. Trans. 56, 607–614 (2013). https://doi.org/10.1080/10402004.2012.745104
Johansson, P., Marklund, P., Björling, M., Shi, Y.: Effect of humidity and counterface material on the friction and wear of carbon fiber reinforced PTFE composites. Tribol. Int. (2021). https://doi.org/10.1016/J.TRIBOINT.2021.106869
Schubert, R.: The influence of a gas atmosphere and its moisture on sliding wear in PTFE compositions. ASME J. Lubr. Technol. 93, 216–223 (1971). https://doi.org/10.1115/1.3451544
Fuchsluger, J.H., Taber, R.D.: The effect of atmospheric environment on the wear properties of filled TFE materials. J. Tribol. 93, 423–429 (1971). https://doi.org/10.1115/1.3451611
Theiler, G., Gradt, T.: Environmental effects on the sliding behaviour of PEEK composites. Wear 368–269, 278–286 (2016). https://doi.org/10.1016/j.wear.2016.09.019
Yen, B.K.: Influence of water vapor and oxygen on the tribology of carbon materials with sp2 valence configuration. Wear 192, 208–215 (1996)
Yen, B.K., Ishihara, T., Yamamoto, I.: Influence of environment and temperature on “‘dusting’” wear transitions of carbon-carbon composites. J. Mater. Sci. 32, 681–686 (1997)
Lancaster, J.K., Pritchard, J.R.: The influence of environment and pressure on the transition to dusting wear of graphite. J. Phys. D 14, 747–762 (1981). https://doi.org/10.1088/0022-3727/14/4/027
Savage, R.H.: Graphite lubrication. J. Appl. Phys. 19, 1–10 (1948). https://doi.org/10.1063/1.1697867
Campbell, W.E., Kozak, R.: Studies in boundary lubrication–III: the wear of carbon brushes in dry atmospheres. Trans. ASME 70, 491–497 (1948). https://doi.org/10.1115/1.4017775
Morstein, C.E., Klemenz, A., Dienwiebel, M., Moseler, M.: Humidity-dependent lubrication of highly loaded contacts by graphite and a structural transition to turbostratic carbon. Nat. Commun. 13, 5958 (2022). https://doi.org/10.1038/s41467-022-33481-9
Yen, B.K., Schwickert, B.E., Toney, M.F.: Origin of low-friction behavior in graphite investigated by surface x-ray diffraction. Appl. Phys. Lett. 84, 4702–4704 (2004). https://doi.org/10.1063/1.1760597
Deacon, R.F., Goodman, J.F.: Lubrication by lamellar solids. Proc. R. Soc. Lond. A 243, 464–482 (1958). https://doi.org/10.1098/rspa.1958.0013
Johansson, P., Kalliorinne, K., Marklund, P., Björling, M.: Precise control of operating conditions in tribotesting with respect to trace humidity and contact temperature. MethodsX 8, 101362 (2021). https://doi.org/10.1016/J.MEX.2021.101362
Schulze, M., Bolwin, K., Giilzow, E., Schnurnberger, W.: XPS analysis of PTFE decomposition due to ionizing radiation. Fresenius J. Anal. Chem. 353, 778–784 (1995). https://doi.org/10.1007/BF00321370
Schulze, M., Lorenz, M., Kaz, T.: XPS study of electrodes formed from a mixture of carbon black and PTFE powder. Surf. Interface Anal. 34, 646–651 (2002). https://doi.org/10.1002/sia.1378
Takahagi, T., Ishitani, A.: X-ray photoelectron spectroscopy study of an ion sputtering process of fluoro polymers using monte carlo simulation. Phys. Rev. Lett. 20, 404–407 (1987). https://doi.org/10.1021/ma00168a029
Girardeaux, C., Pireaux, J.-J.: Analysis of poly(tetrafluoroethylene) (PTFE) by XPS. Surf. Sci. Spectra 4, 138–141 (1996). https://doi.org/10.1116/1.1247814
Beard, B.C., Brizzolara, R.A.: Polytetrafluoroethylene characterized by XPS, with Mg source. Surf. Sci. Spectra 2, 85–88 (1993). https://doi.org/10.1116/1.1247714
Ackeret, M.: Polytetrafluoroethylene by XPS. Surf. Sci. Spectra 1, 100–103 (1992). https://doi.org/10.1116/1.1247678
Piwowarczyk, J., Jedrzejewski, R., Moszyński, D., Kwiatkowski, K., Niemczyk, A., Baranowska, J.: XPS and FTIR studies of polytetrafluoroethylene thin films obtained by physical methods. Polymers (Basel) (2019). https://doi.org/10.3390/polym11101629
Chen, G., Zhang, J., Yang, S.: Fabrication of hydrophobic fluorinated amorphous carbon thin films by an electrochemical route. Electrochem. Commun. 10, 7–11 (2008). https://doi.org/10.1016/j.elecom.2007.10.006
Yu, G.Q., Tay, B.K., Sun, Z.: Fluorinated amorphous diamond-like carbon films deposited by plasma-enhanced chemical vapor deposition. Surf. Coat. Technol. 191, 236–241 (2005). https://doi.org/10.1016/j.surfcoat.2004.04.060
Goloveshkin, A.S., Golub, A.S., Grayfer, E.D., Makotchenko, V.G., Fedorov, V.E.: Atomic structure and bonding in fluorinated graphite intercalated with a strong fluoroxidant. Diam. Relat. Mater. (2023). https://doi.org/10.1016/j.diamond.2023.109851
An, K.H., Heo, J.G., Jeon, K.G., Bae, D.J., Jo, C., Yang, C.W., et al.: X-ray photoemission spectroscopy study of fluorinated single-walled carbon nanotubes. Appl. Phys. Lett. 80, 4235–4237 (2002). https://doi.org/10.1063/1.1482801
Dilks, A., Kay, E.: Plasma polymerization of ethylene and the series of fluoroethylenes: plasma effluent mass spectrometry and ESCA studies. Macromolecules 14, 855–862 (1981). https://doi.org/10.1021/ma50004a074
Badway, F., Pereira, N., Cosandey, F., Amatucci, G.G.: Carbon-metal fluoride nanocomposites: structure and electrochemistry of FeF3:C. J. Electrochem. Soc. 150, A1209 (2003). https://doi.org/10.1149/1.1596162
Li, L., Meng, F., Jin, S.: High-capacity lithium-ion battery conversion cathodes based on iron fluoride nanowires and insights into the conversion mechanism. Nano Lett. 12, 6030–6037 (2012). https://doi.org/10.1021/nl303630p
Xu, X., Li, F., Zhang, D., Ji, S., Huo, Y., Liu, J.: FeF3@C nanotube arrays grown on carbon fabric as a free-standing cathode for lithium-ion batteries. Mater. Chem. Front. 6, 3512–3521 (2022). https://doi.org/10.1039/d2qm00908k
Kojima, H., Sawae, Y., Morita, T., Sugimura, J.: Effect of trace moisture content on friction of carbon fiber filled PTFE in high purity gas. In: Proceedings of Malaysian international tribology conference, pp. 125–126. (2015)
Jintang, G.: Tribochemical effects in formation of polymer transfer film. Wear 245, 100–106 (2000). https://doi.org/10.1016/S0043-1648(00)00470-1
Pogosian, A., Hovhannisyan, K., Isajanyan, A.: Polymer friction transfer (FT). In: Encyclopedia of tribology. Springer, Boston (2013)
Skinner, J., Gane, N., Tabor, D.: Micro-friction of graphite. Nat. Phys. Sci. 232, 195–196 (1971). https://doi.org/10.1038/physci232195a0
Robert, F., Csapo, E., Zaidi, H., Paulmier, D.: Influence of the current and environment on tiee superficial structure of a grapihte electrical collector. Int. J. Mach. Tools Manuf. 35, 259–262 (1995). https://doi.org/10.1016/0890-6955(94)P2381-O
Qiu, S.R., Lai, H.F., Yarmoff, J.A.: Self-limiting growth of metal fluoride thin films by oxidation reactions employing molecular precursors. Phys. Rev. Lett. 85, 1492–1495 (2000). https://doi.org/10.1103/PhysRevLett.85.1492
Zuo, Z., Yang, Y., Qi, X., Su, W., Yang, X.: Analysis of the chemical composition of the PTFE transfer film produced by sliding against Q235 carbon steel. Wear 320, 87–93 (2014). https://doi.org/10.1016/j.wear.2014.08.019
Kumar, N., Dash, S., Tyagi, A.K., Raj, B.: Super low to high friction of turbostratic graphite under various atmospheric test conditions. Tribol. Int. 44, 1969–1978 (2011). https://doi.org/10.1016/j.triboint.2011.08.012
Ramadanoff, D., Glass, S.W.: High-altitude brush problem. Electr. Eng. 63, 825–829 (1944). https://doi.org/10.1109/EE.1944.6440571
Zaidi, H., Néry, H., Paulmier, D.: Stability of lubricating properties of graphite by orientation of the crystallites in the presence of water vapour. Appl. Surf. Sci. 70, 180–185 (1993). https://doi.org/10.1016/0169-4332(93)90423-9
Zaidi, H., Paulmier, D., Jeanmaire, A., Nery, H.: Behaviour of graphite in friction under various environments. Surf. Sci. 252, 778–781 (1991). https://doi.org/10.1016/0039-6028(91)91097-h
Short, M.A., Walker, P.L.: Measurement of interlayer spacings and crystal sizes in turbostratic carbonS. Carbon N Y 1, 3–9 (1963). https://doi.org/10.1016/0008-6223(63)90003-4
Li, Z.Q., Lu, C.J., Xia, Z.P., Zhou, Y., Luo, Z.: X-ray diffraction patterns of graphite and turbostratic carbon. Carbon N Y 45, 1686–1695 (2007). https://doi.org/10.1016/j.carbon.2007.03.038
Lancaster, J.K.: Transitions in the friction and wear of carbons and graphites sliding against themselves. ASLE Trans. 18, 187–201 (1975). https://doi.org/10.1080/05698197508982761
Stout, J.W., Reed, S.A.: The crystal structure of mnf2, fef2, cof2, nif2 and znf2. J. Am. Chem. Soc. 76, 5279–5281 (1954)
Materials Project n.d. https://materialsproject.org/materials/mp-556911. Accessed May 11 2023
Momma, K., Izumi, F.: VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011)
Vuković, F., Walsh, T.R.: Practical atomistic models of carbon fiber surfaces with tuneable topology and topography. Compos. Sci. Technol. (2021). https://doi.org/10.1016/j.compscitech.2021.109049
Stevenson, W.T.K., Garton, A., Ripmeester, J.A., Wiles, D.M.: A spectroscopic study of the carbonisation of poly(acrylonitrile) and poly(acrylonitrile-co-acrylic acid). Polym. Degrad. Stab. 15, 125–141 (1986)
Thompson, A.P., Aktulga, H.M., Berger, R., Bolintineanu, D.S., Brown, W.M., Crozier, P.S., et al.: LAMMPS-a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 271, 108171 (2022)
Eder, S.J., Vernes, A., Betz, G.: On the Derjaguin offset in boundary-lubricated nanotribological systems. Langmuir 29, 13760–13772 (2013)
Barry, P.R., Chiu, P.Y., Perry, S.S., Sawyer, W.G., Sinnott, S.B., Phillpot, S.R.: Effect of temperature on the friction and wear of PTFE by atomic-level simulation. Tribol. Lett. (2015). https://doi.org/10.1007/s11249-015-0529-y
Ayestarán Latorre, C., Remias, J.E., Moore, J.D., Spikes, H.A., Dini, D., Ewen, J.P.: Mechanochemistry of phosphate esters confined between sliding iron surfaces. Commun. Chem. (2021). https://doi.org/10.1038/s42004-021-00615-x
Cadman, P., Gossedge, G.M.: The chemical interaction of metals with polytetrafluoroethylene. J. Mater. Sci. 14, 2672–2678 (1979). https://doi.org/10.1007/BF00610638
Cadman, P., Gossedge, G.M.: The chemical nature of metal-polytetrafluoroethylene tribological interactions as studied by X-ray photoelectron spectroscopy. Wear 54, 211–215 (1979). https://doi.org/10.1016/0043-1648(79)90115-7
Shen, J.T., Pei, Y.T., De Hosson, J.T.M.: Formation of metal-F bonds during frictional sliding: influence of water and applied load. Appl. Surf. Sci. 368, 427–434 (2016). https://doi.org/10.1016/j.apsusc.2016.02.012
Deli, G., Qunji, X., Hongli, W.: ESCA study on tribochemical characteristics of filled PTFE. Wear 148, 161–169 (1991). https://doi.org/10.1016/0043-1648(91)90214-F
Blanchet, T.A., Kennedy, F.E., Jayne, D.T.: Xps analysis of the effect of fillers on ptfe transfer film development in sliding contacts. Tribol. Trans. 36, 535–544 (1993). https://doi.org/10.1080/10402009308983193
Gardos, M.N., Davis, P.S., Meldrum, G.R.: Crystal-structure-controlled tribological behavior of carbon-graphite seal materials in partial pressures of helium and hydrogen. II. SEM tribometry. Tribol. Lett. 3, 185–198 (1997). https://doi.org/10.1023/A:1019120819675
Robert, F., Paulmier, D., Zaidi, H.: Influence of argon and helium on the friciton of a graphite-graphite couple in pin-disc system. Tribologia 11, 65–73 (1992)
Butova, V.V., Aboraia, A.M., Shapovalov, V.V., Dzhangiryan, N.A., Papkovskaya, E.D., Ilin, O.I., et al.: Iron (II) fluoride cathode material derived from MIL-88A. J. Alloys Compd. (2022). https://doi.org/10.1016/j.jallcom.2022.165438
Funding
Open access funding provided by Lulea University of Technology. The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
PJ designed the concept and methodology of the study, conducted the experiments, analyzed the results and wrote the first draft of the manuscript. RE conducted the XPS analysis, wrote the related method and results and reviewed the manuscript. VFN conducted the MD simulations and wrote the detailed simulation report, found in Supplementary Information. PM, MB and YS supervised the study and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MPG 45846 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Johansson, P., Elo, R., Naeini, V.F. et al. Insights of the Ultralow Wear and Low Friction of Carbon Fiber Reinforced PTFE in Inert Trace Moisture Environment. Tribol Lett 71, 100 (2023). https://doi.org/10.1007/s11249-023-01770-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11249-023-01770-z