Abstract
Recent results from inelastic neutron scattering (INS) measurements are reviewed that show the synergy between research on hydrogen adsorption/absorption on/in catalysts and chemical engineering. The proton dynamics of catalysts for large scale reactor technology are compared. The focus is the identification and quantification of hydrogenous species on, and inside, fresh, hydrogenated and dehydrogenated palladium black and supported palladium catalysts of different particle size, type of support and, therefore, morphology. INS studies of in-situ hydrogenation/ dehydrogenation of catalysts of 25–60 g size per sample are carried out in stainless steel cans as a function of varying hydrogen pressures. Differences in hysteresis effects in retaining hydrogen inside the catalyst in desorption from palladium hydrides and hydrogen bonding in adsorption sites in varying particle morphologies on different supports were evaluated. This aids the understanding of hydrogen/palladium interactions in catalytic processes with varying local hydrogen partial pressure, e.g. in loop reactors. The importance of the measured on-top Pd–H site in catalytic hydrogenation reactions is discussed together with previous INS results on the degree of deactivation of a technical catalyst by molecular blocking of the on-top site.


Reproduced from [12]

Top part reproduced from [12] under a Creative Commons Attribution Unported License 3.0 (CC BY)

Reproduced from [13] under a Creative Commons Attribution Unported License 3.0 (CC BY)

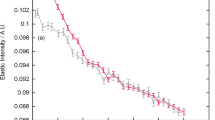
Reproduced from [13] under a Creative Commons Attribution Unported License 3.0 (CC BY)

Reproduced from [13] under a Creative Commons Attribution Unported License 3.0 (CC BY)

Reproduced from [13] under a Creative Commons Attribution Unported License 3.0 (CC BY)
Similar content being viewed by others
References
Albers PW, Lennon D, Parker SF (2017) Catalysis. In: Fernandez-Alonso F, Price DL (eds) Experimental methods in the physical sciences, neutron scattering: applications in chemistry, materials science, and biology, vol 49. Academic Press, Oxford, pp 281–350
Ruth K, Albers P (2018) Materials for solid catalysts. In: Warlimont H, Martienssen W (eds) Springer handbook of materials data, 2nd edn. Springer, Cham, pp 935–955
Richter D, Hempelmann R, Bowman RC Jr (1992) Dynamics of hydrogen in intermetallic compounds. In: Schlapbach L (ed) Hydrogen in intermetallic compounds ii, surface and dynamic properties, applications, topics in applied physics, vol 67. Springer, Berlin, pp 97–164
Ross DK (1997) Neutron scattering studies of metal-hydrogen systems. In: Wipf H (ed) Hydrogen in metals III: properties and applications topics in applied physics, vol 73. Springer, Berlin, pp 153–214
Celli M, Colognesi D, Zoppi M (2009) Hydrogen and hydrogen-storage materials. In: Liang L, Rinaldi R, Schober H (eds) Neutron applications in earth, energy and environmental sciences. Springer, Cham, pp 417–438
Ross DK, Roach DL (2016) Inelastic and quasi-elastic neutron scattering. In: Fritzsche H, Huot J, Fruchard D (eds) Neutron scattering and other nuclear techniques for hydrogen in materials. Springer, Cham, pp 245–276
Drexel W, Murani A, Tocchetti D, Kley W, Sosnowska I, Ross DK (1976) The motions of hydrogen impurities in α-palladium-hydride. J Phys Chem Solids 37:1135–1139
Ross DK, Martin PF, Oates WA, Khoda Bakhsh R (1979) Inelastic neutron scattering measurements of optical vibration frequency distributions in hydrogen-metal systems. Z Phys Chem 114:221–230
Wicke E, Brodowski H (1978) Hydrogen in palladium and palladium alloys. In: Alefeld G, Völkl J (eds) Hydrogen in metals II: application-oriented properties, topics in applied physics, vol 29. Springer, Berlin, pp 73–155
Parker SF, Lennon D, Albers PW (2011) Vibrational spectroscopy with neutrons—new directions. Appl Spectrosc 65:1325–1341
Parker SF, Ramirez-Cuesta AJ, Albers PW, Lennon D (2014) The use of direct geometry spectrometers in molecular spectroscopy. DMMII, J Phys Conf Ser 554:012004
Parker SF, Adroja D, Jimenez-Ruiz M, Tischer M, Möbus K, Wieland SD, Albers P (2016) Characterisation of the surface of freshly prepared precious metal catalysts. Phys Chem Chem Phys 18:17196–17201
Parker SF, Walker HC, Callear SK, Grünewald E, Petzold T, Wolf D, Möbus K, Adam J, Wieland SD, Jiménez-Ruiz M, Albers PW (2019) The effect of particle size, morphology and support on the formation of palladium hydride in commercial catalysts. Chem Sci 10:480–489
Kosak JR (1996) Precious metal synergism in catalytic hydrogenation. In: Malz RE Jr. (ed) Catalysis of organic reactions (chemical industries). Dekker, New York, pp 31–41
Booth G (2007) Nitro compounds, aromatic. Ullmann’s encyclopedia of industrial chemistry, vol 24, 7th edn. Wiley-VCH, Weinheim, pp 301–350
Randall D, Lee S (2002) The polyurethanes book. Wiley, New York
Parker GL, Smith LK, Baxendale IR (2016) (2016) Development of the industrial synthesis of vitamin A. Tetrahedron 72:1645–1652
Weigert W (1978) Wasserstoffperoxid und seine Derivate: Chemie u. Anwendungen. Hüthig-Verlag, Heidelberg
Jones CW (1999) Applications of hydrogen peroxide and derivatives. RSC clean technology monographs. Royal Society of Chemistry, Cambridge
Chen Q (2008) Development of an anthraquinone process for the production of hydrogen peroxide in a trickle bed reactor—from bench scale to industrial scale. Chem Eng Proc: Proc Intensification 47:787–792
Goor G, Glenneberg J, Jacobi S (2012) Hydrogen peroxide. Ullmanns’s encyclopedia of industrial chemistry, vol 18, 7th edn. Wiley-VCH, Weinheim, pp 393–427
Duveen RF (1998) High performance gas-liquid reaction technology. HH Technology Corp., Sissach, Switzerland, http://www.hhcorp.net/PDF/Rene_High_Performance.pdf. Accessed 28 Jan 2021
Warmeling H, Behr A, Vorholt AJ (2016) Jet loop reactors as a versatile reactor set up—intensifying catalytic reactions: a review. Chem Eng Sci 140:229–248
Wood J (2016) Three-phase catalytic reactors for hydrogenation and oxidation reactions. Phys Sci Rev 1:20150019
Satterfield CN (1975) Trickle-bed reactors. AIChE J 21:209–228
Gelder EA, Jackson SD, Lok CM (2002) A study of nitrobenzene hydrogenation over palladium/carbon catalysts. Catal Lett 84:205–208
Pundt A, Suleiman M, Bähtz C, Reetz MT, Kirchheim R, Jisrawi NM (2004) hydrogen and Pd-clusters. Mat Sci Eng B 108:19–23
Pundt A, Sachs C, Winter M, Reetz MT, Frisch D, Kirchheim R (1999) Hydrogen sorption in elastically soft stabilized Pd-clusters. J Alloys Compd 293–295:480–483
Sachs C, Pundt A, Kirchheim R, Winter M, Reetz MT, Frisch D (2001) Solubility of hydrogen in single-sized palladium clusters. Phys Rev B 64:075408
Suleiman M, Jisrawi NM, Dankert O, Reetz MT, Bähtz C, Kirchheim R (2003) Phase transition and lattice expansion during hydrogen loading of nanometer sized palladium clusters. J Alloys Compd 356(357):644–648
Robinson IK (2015) Computational studies of hydrogen in palladium. PhD Thesis, University of Salford
Suzana A, Wu L, Assafa T, Williams B, Harder R, Cha W, Kuo C-H, Tsung C-K, Robinson I (2020) Structure of a single nanoparticle and its dynamics during the hydride phase transformation. Brookhaven National Lab, Upton. https://doi.org/10.21203/rs.3.rs-65592/v1
Houari A, Matar SF, Eyert V (2014) Electronic structure and crystal phase stability of palladium hydrides. J Appl Phys 116:173706
Briggs D, Seah MP (1990) Practical surface analysis. Auger and X-ray photoelectron spectroscopy, vol 1, 2nd edn. Wiley, Chichester, pp 613–634 (Literature cited therein)
Parker SF, Refson K, Hannon AC, Barney ER, Robertson SJ, Albers P (2010) Characterisation of hydrous palladium oxide: Implications for low temperature carbon monoxide oxidation. J Phys Chem C 114:14164–14171
Albers PW, Möbus K, Wieland SD, Parker SF (2015) The fine structure of Pearlman’s catalyst. Phys Chem Chem Phys 17:5274–5278
Borodziński A, Bond GC (2006) Selective hydrogenation of ethyne in ethene-rich streams on palladium catalysts. Part 1. Effects of changes to catalyst during reaction. Catal Rev 48:91–144
Parker SF, Bowron DT, Imberti S, Soper AK, Refson K, Lox ES, Lopez M, Albers P (2010) Structure determination of adsorbed hydrogen on a real catalyst. Chem Commun 46:2959–2961
Loewert M, Serrer M-A, Carambia T, Stehle M, Zimina A, Kalz KF, Lichtenberg H, Saraçi E, Pfeifer P, Grunwaldt JD (2020) Bridging the gap between industry and synchrotron: an operando study at 30 bar over 300 h during Fischer-Tropsch synthesis. React Chem Eng 5:1071–1082
Davidson AL, Lennon D, Webb PB, Albers PW, Berweiler M, Poss R, Roos M, Reinsdorf A, Wolf D, Parker SF (2021) The characterization of hydrogen on nickel and cobalt catalysts. Topics Catal (accepted for publication)
Albers P, Angert H, Prescher G, Seibold K, Parker SF (1999) Catalyst poisoning by methyl groups. Chem. Commun. 17:1619–1620
Albers P, Pietsch J, Parker SF (2001) Poisoning and deactivation of palladium catalysts. J Mol Catal A 173:275–286
Albers PW, Parker SF (2007) Inelastic incoherent neutron scattering in catalysis research. Adv Catal 51:99–132
Schulz H (2003) major and minor reactions in Fischer-Tropsch synthesis on cobalt catalysts. Top Catal 26:73–85
Paul JF, Sautet P (1998) Chemisorption and transformation of CHx fragments (x=0-3) on a Pd(111) surface: a periodic density functional study. J Phys Chem B 102:1578–1585
Kellner CS, Bell AT (1981) The kinetics and mechanism of carbon monoxide hydrogenation over alumina supported ruthenium. J Catal 70:418–432
Acknowledgements
The STFC Rutherford Appleton Laboratory is thanked for access to the neutron beam facilities of TOSCA, MAPS, MERLIN and SANDALS. The Institut Max von Laue-Paul Langevin (ILL), Grenoble, France is thanked for access to the high flux reactor facilities (IN1 Lagrange).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Research Involving Human and/or Animal Participants
There were no human or animal subjects involved in this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Albers, P.W., Parker, S.F. Applications of Neutron Scattering in Technical Catalysis: Characterisation of Hydrogenous Species on/in Unsupported and Supported Palladium. Top Catal 64, 603–613 (2021). https://doi.org/10.1007/s11244-021-01424-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-021-01424-1