Abstract
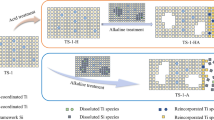
Solid-state NMR spectroscopy was utilized for investigating the post-synthetic formation of surface acid sites via AlCl3 modification of dealuminated zeolite Y (DeaY) and siliceous mesoporous SBA-15 and subsequent thermal treatment. Upon calcination at 723 K, aluminum atoms introduced by the AlCl3 modification coordinate at Q2 (Si(2Si,2OH)) and Q3 (Si(3Si,1OH)) sites and form tetrahedrally coordinated (AlIV) framework aluminum species. Most of the spectroscopically observed pentacoordinated (AlV) and octahedrally coordinated (AlVI) aluminum atoms are extra-framework species, partially acting as Lewis acid sites (LAC). The aluminum coordination at the framework is accompanied by the formation of Brønsted acidic SiOH groups with weak acid strength. The weak Brønsted acidity of these SiOH groups is explained by neighboring framework aluminum atoms with strongly disturbed tetrahedral oxygen coordination. For zeolite DeaY, in addition to the aluminum incorporation into silanol nests, also the aluminum incorporation via surface reactions of AlCl3 with the intact SiO2 framework occurs. In the case of mesoporous SBA-15, with a fivefold higher density of SiOH groups of the parent material compared with that of the parent zeolite DeaY, the aluminum incorporation into silanol nests is the dominating mechanism. By solid-state NMR spectroscopy of the dehydrated samples loaded with probe molecules, significantly larger densities (factor of 3–8) of LAC compared with those of Brønsted acid sites were determined for the AlCl3-modified and calcined zeolite DeaY and mesoporous SBA-15.

















Similar content being viewed by others
References
Kerr GT (1969) Chemistry of crystalline aluminosilicates. VI. Preparation and properties of ultrastable hydrogen zeolite Y. J Phys Chem 73:2780–2782
Yoshida A, Nakamoto H, Okanishi K et al (1982) Preparation and properties of dealuminated Y type zeolite. Bull Chem Soc Jpn 55:581–586
Barrer RM, Makki MB (1964) Molecular sieve sorbents from clinoptilolite. Can J Chem 42:1481–1487
Debras G, Nagy JB, Gabelica Z et al (1983) Determination of silicon-aluminium orderings in mordenite and its aluminium deficient forms using high-resolution magic-angle-spinning 29Si-NMR. Chem Lett 12:199–202
Springuel-Huet MA, Fraissard JP (1992) A 129Xe N.M.R. study of dealuminated mordenites. Zeolites 12:841–845
O’Donovan AW, O’Connor CT, Koch KR (1995) Effect of acid and steam treatment of Na- and H-mordenite on their structural, acidic and catalytic properties. Microporous Mater 5:185–202
Apelian MR, Fung AS, Kennedy GJ et al (1996) Dealumination of zeolite β via dicarboxylic acid treatment. J Phys Chem 100:16577–16583
Corma A, García H (2002) Lewis acids as catalysts in oxidation reactions: from homogeneous to heterogeneous systems. Chem Rev 102:3837–3892
Román-Leshkov Y, Davis ME (2011) Activation of carbonyl-containing molecules with solid Lewis acids in aqueous media. ACS Catal 1:1566–1580
Moliner M (2014) State of the art of Lewis acid-containing zeolites: lessons from fine chemistry to new biomass transformation processes. Dalton Trans 43:4197–4208
Corma A, Nemeth LT, Renz M et al (2001) Sn-zeolite Beta as a heterogeneous chemoselective catalyst for Baeyer-Villiger oxidations. Nature 412:423–425
Pacheco JJ, Davis ME (2014) Synthesis of terephthalic acid via Diels–Alder reactions with ethylene and oxidized variants of 5-hydroxymethylfurfural. Proc Natl Acad Sci USA 111:8363–8367
Tang B, Dai W, Wu G et al (2014) Improved postsynthesis strategy to Sn-Beta zeolites as Lewis acid catalysts for the ring-opening hydration of epoxides. ACS Catal 4:2801–2810
Holm MS, Saravanamurugan S, Taarning E (2010) Conversion of sugars to lactic acid derivatives using heterogeneous zeotype catalysts. Science 328:602–605
Moliner M, Roman-Leshkov Y, Davis ME (2010) Tin-containing zeolites are highly active catalysts for the isomerization of glucose in water. Proc Natl Acad Sci USA 107:6164–6168
Cho HJ, Dornath P, Fan W (2014) Synthesis of hierarchical Sn-MFI as Lewis acid catalysts for isomerization of cellulosic sugars. ACS Catal 4:2029–2037
Dai W, Wang C, Tang B et al (2016) Lewis acid catalysis confined in zeolite cages as a strategy for sustainable heterogeneous hydration of epoxides. ACS Catal 6:2955–2964
Tang B, Dai W, Sun X et al (2015) Mesoporous Zr-Beta zeolites prepared by a post-synthetic strategy as a robust Lewis acid catalyst for the ring-opening aminolysis of epoxides. Green Chem 17:1744–1755
Dessau RM, Kerr GT (1984) Aluminum incorporation into high silica zeolites. Zeolites 4:315–318
Shilina MI, Vasilevskii GY, Rostovshchikova TN et al (2015) Unusual coordination state of cobalt ions in zeolites modified by aluminum chloride. Dalton Trans 44:13282–13293
Kühl GH (1999) In: Weitkamp J, Puppe L (eds) Catalysis and zeolites: fundamentals and applications. Springer, Berlin, pp 161–163
Anderson MW, Klinowski J, Xinsheng L (1984) Alumination of highly siliceous zeolites. J Chem Soc Chem Commun 23:1596–1597
Boroujeni KP (2010) Silica gel supported AlCl3 catalyzed Friedel-Crafts acylation of aromatic compounds. Chin Chem Lett 21:1395–1398
Wu Y, Tian F, He M et al (2011) Isomerization of α-pinene over immobilized AlCl3 catalysts. Chin J Catal 32:1138–1142
Xu M, Arnold A, Buchholz A et al (2002) Low-temperature modification of mesoporous MCM-41 material with sublimated aluminum chloride in vacuum. J Phys Chem B 106:12140–12143
Luo M, Wang Q, Li G et al (2014) Enhancing tetralin hydrogenation activity and sulphur-tolerance of Pt/MCM-41 catalyst with Al(NO3)3, AlCl3 and Al(CH3)3. Catal Sci Technol 4:2081–2090
Sumiya S, Oumi Y, Uozumi T et al (2001) Characterization of AlSBA-15 prepared by post-synthesis alumination with trimethylaluminium. J Mater Chem 11:1111–1115
Zhai S-R, Wei L, Qu F-Z et al (2006) Comparative evaluation of steam stability and catalytic properties of four common aluminosilicate mesostructures prepared by post-grafting method. J Chinese Chemical Soc 53:1053–1058
Hunger M (1997) Brønsted acid sites in zeolites characterized by multinuclear solid-state NMR spectroscopy. Catalysis Reviews 39:345–393
Hunger M (2008) In: Ertl G, Knözinger H, Schüth F, Weitkamp J (eds) Handbook of heterogeneous catalysis, 2nd Ed. Wiley, Weinheim, pp 1163–1178
Hunger M (2009) In: Chester AW, Derouane EG (eds) Zeolite characterization and catalysis—a tutorial. Springer, Berlin, pp 65–106
Jiang Y, Huang J, Dai W et al (2011) Solid-state nuclear magnetic resonance investigations of the nature, property, and activity of acid sites on solid catalysts. Solid State Nucl Magn Reson 39:116–141
Haw JF, Nicholas JB, Xu T et al (1996) Physical organic chemistry of solid acids: lessons from in situ NMR and theoretical chemistry. Acc Chem Res 29:259–267
Fang H, Zheng A, Chu Y et al (2010)) 13C chemical shift of adsorbed acetone for measuring the acid strength of solid acids: a theoretical calculation study. J Phys Chem C 114:12711–12718
Filek U, Bressel A, Sulikowski B et al (2008) Structural stability and Brønsted acidity of thermally treated AlPW12O40 in comparison with H3PW12O40. J Phys Chem C 112:19470–19476
Yang J, Janik MJ, Ma D et al (2005) Location, acid strength, and mobility of the acidic protons in Keggin 12-H3PW12O40: a combined solid-state NMR spectroscopy and DFT quantum chemical calculation study. J Am Chem Soc 127:18274–18280
Li S, Zheng A, Su Y et al (2007) Bronsted/Lewis acid synergy in dealuminated HY zeolite: a combined solid-state NMR and theoretical calculation study. J Am Chem Soc 129:11161–11171
Li S, Huang S-J, Shen W et al (2008) Probing the spatial proximities among acid sites in dealuminated H-Y zeolite by solid-state NMR spectroscopy. J Phys Chem C 112:14486–14494
Biaglow AI, Gorte RJ, White D (1994) 13C NMR studies of acetone in dealuminated faujasites: a probe for nonframework alumina. J Catal 150:221–224
Wang Z, Jiang Y, Hunger M et al (2014) Catalytic performance of Brønsted and Lewis acid sites in phenylglyoxal conversion on flame-derived silica–zirconia. ChemCatChem 6:2970–2975
Lang S, Benz M, Obenaus U et al (2016) Novel approach for the characterization of Lewis acidic solid catalysts by solid-state NMR spectroscopy. ChemCatChem 8:2031–2036
Zheng A, Huang S-J, Liu S-B et al (2011) Acid properties of solid acid catalysts characterized by solid-state 31P NMR of adsorbed phosphorous probe molecules. Phys Chem Chem Phys 13:14889–14901
Sutovich KJ, Peters AW, Rakiewicz EF et al (1999) Simultaneous quantification of Brønsted- and Lewis-acid sites in a USY zeolite. J Catal 183:155–158
Kao H-M, Yu C-Y, Yeh M-C (2002) Detection of the inhomogeneity of Brønsted acidity in H-mordenite and H-β zeolites: a comparative NMR study using trimethylphosphine and trimethylphosphine oxide as 31P NMR probes. Micropor Mesopor Mater 53:1–12
Karra MD, Sutovich KJ, Mueller KT (2002) NMR characterization of Bronsted acid sites in faujasitic zeolites with use of perdeuterated trimethylphosphine oxide. J Am Chem Soc 124:902–903
Obenaus U, Dyballa M, Lang S et al (2015) Generation and properties of Brønsted acid sites in bifunctional Rh-, Ir-, Pd-, and Pt-containing zeolites Y investigated by solid-state NMR spectroscopy. J Phys Chem C 119:15254–15262
Huang S-J, Yang C-Y, Zheng A et al (2011) New insights into Keggin-type 12-tungstophosphoric acid from 31P MAS NMR analysis of absorbed trimethylphosphine oxide and DFT calculations. Chem Asian J 6:137–148
Lunsford JH, Rothwell WP, Shen W (1985) Acid sites in zeolite Y: a solid-state NMR and infrared study using trimethylphosphine as a probe molecule. J Am Chem Soc 107:1540–1547
Hayashi S, Jimura K, Kojima N (2014) Adsorption of trimethylphosphine oxide on silicalite studied by solid-state NMR. Bull Chem Soc Jpn 87:69–75
Breck DW (1974) Zeolite molecular sieves. Krieger Publishing Company, Malabar, p 460–636
Hunger B, Hoffmann J, Heitzsch O et al (1990) Temperature-programmed desorption (TPD) of ammonia from HZSM-5 zeolites. J Therm Anal 36:1379–1391
Niwa M, Katada N (2013) New method for the temperature-programmed desorption (TPD) of ammonia experiment for characterization of zeolite acidity: a review. Chem Rec 13:432–455
Yin F, Blumenfeld AL, Gruver V et al (1997) NH3 as a probe molecule for NMR and IR study of zeolite catalyst acidity. J Phys Chem B 101:1824–1830
Zhao D, Feng J, Huo Q et al (1998) Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279:548–552
Treacy MMJ, Higgins JB (2007) Collection of simulated XRD powder patterns for zeolites, 5th Ed. Elsevier, Oxford, p 174
Černý Z, Macháček J, Fusek J et al (2000) 27Al NMR studies of the hydrolysis of aluminium(III)chloride in non-aqueous media. Inorg Chim Acta 300–302:556–564
Jiao J, Kanellopoulos J, Wang W et al (2005) Characterization of framework and extra-framework aluminum species in non-hydrated zeolites Y by 27Al spin-echo, high-speed MAS, and MQMAS NMR spectroscopy at B0 = 9.4 to 17.6 T. Phys Chem Chem Phys 7:3221–3226
Huang J, Jiang Y, Reddy Marthala VR et al (2008) Characterization and acidic properties of aluminum-exchanged zeolites X and Y. J Phys Chem C 112:3811–3818
Thomas JM, Klinowski J, Anderson MW (1983) On the similarity of the high-resolution solid-state 29Si and 27Al spectra of silicalite and dealuminated zeolite ZSM-5. Chem Lett:1555–1556
Klinowski J (1991) Solid-state NMR studies of molecular sieve catalysts. Chem Rev 91:1459–1479
Engelhardt G, Michel D (1987) High resolution solid state NMR of silicates and zeolites. Wiley, Chichester, p 149–156
Pursch M, Sander LC, Albert K (1999) Peer reviewed: understanding reversed-phase LC with solid-state NMR. Anal Chem 71:733A–741A
Brunner E, Sternberg U (1998) Solid-state NMR investigations on the nature of hydrogen bonds. Prog Nucl Magn Reson Spectrosc 32:21–57
Huang J, van Vegten N, Jiang Y et al (2010) Increasing the Bronsted acidity of flame-derived silica/alumina up to zeolitic strength. Angew Chem Int Ed Engl 49:7776–7781
Huang J, Jiang Y, van Vegten N et al (2011) Tuning the support acidity of flame-made Pd/SiO2–Al2O3 catalysts for chemoselective hydrogenation. J Catal 281:352–360
Griffiths DM, Rochester CH (1978) Infrared study of the adsorption of acetone on rutile. J Chem Soc Faraday Trans 1 74:403
Weitkamp J (2000) Zeolites and catalysis. Solid State Ion 131:175–188
Freude D, Ernst H, Wolf I (1994) Solid-state nuclear magnetic resonance studies of acid sites in zeolites. Solid State Nucl Magn Reson 3:271–286
Jiao J, Altwasser S, Wang W et al (2004) State of aluminum in dealuminated, nonhydrated zeolites Y investigated by multinuclear solid-state NMR spectroscopy. J Phys Chem B 108:14305–14310
Jiao J, Kanellopoulos J, Behera B et al (2006) Effects of adsorbate molecules on the quadrupolar interaction of framework aluminum atoms in dehydrated zeolite H,Na-Y. J Phys Chem B 110:13812–13818
Hunger M, Schenk U, Breuninger M et al (1999) Characterization of the acid sites in MCM-41-type materials by spectroscopic and catalytic techniques. Micropor Mesopor Mater 27:261–271
Acknowledgements
Financial support by Deutsche Forschungsgemeinschaft, Baden-Württemberg Stiftung, and European Community with the FASTCARD (FAST industrialization and CAtalyst Research and Development) project in the Seventh Framework Program GA No. 604277 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lang, S., Benz, M., Obenaus, U. et al. Mechanisms of the AlCl3 Modification of Siliceous Microporous and Mesoporous Catalysts Investigated by Multi-Nuclear Solid-State NMR. Top Catal 60, 1537–1553 (2017). https://doi.org/10.1007/s11244-017-0837-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-017-0837-6