Abstract
CO2 utilizations and conversions contribute to the reduction of greenhouse gas emissions and regeneration of industrial exhausts. Reforming and hydrogenation processes can transform CO2, hydrogen and hydrocarbons to syngas and other value-added products. To ensure a high activity, selectivity and stability as well as anti-coking property, efficient adsorption and activation of CO2 exert a profound impact. Among the catalysts adopted in these reactions, metal oxides have been proven active for adsorbing and activating CO2 based on surface acidity/basicity and oxygen defects. In this review, the impacts of these two physicochemical properties of metal oxides on the CO2 adsorption and activation will be comprehensively and systematically summarized in terms of three performance criteria (CO2 conversion—activity, product yield—selectivity, anti-coking property—stability) in two types of reactions relating to thermo-catalytic conversion of CO2 (reforming and hydrogenation). In addition to the critical discussion of the structure-performance relationships, the reaction/deactivation mechanisms and origin of surface acidity/basicity and oxygen defects are also introduced in depth. Finally, conclusive remarks of the main contents and proposed future works are provided.


Reproduced with permission from the authors of [67]. Copyright 2017 Springer Nature

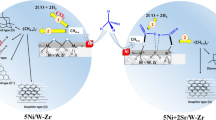
Reproduced with permission from the authors of [129]. Copyright 2017 Royal Society of Chemistry

Reproduced with permission from the authors of [131]. Copyright 2014 Elsevier

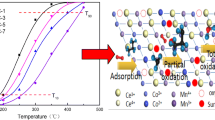
Reproduced with permission from the authors of [68]. Copyright 2021 American Chemical Society. b Formate formation mechanism between the CO2 bonded to an anion vacancy on the Zn-terminated polar face and a vicinally adsorbed H atom. Reproduced with permission from the authors of [149]. Copyright 2021 Springer

Reproduced with permission from the authors of [160]. Copyright 2019 Elsevier

Reproduced with permission from the authors of [157]. Copyright 2021 Elsevier
Similar content being viewed by others
Data Availability
All data included in this study are available upon the permission from the publishers.
References
Gao X, Yang S, Hu L, Cai S, Wu L, Kawi S (2022) Carbonaceous materials as adsorbents for CO2 capture: synthesis and modification. Carbon Capture Sci Technol 3:100039
Karimi IA, Kawi S (2016) Preface to the ICCDU-2015 special issue. Ind Eng Chem Res 55:7839–7841
Posada-Pérez S, Viñes F, Rodriguez JA, Illas F (2015) Fundamentals of methanol synthesis on metal carbide based catalysts: activation of CO2 and H2. Top Catal 58:159–173
Zhang S, Wu Z, Liu X, Hua K, Shao Z, Wei B, Huang C, Wang H, Sun Y (2021) A short review of recent advances in direct CO2 hydrogenation to alcohols. Top Catal 64:371–394
Gao X, Lin X, Xie X, Li J, Wu X, Li Y, Kawi S (2022) Modification strategies of heterogeneous catalysts for water–gas shift reactions. React Chem Eng 7:551–565
Shahed GV, Taherian Z, Khataee A, Meshkani F, Orooji Y (2020) Samarium-impregnated nickel catalysts over SBA-15 in steam reforming of CH4 process. J Ind Eng Chem 86:73–80
Dewangan N, Hui WM, Jayaprakash S, Bawah AR, Poerjoto AJ, Jie T, Ashok J, Hidajat K, Kawi S (2020) Recent progress on layered double hydroxide (LDH) derived metal-based catalysts for CO2 conversion to valuable chemicals. Catal Today 356:490–513
Golunski S, Burch R (2021) CO2 hydrogenation to methanol over copper catalysts: learning from syngas conversion. Top Catal 64:974–983
Das S, Ashok J, Xi S, Borgna A, Hidajat K, Kawi S (2020) Highly dispersed Ni/silica by carbonization–calcination of a chelated precursor for coke-free dry reforming of methane. ACS Appl Energy Mater 3:7719–7735
Das S, Pérez-Ramírez J, Gong J, Dewangan N, Hidajat K, Gates BC, Kawi S (2020) Core–shell structured catalysts for thermocatalytic, photocatalytic, and electrocatalytic conversion of CO2. Chem Soc Rev 49:2937–3004
Hu J, Poelman H, Marin GB, Detavernier C, Kawi S, Galvita VV (2020) FeO controls the sintering of iron-based oxygen carriers in chemical looping CO2 conversion. J CO2 Util 40:101216
Shoji S, Peng X, Yamaguchi A, Watanabe R, Fukuhara C, Cho Y, Yamamoto T, Matsumura S, Yu MW, Ishii S, Fujita T, Abe H, Miyauchi M (2020) Photocatalytic uphill conversion of natural gas beyond the limitation of thermal reaction systems. Nat Catal 3:148–153
Zhou L, Martirez JMP, Finzel J, Zhang C, Swearer DF, Tian S, Robatjazi H, Lou M, Dong L, Henderson L, Christopher P, Carter EA, Nordlander P, Halas NJ (2020) Light-driven methane dry reforming with single atomic site antenna-reactor plasmonic photocatalysts. Nat Energy 5:61–70
Bian Z, Xia H, Wang Z, Jiang B, Yu Y, Yu K, Zhong W, Kawi S (2020) CFD simulation of a hydrogen-permeable membrane reactor for CO2 reforming of CH4: the interplay of the reaction and hydrogen permeation. Energy Fuels 34:12366–12378
Ashok J, Bian Z, Wang Z, Kawi S (2018) Ni-phyllosilicate structure derived Ni–SiO2–MgO catalysts for bi-reforming applications: acidity, basicity and thermal stability. Catal Sci Technol 8:1730–1742
Gao XY, Lin ZT, Li TT, Huang LT, Zhang JM, Askari S, Dewangan N, Jangam A, Kawi S (2021) Recent developments in dielectric barrier discharge plasma-assisted catalytic dry reforming of methane over Ni-based catalysts. Catalysts 11:455
Gao XY, Tan ZW, Hidajat K, Kawi S (2017) Highly reactive Ni–Co/SiO2 bimetallic catalyst via complexation with oleylamine/oleic acid organic pair for dry reforming of methane. Catal Today 281:250–258
Li ZW, Li M, Bian ZF, Kathiraser Y, Kawi S (2016) Design of highly stable and selective core/yolk–shell nanocatalysts-a review. Appl Catal B 188:324–341
Sekine Y, Yamagishi K, Nogami Y, Manabel R, Oshimal K, Ogo S (2016) Low temperature catalytic water gas shift in an electric field. Catal Lett 146:1423–1428
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38
Cui Y, Zhang H, Xu H, Li W (2007) Kinetic study of the catalytic reforming of CH4 with CO2 to syngas over Ni/α-Al2O3 catalyst: the effect of temperature on the reforming mechanism. Appl Catal A 318:79–88
Gaddalla AM, Sommer ME (1989) Carbon dioxide reforming of methane on nickel catalysts. Chem Eng Sci 44:2825–2829
Akri M, Zhao S, Li X, Zang K, Lee AF, Isaacs MA, Xi W, Gangarajula Y, Luo J, Ren Y, Cui YT, Li L, Su Y, Pan X, Wen W, Pan Y, Wilson K, Li L, Qiao B, Ishii H, Liao YF, Wang A, Wang X, Zhang T (2019) Atomically dispersed nickel as coke-resistant active sites for methane dry reforming. Nat Commun 10:5181
Dang CX, Long J, Li HK, Cai WQ, Yu H (2020) Pd-promoted Ni-Ca-Al bi-functional catalyst for integrated sorption-enhanced steam reforming of glycerol and methane reforming of carbonate. Chem Eng Sci 230:116226
Dang S, Qin B, Yang Y, Wang H, Cai J, Han Y, Li S, Gao P, Sun Y (2020) Rationally designed indium oxide catalysts for CO2 hydrogenation to methanol with high activity and selectivity. Sci Adv 6:eaaz2060
Meng C, Zhao G, Shi XR, Chen P, Liu Y, Lu Y (2021) Oxygen-deficient metal oxides supported nano-intermetallic InNi3C0.5 toward efficient CO2 hydrogenation to methanol. Sci Adv 7:eabi6012
Huang W, Johnston-Peck AC, Wolter T, Yang WCD, Xu L, Oh J, Reeves BA, Zhou C, Holtz ME, Herzing AA, Lindenberg AM, Mavrikakis M, Cargnello M (2021) Steam-created grain boundaries for methane C-H activation in palladium catalysts. Science 373:1518–1523
Chen T, Wang Z, Liu L, Pati S, Wai MH, Kawi S (2020) Coupling CO2 separation with catalytic reverse water-gas shift reaction via ceramic-carbonate dual-phase membrane reactor. Chem Eng J 379:122182
Liu L, Das S, Chen T, Dewangan N, Ashok J, Xi S, Borgna A, Li Z, Kawi S (2020) Low temperature catalytic reverse water-gas shift reaction over perovskite catalysts in DBD plasma. Appl Catal B 265:118573
Liu L, Zhang Z, Das S, Xi S, Kawi S (2020) LaNiO3 as a precursor of Ni/La2O3 for reverse water-gas shift in DBD plasma: effect of calcination temperature. Energy Convers Manage 206:112475
Hongmanorom P, Ashok J, Zhang G, Bian Z, Wai MH, Zeng Y, Xi S, Borgna A, Kawi S (2021) Enhanced performance and selectivity of CO2 methanation over phyllosilicate structure derived Ni-Mg/SBA-15 catalysts. Appl Catal B 282:119564
Ashok J, Ang ML, Kawi S (2017) Enhanced activity of CO2 methanation over Ni/CeO2–ZrO2 catalysts: Influence of preparation methods. Catal Today 281:304–311
Ashok J, Pati S, Hongmanorom P, Zhang T, Chen J, Kawi S (2020) A review of recent catalyst advances in CO2 methanation processes. Catal Today 356:471–489
Yu Y, Bian Z, Song F, Wang J, Zhong Q, Kawi S (2018) Influence of calcination temperature on activity and selectivity of Ni–CeO2 and Ni–Ce0.8Zr0.2O2 catalysts for CO2 methanation. Top Catal 61:1514–1527
Yu Y, Chan YM, Bian Z, Song F, Wang J, Zhong Q, Kawi S (2018) Enhanced performance and selectivity of CO2 methanation over g-C3N4 assisted synthesis of Ni–CeO2 catalyst: kinetics and DRIFTS studies. Int J Hydrog Energy 43:15191–15204
Pati S, Ashok J, Dewangan N, Chen T, Kawi S (2020) Ultra-thin (~1 µm) Pd–Cu membrane reactor for coupling CO2 hydrogenation and propane dehydrogenation applications. J Membr Sci 595:117496
Gao X, Liang J, Wu L, Wu L, Kawi S (2022) Dielectric barrier discharge plasma-assisted catalytic CO2 hydrogenation: synergy of catalyst and plasma. Catalysts 12:66
Chen L, Xu Q (2020) Fewer defects, better catalysis? Science 367:737–737
Song Y, Ozdemir E, Ramesh S, Adishev A, Subramanian S, Harale A, Albuali M, Fadhel BA, Jamal A, Moon D, Choi SH, Yavuz CT (2020) Dry reforming of methane by stable Ni–Mo nanocatalysts on single-crystalline MgO. Science 367:777–781
Gao X, Ge Z, Zhu G, Wang Z, Ashok J, Kawi S (2021) Anti-coking and anti-sintering Ni/Al2O3 catalysts in the dry reforming of methane: recent progress and prospects. Catalysts 11:1003
Bian Z, Zhong W, Yu Y, Wang Z, Jiang B, Kawi S (2021) Dry reforming of methane on Ni/mesoporous-Al2O3 catalysts: effect of calcination temperature. Int J Hydrog Energy 46:31041–31053
Gao X, Ashok J, Kawi S (2020) Smart designs of anti-coking and anti-sintering Ni-based catalysts for dry reforming of methane: a recent review. Reactions 1:162–194
Li Z, Lin Q, Li M, Cao J, Liu F, Pan H, Wang Z, Kawi S (2020) Recent advances in process and catalyst for CO2 reforming of methane. Renew Sust Energy Rev 134:110312
Bian Z, Kawi S (2020) Preparation, characterization and catalytic application of phyllosilicate: a review. Catal Today 339:3–23
Gao X, Ashok J, Kawi S (2022) A review on roles of pretreatment atmospheres for the preparation of efficient Ni-based catalysts. Catal Today 397–399:581–591
Gao XY, Ashok J, Widjaja S, Hidajat K, Kawi S (2015) Ni/SiO2 catalyst prepared via Ni-aliphatic amine complexation for dry reforming of methane: effect of carbon chain number and amine concentration. Appl Catal A 503:34–42
Gao XY, Hidajat K, Kawi S (2016) Facile synthesis of Ni/SiO2 catalyst by sequential hydrogen/air treatment: a superior anti-coking catalyst for dry reforming of methane. J CO2 Util 15:146–153
Gao XY, Liu HJ, Hidajat K, Kawi S (2015) Anti-coking Ni/SiO2 catalyst for dry reforming of methane: role of oleylamine/oleic acid organic pair. ChemCatChem 7:4188–4196
Li ZW, Das S, Hongmanorom P, Dewangan N, Wai MH, Kawi S (2018) Silica-based micro- and mesoporous catalysts for dry reforming of methane. Catal Sci Technol 8:2763–2778
Li ZW, Jiang B, Wang ZG, Kawi S (2018) High carbon resistant Ni@Ni phyllosilicate@SiO2 core shell hollow sphere catalysts for low temperature CH4 dry reforming. J CO2 Util 27:238–246
Li ZW, Kawi S (2018) Multi-Ni@Ni phyllosilicate hollow sphere for CO2 reforming of CH4: Influence of Ni precursors on structure, sintering, and carbon resistance. Catal Sci Technol 8:1915–1922
Liu Z, Gao F, Zhu YA, Liu Z, Zhu K, Zhou X (2020) Bi-reforming of methane with steam and CO2 under pressurized conditions on a durable Ir–Ni/MgAl2O4 catalyst. Chem Commun 56:13536–13539
He D, Zhang Y, Wang Z, Mei Y, Jiang Y (2020) Bi-reforming of methane with carbon dioxide and steam on nickel-supported binary Mg−Al metal oxide catalysts. Energy Fuels 34:4822–4827
Pham Minh D, Pham XH, Siang TJ, Vo DVN (2021) Review on the catalytic tri-reforming of methane - Part I: impact of operating conditions, catalyst deactivation and regeneration. Appl Catal A 621:118202
Díez-Ramírez J, Dorado F, Martínez-Valiente A, García-Vargas JM, Sanchez P (2016) Kinetic, energetic and exergetic approach to the methane tri-reforming process. Int J Hydrog Energy 41:19339–19348
Kozonoe CE, de Paiva Floro BR, Alves RMB, Schmal M (2019) The Fe-Co-Cu supported on MWCNT as catalyst for the tri-reforming of methane – investigating the structure changes of the catalysts. Fuel 256:115917
Samanta S, Srivastava R (2020) Catalytic conversion of CO2 to chemicals and fuels: the collective thermocatalytic/photocatalytic/electrocatalytic approach with graphitic carbon nitride. Mater Adv 1:1506–1545
Papp H, Schuler P, Zhuang Q (1996) CO2 reforming and partial oxidation of methane. Top Catal 3:299–311
Tran NT, Kumar PS, Le QV, Cuong NV, Phuong PTT, Jalil AA, Sharma G, Kumar A, Sharma A, Ayodele BV, Abidin SZ, Vo DVN (2021) CO2 reforming of CH4 on mesoporous alumina-supported cobalt catalyst: optimization of lanthana promoter loading. Top Catal 64:338–347
Wang W, Tongo DWK, Song L, Qu Z (2021) Effect of Au addition on the catalytic performance of CuO/CeO2 catalysts for CO2 hydrogenation to methanol. Top Catal 64:446–455
Taherian Z, Gharahshiran VS, Khataee A, Orooji Y (2022) Synergistic effect of freeze-drying and promoters on the catalytic performance of Ni/MgAl layered double hydroxide. Fuel 311:122620
Megha M, Mondal K, Banerjee A, Ghanty TK (2020) Adsorption and activation of CO2 on Zrn (n = 2–7) clusters. Phys Chem Chem Phys 22:16877–16886
Austin N, Ye J, Mpourmpakis G (2017) CO2 activation on Cu-based Zr-decorated nanoparticles. Catal Sci Technol 7:2245–2251
Huygh S, Bogaerts A, Neyts EC (2016) How oxygen vacancies activate CO2 dissociation on TiO2 anatase (001). J Phys Chem C 120:21659–21669
Taherian Z, Khataee A, Orooji Y (2020) Facile synthesis of yttria-promoted nickel catalysts supported on MgO-MCM-41 for syngas production from greenhouse gases. Renew Sust Energ Rev 134:110130
Jin X, Lv C, Zhou X, Ye L, Xie H, Liu Y, Su H, Zhang B, Chen G (2019) Oxygen vacancy engineering of Bi24O31Cl10 for boosted photocatalytic CO2 conversion. Chemsuschem 12:2740–2747
Gao P, Li S, Bu X, Dang S, Liu Z, Wang H, Zhong L, Qiu M, Yang C, Cai J (2017) Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat Chem 9:1019–1024
Feng WH, Yu MM, Wang LJ, Miao YT, Shakouri M, Ran J, Hu Y, Li Z, Huang R, Lu YL, Gao D, Wu JF (2021) Insights into bimetallic oxide synergy during carbon dioxide hydrogenation to methanol and dimethyl ether over GaZrOx oxide catalysts. ACS Catal 11:4704–4711
Taherian Z, Khataee A, Orooji Y (2020) Promoted nickel-based catalysts on modified mesoporous silica support: the role of yttria and magnesia on CO2 methanation. Micropor Mesopor Mat 306:110455
Zu Q, Wang X, Dong L, Su T, Li B, Zhou Y, Jiang Y, Luo X, Ji H (2019) CO2 methanation on CO/TiO2 catalyst: effects of Y on the support. Chem Eng Sci 210:115–245
Vrijburg WL, Moioli E, Chen W, Zhang M, Terlingen BJP, Zijlstra B, Filot IAW, Zuttel A, Pidko EA, Hensen EJM (2019) Efficient base-metal NiMn/TiO2 catalyst for CO2 methanation. ACS Catal 9:7823–7839
Yang X, Su X, Chen X, Duan H, Liang B, Liu Q, Liu X, Ren Y, Huang Y, Zhang T (2017) Promotion effects of potassium on the activity and selectivity of Pt/zeolite catalysts for reverse water gas shift reaction. Appl Catal B 216:95–105
Zhang Z, Hu X, Wang Y, Hu S, Xiang J, Li C, Chen G, Liu Q, Wei T, Dong D (2019) Regulation the reaction intermediates in methanation reactions via modification of nickel catalysts with strong base. Fuel 237:566–579
Chai S, Men Y, Wang J, Liu S, Song Q, An W, Kolb G (2019) Boosting CO2 methanation activity on Ru/TiO2 catalysts by exposing (001) facets of anatase TiO2. J CO2 Util 33:242–252
Jia X, Zhang X, Rui N, Xue X, Liu C (2019) Structural effect of Ni/ZrO2 catalyst on CO2 methanation with enhanced activity. Appl Catal B 244:159–169
Varvoutis G, Lykaki M, Stefa S, Papista E, Carabineiro SA, Marnellos GE, Konsolakis M (2020) Remarkable efficiency of Ni supported on hydrothermally synthesized CeO2 nanorods for low-temperature CO2 hydrogenation to methane. Catal Commun 142:106036
Van NTT, Loc LC, Anh NP, Cuong HT, Tri N (2020) Positive effects of CeO2 promoter and co-reactant/CO on methanation of CO2-rich gas over Ni/SBA-15 catalyst. Mater Trans 61:1332–1338
Wang J, Liu CY, Senftle TP, Zhu J, Zhang G, Guo X, Song C (2020) Variation in the In2O3 crystal phase alters catalytic performance toward the reverse water gas shift reaction. ACS Catal 10:3264–3273
Mou J, Fan X, Liu F, Wang X, Zhao T, Chen P, Li Z, Yang C, Cao J (2021) CO2 hydrogenation to lower olefins over Mn2O3–ZnO/SAPO-34 tandem catalysts. Chem Eng J 421:129978
Liu W, Li L, Zhang X, Wang Z, Wang X, Peng H (2018) Design of Ni–ZrO2@SiO2 catalyst with ultra-high sintering and coking resistance for dry reforming of methane to prepare syngas. J CO2 Util 27:297–307
Kim BJ, Jeon KW, Na HS, Lee YL, Ahn SY, Kim KJ, Jang WJ, Shim JO, Roh HS (2020) Reducible oxide (CeO2, ZrO2, and CeO2–ZrO2) promoted Ni–MgO catalysts for carbon dioxide reforming of methane reaction. Korean J Chem Eng 37:1130–1136
Kumara R, Kumara K, Choudary NV, Panta KK (2019) Effect of support materials on the performance of Ni-based catalysts in tri-reforming of methane. Fuel Process Technol 186:40–52
Khoja AH, Tahir M, Amin NAS (2019) Evaluating the performance of a Ni catalyst supported on La2O3–MgAl2O4 for dry reforming of methane in a packed bed dielectric barrier discharge plasma reactor. Energy Fuels 33:11630–11647
de Roseno KTC, Antunes RA, Alves RMB, Schmal M (2021) Tri-reforming of methane over NdM0.25Ni0.75O3 (M = Cr, Fe) catalysts and the effect of CO2 composition. Calat Lett 151:3639–3655
Hu X, Jia X, Zhang X, Liu Y, Liu C (2019) Improvement in the activity of Ni/ZrO2 by cold plasma decomposition for dry reforming of methane. Catal Commun 128:105720
Pham XH, Ashik UPM, Hayashi JI, Alonso AP, Pla D, Gómez M, Minh DP (2021) Review on the catalytic tri-reforming of methane - Part II: catalyst development. Appl Catal A 623:118286
Yentekakis IV, Panagiotopoulou P, Artemakis G (2021) A review of recent efforts to promote dry reforming of methane (DRM) to syngas production via bimetallic catalyst formulations. Appl Catal B 296:120210
Teh LP, Setiabudi HD, Timmiati SN, Aziz MAA, Annuar NHR, Ruslan NN (2021) Recent progress in ceria-based catalysts for the dry reforming of methane: a review. Chem Eng Sci 239:116606
Xu J, Xia P, Zhang Q, Guo F, Xia Y, Tian H (2021) Coke resistance of Ni-based catalysts enhanced by cold plasma treatment for CH4–CO2 reforming: review. Int J Hydrog Energy 46:23174–23189
Taherian Z, Khataee A, Han N, Orooji Y (2022) Hydrogen production through methane reforming processes using promoted-Ni/mesoporous silica: a review. J Ind Eng Chem 107:20–30
Etim UJ, Zhang C, Zhong Z (2021) Impacts of the catalyst structures on CO2 activation on catalyst surfaces. Nanomaterials 11:3265
Li J, Cheng X, Zhang C, Yang Y, Li Y (2015) Effects of alkali on iron-based catalysts for fischer-tropsch synthesis: CO chemisorptions study. J Mol Catal A-Chem 396:174–180
Gao X, Li J, Zheng M, Cai S, Zhang J, Askari S, Dewangan N, Ashok J, Kawi S (2021) Recent progress in anti-coking Ni catalysts for thermo-catalytic conversion of greenhouse gases. Process Saf Environ 156:598–616
Lalinde JAH, Roongruangsree P, Ilsemann J, Bäumer M, Kopyscinski J (2020) CO2 methanation and reverse water gas shift reaction. Kinetic study based on in situ spatially-resolved measurements. Chem Eng J 390:124629
Kreitz B, Sargsyan K, Blöndal K, Mazeau EJ, West RH, Wehinger GD, Turek T, Goldsmith CF (2021) Quantifying the impact of parametric uncertainty on automatic mechanism generation for CO2 hydrogenation on Ni(111). JACS Au 1:1656–1673
Navarro JC, Centeno MA, Laguna OH, Odriozola JA (2018) Policies and motivations for the CO2 valorization through the sabatier reaction using structured catalysts. A review of the most recent advances. Catalysts 8:578
Qiu M, Tao H, Li Y, Zhang Y (2019) Insight into the mechanism of CO2 and CO methanation over Cu(100) and Co-modified Cu(100) surfaces: a DFT study. Appl Surf Sci 495:143457
Wang F, He S, Chen H, Wang B, Zheng L, Wei M, Evans DG, Duan X (2016) Active site dependent reaction mechanism over Ru/CeO2 catalyst toward CO2 methanation. J Am Chem Soc 138:6298–6305
Zhang Z, Zhang X, Zhang L, Gao J, Shao Y, Dong D, Zhang S, Liu Q, Xu L, Hu X (2020) Impacts of alkali or alkaline earth metals addition on reaction intermediates formed in methanation of CO2 over cobalt catalysts. J Energy Inst 93:1581–1596
Pan Q, Peng J, Wang SS (2014) In situ FTIR spectroscopic study of the CO2 methanation mechanism on Ni/Ce0.5Zr0.5O2. Catal Sci Technol 4:502–509
Huynh HL, Zhu J, Zhang G, Shen Y, Tucho WM, Ding Y, Yu Z (2020) Promoting effect of Fe on supported Ni catalysts in CO2 methanation by in situ DRIFTS and DFT study. J Catal 392:266–277
Sajjadi SM, Haghighi M, Rahmani F (2014) Dry reforming of greenhouse gases CH4/CO2 over MgO-promoted Ni–Co/Al2O3–ZrO2 nanocatalyst: effect of MgO addition via sol-gel method on catalytic properties and hydrogen yield. J Sol-Gel Sci Technol 70:111–124
Guo L, Sun J, Ge Q, Tsubaki N (2018) Recent advances in direct catalytic hydrogenation of carbon dioxide to valuable C2+ hydrocarbons. J Mater Chem A 6:23244–23262
Zhang CY, Zhang RK, Liu HW, Wei QH, Gong DD, Mo LY, Tao HC, Cui S, Wang LH (2020) One-Step synthesis of highly dispersed and stable Ni nanoparticles confined by CeO2 on SiO2 for dry reforming of methane. Energies 13:5956
Zhang FS, Song ZL, Zhu JZ, Liu L, Sun J, Zhao XQ, Mao YP, Wang WL (2018) Process of CH4–CO2 reforming over Fe/SiC catalyst under microwave irradiation. Sci Total Environ 639:1148–1155
Zhang M, Zijlstra B, Filot IAW, Li F, Wang H, Li J, Hensen EJM (2020) A theoretical study of the reverse water–gas–shift reaction on Ni(111) and Ni(311) surfaces. Can J Chem Eng 98:740–748
Zhang TT, Liu ZX, Zhu YA, Liu ZC, Sui ZJ, Zhu KK, Zhou XG (2020) Dry reforming of methane on Ni–Fe–MgO catalysts: influence of Fe on carbon-resistant property and kinetics. Appl Catal B 264:118497
Chen X, Chen Y, Song C, Ji P, Wang N, Wang W, Cui L (2020) Recent advances in supported metal catalysts and oxide catalysts for the reverse water-gas shift reaction. Front Chem 8:709
Chen CS, Lin JH, You JH, Yang KH (2010) Effects of potassium on Ni−K/Al2O3 catalysts in the synthesis of carbon nanofibers by catalytic hydrogenation of CO2. J Phys Chem A 114:3773–3781
Chen WH, Chiu TW, Hung CI (2010) Hydrogen production from methane under the interaction of catalytic partial oxidation, water gas shift reaction and heat recovery. Int J Hydrog Energy 35:12808–12820
Nielsen DU, Hu XM, Daasbjerg K, Skrydstrup T (2018) Chemically and electrochemically catalysed conversion of CO2 to CO with follow-up utilization to value-added chemicals. Nat Catal 1:244–254
Arora S, Prasad R (2016) An overview on dry reforming of methane: strategies to reduce carbonaceous deactivation of catalysts. RSC Adv 6:108668–108688
Akpana E, Suna Y, Kumarb P, Ibrahima H, Aboudheirb A, Idema R (2007) Kinetics, experimental and reactor modeling studies of the carbon dioxide reforming of methane (CDRM) over a new Ni/CeO2–ZrO2 catalyst in a packed bed tubular reactor. Chem Eng Sci 62:4012–4024
Kawi S, Kathiraser Y, Ni J, Oemar U, Li Z, Saw ET (2015) Progress in synthesis of highly active and stable nickel-based catalysts for carbon dioxide reforming of methane. Chemsuschem 8:3556–3575
Tao X, Yang C, Huang L, Xu D (2020) DBD plasma combined with catalysts derived from NiMgAlCe hydrotalcite for CO2 reforming of CH4. Mater Chem Phys 250:123118
Olah GA, Goeppert A, Czaun M, Prakash GKS (2012) Bi-reforming of methane from any source with steam and carbon dioxide exclusively to metgas (CO−2H2) for methanol and hydrocarbon synthesis. J Am Chem Soc 135:648–650
Olah GA, Goeppert A, Czaun M, Mathew T, May RB, Prakash GKS (2015) Single step bi-reforming and oxidative bi-reforming of methane (natural gas) with steam and carbon dioxide to metgas (CO-2H2) for methanol synthesis: self-sufficient effective and exclusive oxygenation of methane to methanol with oxygen. J Am Chem Soc 137:8720–8729
Li M, Van Veen AC (2018) Coupled reforming of methane to syngas (2H2–CO) over Mg–Al oxide supported Ni catalyst. Appl Catal A 550:176–183
Lovell E, Jiang Y, Scott J, Wang F, Suhardja Y, Chen M, Huang J, Amal R (2014) CO2 reforming of methane over MCM-41-supported nickel catalysts: altering support acidity by one-pot synthesis at room temperature. Appl Catal A 473:51–58
Walker DM, Pettit SL, Wolan JT, Kuhn JN (2012) Synthesis gas production to desired hydrogen to carbon monoxide ratios by tri-reforming of methane using Ni–MgO–(Ce, Zr)O2 catalysts. Appl Catal A 445–446:61–68
Świrk K, Grzybek T, Motak M (2017) Tri-reforming as a process of CO2 utilization and a novel concept of energy storage in chemical products. E3S Web Conf 14:02038
Sukonket T, Khan A, Saha B, Ibrahim H, Tantayanon S, Kumar P, Idem R (2011) Influence of the catalyst preparation method, surfactant amount, and steam on CO2 reforming of CH4 over 5Ni/Ce0.6Zr0.4O2 catalysts. Energy Fuels 25:864–877
Rostrup-Nielsen JR, Hansen JHB (1993) CO2 reforming of methane over transition metals. J Catal 144:38–49
Song C, Pan W (2004) Tri-reforming of methane: a novel concept for catalytic production of industrially useful synthesis gas with desired H2/CO ratios. Catal Today 98:463–484
Pino L, Vita A, Cipitì F, Laganà M, Recupero V (2011) Hydrogen production by methane tri-reforming process over Ni–ceria catalysts: effect of La-doping. Appl Catal B 104:64–73
Barthomeuf D (1996) Basic zeolites: characterization and uses in adsorption and catalysis. Catal Rev Sci Eng 38:521–612
Corma A, Iborra S (2006) Optimization of alkaline earth metal oxide and hydroxide catalysts for base-catalyzed reactions. Adv Catal 49:239–302
Hattori H (1995) Heterogeneous basic catalysis. Chem Rev 95:537–558
Jia J, Qian C, Dong Y, Li YF, Wang H, Ghoussoub M, Butler KT, Walsh A, Ozin GA (2017) Heterogeneous catalytic hydrogenation of CO2 by metal oxides: defect engineering–perfecting imperfection. Chem Soc Rev 46:4631–4644
Boehm HP (1971) Acidic and basic properties of hydroxylated metal oxides surface. Discuss Faraday Soc 52:264–275
Pan QS, Peng JX, Sun TJ, Wang S, Wang SD (2014) Insight into the reaction route of CO2 methanation: promotion effect of medium basic sites. Catal Commun 45:74–78
Kim J, Park WH, Doh WH, Lee SW, Noh MC, Gallet JJ, Bournel F, Kondoh H, Mase K, Jung Y, Mun BS, Park JY (2018) Adsorbate-driven reactive interfacial Pt-NiO1−x nanostructure formation on the Pt3Ni(111) alloy surface. Sci Adv 4:eaat3151
Liang C, Zhang L, Zheng Y, Zhang S, Liu Q, Gao G, Dong D, Wang Y, Xu L, Hu X (2020) Methanation of CO2 over nickel catalysts: impacts of acidic/basic sites on formation of the reaction intermediates. Fuel 262:116521
Boffa A, Lin C, Bell AT, Somorjai GA (1994) Promotion of CO and CO2 hydrogenation over Rh by metal oxides: the influence of oxide Lewis acidity and reducibility. J Catal 149:149–158
Boffa AB, Lin C, Bell AT, Somorjai GA (1994) Lewis acidity as an explanation for oxide promotion of metals: implications of its importance and limits for catalytic reactions. Catal Lett 27:243–249
Cheng CK, Foo SY, Adesina AA (2010) Glycerol steam reforming over Bimetallic Co-Ni/Al2O3. Ind Eng Chem Res 49:10804–10817
Hattori H (1988) Catalysis by basic metal oxides. Mater Chem Phys 18:533–552
Hattori H (2001) Solid base catalysts: generation of basic sites and application to organic synthesis. Appl Catal A 222:247–259
Westermann A, Azambre B, Bacariza MC, Graça I, Ribeiro MF, Lopes JM, Henriques C (2015) Insight into CO2 methanation mechanism over NiUSY zeolites: an operando IR study. Appl Catal B 174–175:120–125
Benitez JJ, Carrizosa I, Odriozola JA (1993) HCOOH hydrogenation over Lanthanide-oxide-promoted Rh/Al2O3 catalyst. Appl Surf Sci 68:565–573
Phan TS, Sane AR, de Vasconcelos BR, Nzihou A, Sharrock P, Grouset D, Minh DP (2018) Hydroxyapatite supported bimetallic cobalt and nickel catalysts for syngas production from dry reforming of methane. Appl Catal B 224:310–321
Aziz MAA, Jalil AA, Wongsakulphasatch S, Vo DVN (2020) Understanding the role of surface basic sites of catalysts in CO2 activation in dry reforming of methane: a short review. Catal Sci Technol 10:35–45
Debek R, Motak M, Galvez ME, Grzybek T, Da Costa P (2017) Influence of Ce/Zr molar ratio on catalytic performance of hydrotalcite-derived catalysts at low temperature CO2 methane reforming. Int J Hydrog Energy 42:23556–23567
Zhang J, Yin R, Shao Q, Zhu T, Huang X (2019) Oxygen vacancies in amorphous InOx nanoribbons enhance CO2 adsorption and activation for CO2 electroreduction. Angew Chem Int Ed 58:5609–5613
Budiman AW, Song SH, Chang TS, Shin CH, Choi MJ (2012) Dry reforming of methane over cobalt catalysts: a literature review of catalyst development. Catal Surv Asia 16:183–197
Liu L, Zhao H, Andino JM, Li Y (2012) Photocatalytic CO2 reduction with H2O on TiO2 nanocrystals: comparison of anatase, rutile, and brookite polymorphs and exploration of surface chemistry. ACS Catal 2:1817–1828
Pan Q, Peng J, Wang S, Wang SD (2014) In situ FTIR spectroscopic study of the CO2 methanation mechanism on Ni/Ce0.5Zr0.5O2. Catal Sci Technol 4:502–509
Ye J, Liu C, Mei D, Ge Q (2013) Active oxygen vacancy site for methanol synthesis from CO2 hydrogenation on In2O3(110): a DFT study. ACS Catal 3:1296–1306
Tabatabaei J, Sakakini BH, Waugh KC (2006) On the mechanism of methanol synthesis and the water-gas shift reaction on ZnO. Catal Lett 110:77–84
Liu ZY, Grinter DC, Lustemberg PG, Nguyen-Phan TD, Zhou YH, Luo S, Waluyo I, Crumlin EJ, Stacchiola DJ, Zhou J, Carrasco J, Fabio Busnengo H, Verónica Ganduglia-Pirovano M, Senanayake SD, Rodriguez JA (2016) Dry reforming of methane on a highly-active Ni–CeO2 catalyst: effects of metal-support interactions on C−H bond breaking. Angew Chem Int Ed 55:7455–7459
Abdulrasheed A, Jalil AA, Gambo Y, Ibrahim M, Hambali HU, Hamid MYS (2019) A review on catalyst development for dry reforming of methane to syngas: recent advances. Renew Sustain Energy Rev 108:175–193
Macario A, Frontera P, Candamano S, Crea F, Luca PD, Antonucci PL (2019) Nanostructured catalysts for dry-reforming of METHANE. J Nanosci Nanotechnol 19:3135–3147
Khoja AH, Tahir M, Amin NAS (2018) Cold plasma dielectric barrier discharge reactor for dry reforming of methane over Ni/ɤ-Al2O3–MgO nanocomposite. Fuel Process Technol 178:166–179
Yang H, Xu L, Chen M, Lv C, Cui Y, Wen X, Wu C, Yang B, Miao Z, Hu X, Shou Q (2020) Facilely fabricating highly dispersed Ni-based catalysts supported on mesoporous MFI nanosponge for CO2 methanation. Micropor Mesopor Mat 302:110250
Le TA, Kim J, Kang JK, Park DE (2020) CO and CO2 methanation over Ni/Al@Al2O3 core–shell catalyst. Catal Today 365:622–630
Hussain I, Jalil AA, Hassan NS, Hambali HU, Jusoh NWC (2020) Fabrication and characterization of highly active fibrous silica-mordenite (FS@SiO2-MOR) cockscomb shaped catalyst for enhanced CO2 methanation. Chem Eng Sci 228:115978
Jin B, Li S, Liang X (2021) Enhanced activity and stability of MgO-promoted Ni/Al2O3 catalyst for dry reforming of methane: role of MgO. Fuel 284:119082
Wang H, Zhao B, Qin L, Wang Y, Yu F, Han J (2020) Non-thermal plasma-enhanced dry reforming of methane and CO2 over Ce promoted Ni/C catalysts. Mol Catal 485:110821
Guo Y, Tian L, Yan W, Qi R, Tu W, Wang Z (2021) CeO2-promoted Ni/SiO2 catalysts for carbon dioxide reforming of methane: the effect of introduction methodologies. Catal Lett 151:2144–2152
Lino AVP, Calderon YNC, Mastelaro VR, Assaf EM, Assaf JM (2019) Syngas for Fischer-Tropsch synthesis by methane tri-reforming using nickel supported on MgAl2O4 promoted with Zr, Ce and Ce–Zr. Appl Surf Sci 481:727–761
Zhang Z, Zhao G, Bi G, Guo Y, Xie J (2021) Monolithic SiC-foam supported Ni–La2O3 composites for dry reforming of methane with enhanced carbon resistance. Fuel Process Technol 212:106627
Al-Fatesh AS, Arafat Y, Ibrahim AA, Atia H, Fakeeha AH, Armbruster U, Abasaeed AE, Frusteri F (2018) Evaluation of Co–Ni/Sc–SBA-15 as a novel coke resistant catalyst for syngas production via CO2 reforming of methane. Appl Catal A 567:102–111
Pan XY, Kuai P, Liu Y, Ge Q, Liu CJ (2010) Promotion effects of Ga2O3 on CO2 adsorption and conversion over a SiO2-supported Nicatalyst. Energy Environ Sci 3:1322–1325
Świrk K, Gálvez ME, Motak M, Grzybek T, Rønning M, Da Costa P (2018) Yttrium promoted Ni-based double-layered hydroxides for dry methane reforming. J CO2 Util 27:247258
Ghani NAA, Azapour A, Muhammad AFS, Abdullah B (2019) Dry reforming of methane for hydrogen production over Ni–Co catalysts: effect of Nb–Zr promoters. Int J Hydrog Energ 44:20881–20888
Boukha Z, Yeste MP, Cauqui MÁ, González-Velasco JR (2019) Influence of Ca/P ratio on the catalytic performance of Ni/hydroxyapatite samples in dry reforming of methane. Appl Catal A 580:34–45
Proaño L, Tello E, Arellano-Trevino MA, Wang S, Farrauto RJ, Cobo M (2019) In-situ DRIFTS study of two-step CO2 capture and catalytic methanation over Ru, “Na2O”/Al2O3 dual functional material. Appl Surf Sci 479:25–30
Bermejo-López A, Pereda-Ayo B, González-Marcos JA, González-Velasco JR (2019) Ni loading effects on dual function materials for capture and in-situ conversion of CO2 to CH4 using CaO or Na2CO3. J CO2 Util 34:576–587
Xu L, Wang F, Chen M, Yang H, Nie D, Qi L, Lian X (2017) Alkaline-promoted Ni based ordered mesoporous catalysts with enhanced low-temperature catalytic activity toward CO2 methanation. RSC Adv 7:18199–18210
Makdee A, Chanapattharapol KC, Kidkhunthod P, Poo-arporn Y, Ohno T (2020) The role of Ce addition in catalytic activity enhancement of TiO2-supported Ni for CO2 methanation reaction. RSC Adv 10:26952–26971
Gac W, Zawadzki W, Rotko M, Slowik G, Greluk M (2019) CO2 Methanation in the presence of Ce-promoted alumina supported nickel catalysts: H2S deactivation studies. Top Catal 62:524–534
Li Y, Men Y, Liu S, Wang J, Wang K, Tang Y, An W, Pan X, Li L (2021) Remarkably efficient and stable Ni/Y2O3 catalysts for CO2 methanation: effect of citric acid addition. Appl Catal B 293:120206
He L, Lin Q, Liu Y, Huang Y (2014) Unique catalysis of Ni-Al hydrotalcite derived catalyst in CO2 methanation: cooperative effect between Ni nanoparticles and a basic support. J Energy Chem 23:587–592
Zhou Y, Jiang Y, Qin Z, Xie Q, Ji H (2018) Influence of Zr, Ce, and La on Co3O4 catalyst for CO2 methanation at low temperature. Chinese J Chem Eng 26:768–774
Cai M, Wen J, Chu W, Cheng X, Li Z (2011) Methanation of carbon dioxide on Ni/ZrO2–Al2O3 catalysts: effects of ZrO2 promoter and preparation method of novel ZrO2–Al2O3 carrier. J Nat Gas Chem 20:318–324
Santos J, Bobadilla L, Centeno M, Odriozola J (2018) Operando DRIFTS–MS study of WGS and rWGS reaction on biochar-based Pt catalysts: the promotional effect of Na. C 4:47
Machocki A, Ioannides T, Stasinska B, Gac W, Avgouropoulos G, Delimaris D, Grzegorczyk W, Pasieczna S (2004) Manganese–lanthanum oxides modified with silver for the catalytic combustion of methane. J Catal 227:282–296
Wu Q, Christensen JM, Chiarello GL, Duchstein LD, Wagner JB, Temel B, Grunwaldt JD, Jensen AD (2013) Supported molybdenum carbide for higher alcohol synthesis from syngas. Catal Today 215:162–168
Li T, Virginie M, Khodakov AY (2017) Effect of potassium promotion on the structure and performance of alumina supported carburized molybdenum catalysts for Fischer-Tropsch synthesis. Appl Catal A 542:154–162
Abdullah N, Ainirazali N, Chong CC, Razak HA, Setiabudi HD, Jalil AA, Vo DVN (2020) Influence of impregnation assisted methods of Ni/SBA-15 for production of hydrogen via dry reforming of methane. Int J Hydrog Energy 45:18426–18439
Nikolaraki E, Goula G, Panagiotopoulou P, Taylor MJ, Kousi K, Kyriakou G, Kondarides DI, Lambert RM, Yentekakis IV (2021) Support induced effects on the Ir nanoparticles activity, selectivity and stability performance under CO2 reforming of methane. Nanomaterials 11:2880
Quindimil A, De-La-Torre U, Pereda-Ayo B, González-Marcos JA, González-Velasco JR (2018) Ni catalysts with La as promoter supported over Y- and BETA- zeolites for CO2 methanation. Appl Catal B 238:393–403
Garbarino G, Wang C, Cavattoni T, Finocchio E, Riani P (2019) Flytzani-Stephanopoulos, M.; Busca, G. A study of Ni/La–Al2O3 catalysts: a competitive system for CO2 methanation. Appl Catal B 248:286–297
Eckle S, Anfang HG, Behm RJ (2010) Reaction intermediates and side products in the methanation of CO and CO2 over supported Ru catalysts in H2-rich reformate gases. J Phys Chem C 115:1361–1367
Wang X, Bai X, Guo Y, Liu Q, Ji S, Wang Z (2021) A nanoscale Ni/ZrO2 catalyst coated with Al2O3 for carbon dioxide reforming of methane. J Chem Technol Biot 96:474–480
González-Castano M, de Miguel JCN, Sinha F, Wabo SG, Klepel O, Arellano-Garcia H (2021) Cu supported Fe–SiO2 nanocomposites for reverse water gas shift reaction. J CO2 Util 46:101493
Zhang M, Zhang JF, Zhou ZL, Chen SY, Zhang T, Song F, Zhang Q, Tsubaki N, Tan Y, Han Y (2020) Effects of the surface adsorbed oxygen species tuned by rare-earth metal doping on dry reforming of methane over Ni/ZrO2 catalyst. Appl Catal B 264:118522
Gao X, Wang Z, Ashok J, Kawi S (2020) A comprehensive review of anti-coking, anti-poisoning and anti-sintering catalysts for biomass tar reforming reaction. Chem Eng Sci 7:100065
Silaghi MC, Comas-Vives A, Coperet C (2016) CO2 Activation on Ni/γ–Al2O3 catalysts by first-principles calculations: From ideal surfaces to supported nanoparticles. ACS Catal 6:4501–4505
Rodriguez JA, Liu P, Stacchiola DJ, Senanayake SD, White MG, Chen JG (2015) Hydrogenation of CO2 to methanol: Importance of metal–oxide and metal–carbide interfaces in the activation of CO2. ACS Catal 5:6696–6706
Liang C, Ye Z, Dong D, Zhang S, Liu Q, Chen G, Li C, Wang Y, Hu X (2019) Methanation of CO2: Impacts of modifying nickel catalysts with variable valence additives on reaction mechanism. Fuel 254:115–654
Dedov AG, Loktev AS, Komissarenko DA, Parkhomenko KV, Roger AC, Shlyakhtin OA, Mazo GN, Moiseev II (2016) High-selectivity partial oxidation of methane into synthesis gas: the role of the red-ox transformations of rare earth - alkali earth cobaltate-based catalyst components. Fuel Process Technol 148:128–137
Jia C, Dai Y, Yang Y, Chew J (2019) Nickel-cobalt catalyst supported on TiO2-coated SiO2 spheres for CO2 methanation in a fluidized bed. Int J Hydrog Energy 44:13443–13455
Xu L, Wen X, Chen M, Lv C, Cui Y, Wu X, Wu C, Yang B, Miao Z, Hu X (2020) Mesoporous Ce–Zr solid solutions supported Ni-based catalysts for low-temperature CO2 methanation by tuning the reaction intermediates. Fuel 282:118813
Guo D, Lu Y, Ruan Y, Zhao Y, Zhao Y, Wang S, Ma X (2020) Effects of extrinsic defects originating from the interfacial reaction of CeO2-x-nickel silicate on catalytic performance in methane dry reforming. Appl Catal B 277:119–278
Jin B, Shang Z, Li S, Jiang YB, Gu X, Liang X (2020) Reforming of methane with carbon dioxide over cerium oxide promoted nickel nanoparticles deposited on 4-channel hollow fibers by atomic layer deposition. Catal Sci Technol 10:3212–3222
Unwiset P, Chanapattharapol KC, Kidkhunthod P, Poo-arporn Y, Ohtani B (2020) Catalytic activities of titania-supported nickel for carbon-dioxide methanation. Chem Eng Sci 228:115955
Kim MJ, Youn JR, Kim HJ, Seoc MW, Leec D, Goc KS, Leea KB, Jeonc SG (2020) Effect of surface properties controlled by Ce addition on CO2 methanation over Ni/Ce/Al2O3 catalyst. Int J Hydrog Energy 45:24595–24603
Siakavelasa GI, Charisioua ND, Alkhoorid S, Alkhooric AA, Sebastiane V, Hinderg SJ, Bakerg MA, Yentekakish IV, Polychronopoulouc K, Goula MA (2021) Highly selective and stable nickel catalysts supported on ceria promoted with Sm2O3, Pr2O3 and MgO for the CO2 methanation reaction. Appl Catal B 282:119562
Liu K, Xu X, Xu J, Fang X, Liu L, Wang X (2020) The distributions of alkaline earth metal oxides and their promotional effects on Ni/CeO2 for CO2 methanation. J CO2 Util 38:113–124
Wang W, Zhang Y, Wang Z, Yan JM, Ge Q, Liu CJ (2016) Reverse water gas shift over In2O3–CeO2 catalysts. Catal Today 259:402–408
Wang LC, Widmann D, Behm RJ (2015) Reactive removal of surface oxygen by H2, CO and CO/H2 on a Au/CeO2 catalyst and its relevance to the preferential CO oxidation (PROX) and reverse water gas shift (RWGS) reaction. Catal Sci Technol 5:925–941
Yentekakis IV, Goula G, Hatzisymeon M, Betsi-Argyropoulou I, Botzolaki G, Kousi K, Kondarides DI, Taylor MJ, Parlett CMA, Osatiashtiani A, Kyriakou G, Holgado JP, Lambert RM (2019) Effect of support oxygen storage capacity on the catalytic performance of Rh nanoparticles for CO2 reforming of methane. Appl Catal B 243:490–501
Asencios YJO, Assaf EM (2013) Combination of dry reforming and partial oxidation of methane on NiO–MgO–ZrO2 catalyst: effect of nickel content. Fuel Process Technol 106:247–252
Sumarasingha W, Supasitmongkol S, Phongaksorn M (2021) The effect of ZrO2 as different components of Ni-based catalysts for CO2 reforming of methane and combined steam and CO2 reforming of methane on catalytic performance with coke formation. Catalysts 11:984
Yang Z, Zeng M, Wang K, Yue X, Chen X, Dai W, Fu X (2022) Visible light-assisted thermal catalytic reverse water gas reaction over Cu–CeO2: the synergistic of hot electrons and oxygen vacancies induced by LSPR effect. Fuel 315:123186
Wang L, Zhang S, Liu Y (2008) Reverse water gas shift reaction over Co-precipitated Ni–CeO2 catalysts. J Rare Earths 26:66–70
Li B, Yuan X, Li B, Wang X (2021) Ceria-modified nickel supported on porous silica as highly active and stable catalyst for dry reforming of methane. Fuel 301:121027
Pino L, Italiano C, Vita A, Laganà M, Recupero V (2017) Ce0.70La0.20Ni0.10O2-δ catalyst for methane dry reforming: influence of reduction temperature on the catalytic activity and stability. Appl Catal B 218:779–792
Sun N, Wen X, Wang F, Peng W, Zhao N, Xiao FK, Wei W, Sun YH, Kang JT (2011) Catalytic performance and characterization of Ni–CaO–ZrO2 catalysts for dry reforming of methane. Appl Surf Sci 257:9169–9176
Zhang M, Zhang JF, Wu YQ, Pan JX, Zhang QD, Tan YS, Han YZ (2019) Insight into the effects of the oxygen species over Ni/ZrO2 catalyst surface on methane reforming with carbon dioxide. Appl Catal B 244:427–437
Acknowledgements
This research was funded by Green Energy Program (WBS: A-0005323-05-00), FRC MOE T1 (WBS: A-0009184-00-00), A*STAR LCERFI Project (Award ID: U2102d2011; WBS No. A-8000278-00-00), Guangzhou Basic and Applied Basic Research Project in China: 202102020134; Youth Innovation Talents Project of Guangdong Universities (natural science): 2019KQNCX098.
Author information
Authors and Affiliations
Contributions
Conceptualization, data curation, data analysis, writing—original draft, X.G., P.C., Z.W, and X.L; writing—review and editing; X.G.; project administration, supervision, validation, S.K.; funding acquisition, resources, S.K. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, X., Cai, P., Wang, Z. et al. Surface Acidity/Basicity and Oxygen Defects of Metal Oxide: Impacts on Catalytic Performances of CO2 Reforming and Hydrogenation Reactions. Top Catal 66, 299–325 (2023). https://doi.org/10.1007/s11244-022-01708-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-022-01708-0