Abstract
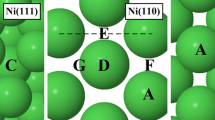
Pursuing an environmentally friendly alternative to the Haber–Bosch process, using density functional theory (DFT) we explore further the merit of transition metal nitride (TMN) surfaces as catalysts for the nitrogen reduction reaction (NRR). Prior studies by our group have investigated the (100) and (111) facets of these TMNs and found the (100) to be favored in all cases. Many of the (100) facets of TMNs showed promising activity for ammonia (NH3) formation. Recent experiments investigating the polycrystalline growth of these TMNs indicated the substantial presence of the (110) facet as well, and so we explore the properties of this surface orientation and compare it to the (100) facets previously reported. The only (110) facets of TMN that showed any promise was VN, but it suffered from high activation energies for N2 adsorption compared to the activation energy of N migration from the bulk, which would likely lead to decomposition of the catalyst. The (110) facets showed overall worse catalytic activity than the (100). The most important outcome of this study is that the presence of the (110) facet of these TMNs in the catalyst structure will be detrimental to the activity of the catalyst. Great care must be taken when investigating the performance of these catalysts by engineering the surface to only include the surface orientation of interest.






Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code Availability
The VASP code was used for the DFT calculations of this manuscript and the corresponding details of that are provided in the manuscript.
References
The Facts About Ammonia (2004) New York State. https://www.health.ny.gov/environmental/emergency/chemical_terrorism/ammonia_tech.htm#:~:text=Ammonia%20is%20also%20used%20as,and%20industrial%2Dstrength%20cleaning%20solutions. Accessed 22 Mar 2021
Chen S et al (2019) Horizons in sustainable industrial chemistry and catalysis. Elsevier, Amsterdam. https://doi.org/10.1016/B978-0-444-64127-4.00002-1
Valera-Medina A et al (2018) Ammonia for power. Prog Energy Combust Sci. https://doi.org/10.1016/j.pecs.2018.07.001
Qing G et al (2020) Recent advances and challenges of electrocatalytic N2 reduction to ammonia. Chem Rev. https://doi.org/10.1021/acs.chemrev.9b00659
Guo X et al (2019) Recent progress in electrocatalytic nitrogen reduction. J Mater Chem A. https://doi.org/10.1039/C8TA11201K
Lee J, Tan LL, Chai SP (2021) Heterojunction photocatalysts for artificial nitrogen fixation: fundamentals, latest advances and future perspectives. Nanoscale. https://doi.org/10.1039/D1NR00783A
Shen H et al (2021) Electrochemical ammonia synthesis: mechanistic understanding and catalyst design. Chem. https://doi.org/10.1016/j.chempr.2021.01.009
Rouwenhorst KHR et al (2021) Techno-economic challenges of green ammonia as an energy vector, 1st edn. Academic Press, Cambridge. https://doi.org/10.1016/B978-0-12-820560-0.00004-7
Xue X, Chen R, Yan C et al (2019) Review on photocatalytic and electrocatalytic artificial nitrogen fixation for ammonia synthesis at mild conditions: advances, challenges and perspectives. Nano Res. https://doi.org/10.1007/s12274-018-2268-5
Giddey S, Badwal SPS, Kulkarni A (2013) Review of electrochemical ammonia production technologies and materials. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2013.09.054
Liu PY et al (2021) Enhanced electrocatalytic nitrogen reduction reaction performance by interfacial engineering of MOF-based sulfides FeNi2S4/NiS hetero-interface. Appl Catal B Environ. https://doi.org/10.1016/j.apcatb.2021.119956
Zheng J et al (2021) Structural insight into [Fe–S2–Mo] motif in electrochemical reduction of N2 over Fe1-supported molecular MoS2. Chem Sci. https://doi.org/10.1039/D0SC04575F
Nørskov JK et al (2004) Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J Phys Chem B. https://doi.org/10.1021/jp047349j
Montoya JH, Tsai C, Vojvodic A, Nørskov JK (2015) The challenge of electrochemical ammonia synthesis: a new perspective on the role of nitrogen scaling relations. Chemsuschem. https://doi.org/10.1002/cssc.201500322
Skúlason E et al (2012) A theoretical evaluation of possible transition metal electrocatalysts for N2 reduction. Phys Chem Chem Phys. https://doi.org/10.1039/C1CP22271F
Doornkamp C, Ponec V (2000) The universal character of the Mars and Van Krevelen mechanism. J Mol Catal A Chem. https://doi.org/10.1016/S1381-1169(00)00319-8
Yang X et al (2018) Mechanistic insights into electrochemical nitrogen reduction reaction on vanadium nitride nanoparticles. J Am Chem Soc. https://doi.org/10.1021/jacs.8b08379
Abghoui Y, Skúlason E (2017) Electrochemical synthesis of ammonia via Mars-van Krevelen mechanism on the (111) facets of group III–VII transition metal mononitrides. Catal Today. https://doi.org/10.1016/j.cattod.2016.06.009
Abghoui Y et al (2015) Enabling electrochemical reduction of nitrogen to ammonia at ambientconditions through rational catalyst design. Phys Chem Chem Phys. https://doi.org/10.1039/C4CP04838E
Abghoui Y et al (2016) Electroreduction of N2 to ammonia at ambient conditions on mononitrides of Zr, Nb, Cr, and V: A DFT guide for experiments. ACS Catal. https://doi.org/10.1021/acscatal.5b01918
Zhang L et al (2018) Efficient electrochemical N2 reduction to NH3 on MoN nanosheets array under ambient conditions. ACS Sustain Chem Eng. https://doi.org/10.1021/acssuschemeng.8b01438
Guo W et al (2021) Understanding the lattice nitrogen stability and deactivation pathways of cu-bic CrN nanoparticles in the electrochemical nitrogen reduction reaction. J Mater Chem A. https://doi.org/10.1039/D0TA11727G
Zhang X et al (2018) Highly efficient electrochemical ammonia synthesis via nitrogen reduction reactions on a VN nanowire array under ambient conditions. Chem Commun. https://doi.org/10.1039/C8CC00459E
Hanifpour F et al (2021) Experimental performance of theoretically derived transition metal nitrides as catalysts for electrochemical reduction of N2 to NH3. Submitted
Hammer B, Hansen LB, Nørskov JK (1999) Improved adsorption energetics within densityfunctional theory using revised Perdew-Burke-Ernzerhof functionals. Phys Rev B. https://doi.org/10.1103/PhysRevB.59.7413
Kresse G, Furthmüller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev. https://doi.org/10.1103/PhysRevB.54.11169
Blöchl PE (1994) Projector augmented-wave method. Phys Rev B. https://doi.org/10.1103/PhysRevB.50.17953
Henkelman G, Uberuaga BP, Jónsson H (2000) A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J Chem Phys. https://doi.org/10.1063/1.1329672
Greenlee LF, Renner JN, Foster SL (2018) The use of controls for consistent and accurate measurements of electrocatalytic ammonia synthesis from dinitrogen. ACS Catal. https://doi.org/10.1021/acscatal.8b02120
Abghoui Y, Skúlason E (2017) Hydrogen evolution reaction catalyzed by transition-metal nitrides. J Phys Chem C. https://doi.org/10.1021/acs.jpcc.7b06811
Abghoui Y (2021) Superiority of the (100) over the (111) facets of the nitrides for hydrogen evolution reaction. Top Catal. https://doi.org/10.1007/s11244-021-01474-5
Pan J, Hansen HA, Vegge T (2020) Vanadium oxynitrides as stable catalysts for electrochemical reduction of nitrogen to ammonia: the role of oxygen. J Mater Chem A. https://doi.org/10.1039/D0TA08313E
Abghoui Y, Skúlason E (2017) Onset potentials for different reaction mechanisms of nitrogen activation to ammonia on transition metal nitride electro-catalysts. Catal Today. https://doi.org/10.1016/j.cattod.2016.11.047
Acknowledgements
Financial support is acknowledged from the Icelandic Research Fund (Grant Numbers 185051-051 and 196437-051) and the Research Fund of the University of Iceland. The calculations were carried out on the Icelandic high-performance computer, Garpur.
Funding
Icelandic research Fund (Grant Numbers 185051-051 and 196437-051), and research fund of the University of Iceland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gudmundsson, M., Ellingsson, V., Skúlason, E. et al. Optimizing Nitrogen Reduction Reaction on Nitrides: A Computational Study on Crystallographic Orientation. Top Catal 65, 252–261 (2022). https://doi.org/10.1007/s11244-021-01485-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-021-01485-2