Abstract
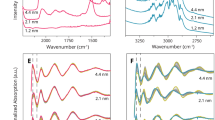
Different nanosized platinum crystallites dispersed on a silica support have been extensively characterized with the help of probe molecule adsorption and instrumental measurements, and their reactivity to the reduction of N2O by H2 as a model reaction has been studied. All measurements using different techniques, i.e., H2–N2O titration, H2 chemisorption, X-ray diffraction and high-resolution transmission electron microscopy, disclosed that the platinum system consisted of nanocrystallites with average sizes of ca. 1.1–22.2 nm, depending on the thermal excursion. In situ diffuse reflectance infrared Fourier transform spectra of CO adsorbed on nanodispersed platinum particles with the indicated range of their sizes after adsorptive dissociation of N2O at 90 °C showed absorption bands near 2188, 2075, and 2088 cm−1 even on the biggest platinum nanocrystallites and these had also some low coordination sites giving the 2188 cm−1 vibration, proposing that all the platinum nanoparticles could exist in three different coordination environments. The reduction of N2O by H2 at 110 °C yielded no significant difference in turnover frequency between different platinum nanocrystallite sizes, disclosing that this reaction was structure-insensitive. The turnover frequency in the model reaction at 125 °C over the finest platinum size sample gave no principal distinction between flowing mixtures containing N2O and H2 whose absolute concentrations varied. All these low temperature reactions could be, to a good approximation, explained by an overall stoichiometry of N2O:H2 = 1:1.






Similar content being viewed by others
References
Behafarid F, Ono LK, Mostafa S, Croy JR, Shafai G, Hong S, Rahman TS, Bare SR, Cuenya BR (2012) Phys Chem Chem Phys 14:11766–11779
Rioux RM, Song H, Yang P, Somorjai GA (2008) In: Corain B, Schmid G, Toshima N (eds) Metal nanoclusters in catalysis and materials science: the issue of size control. Elsevier, Amsterdam
Jin R, Cao YC, Hao E, Metraux GS, Schatz GC, Mirkin CA (2003) Nature 425:487–490
Ozin GA (1992) Adv Mater 4:612–649
Klasovsky F, Claus P (2008) In: Corain B, Schmid G, Toshima N (eds) Metal nanoclusters in catalysis and materials science: the issue of size control. Elsevier, Amsterdam
Sieben JM, Duarte MME, Mayer CE (2008) J Appl Electrochem 38:483–490
Xu T, Lin C, Wang C, Brewe DL, Ito Y, Lu J (2010) J Am Chem Soc 132:2151–2153
Kim MH, Ebner JR, Friedman RM, Vannice MA (2001) J Catal 204:348–357
Kim MH, Ebner JR, Friedman RM, Vannice MA (2002) J Catal 208:381–392
Yang WH, Kim MH (2006) Korean J Chem Eng 23:908–918
Benson JE, Boudart M (1965) J Catal 4:704–710
Wilson GR, Hall WK (1970) J Catal 17:190–206
Vannice MA, Hasselbring LC, Sen B (1985) J Catal 95:57–70
Kim MH, Cho IH, Park JH, Choi SO, Lee IS (2016) J Por Mater 23:291–299
Meyer CI, Regenhardt SA, Zelin J, Sebastian V, Marchi AJ, Garetto TF (2016) Top Catal 59:168–177
Hyde T (2008) Platin Met Rev 52:129–130
Zhuravlev LT (2000) Colloid Surf A 173:1–38
Comas-Vives A (2016) Phys Chem Chem Phys 18:7475–7482
Musso F, Sodupe M, Corno M, Ugliengo P (2009) J Phys Chem C 113:17876–17884
Hadjiivanov K, Vayssilov GN (2002) Adv Catal 47:307–511
Panayotov D, Mihaylov M, Nihtianova D, Spassov T, Hadjiivanov K (2014) Phys Chem Chem Phys 16:13136–13144
Hayden BE, Kretschmar K, Bradshaw AM, Greenler RG (1985) Surf Sci 149:394–406
Somodi F, Werner S, Peng Z, Getsoian AB, Mlinar AN, Yeo BS, Bell AT (2012) Langmuir 28:3345–3349
Brandt RK, Hughes MR, Bourget LP, Truszkowska K, Greenler RG (1993) Surf Sci 286:15–25
Bare SR, Hofman P, King DA (1984) Surf Sci 144:347–369
Hadjiivanov K (1998) J Chem Soc Faraday Trans 94:1901–1904
Balakrishnan K, Sachdev A, Schwank J (1990) J Catal 121:441–455
Barshad Y, Zhou X, Gulari E (1985) J Catal 94:128–141
Kappers MJ, van der Maas JH (1991) Catal Lett 10:365–373
Kim MH, Vannice MA, Kim DG, Lee JH (2003) Korean J Chem Eng 20:247–255
Kondratenko VA, Hahn T, Kondratenko EV (2012) ChemCatChem 4:408–414
Kondratenko EV, Ovsitser O (2008) Angew Chem Int Ed 47:3227–3229
Presto AA, Granite EJ (2008) Platin Met Rev 52:144–154
van Santen RA (2009) Acc Chem Res 42:57–66
Burch R, Attard GA, Daniells ST, Jenkins DJ, Breen JP, Hu P (2002) Chem Commun 22:2738–2739
Burch R, Daniells ST, Breen JP, Hu P (2004) Catal Lett 94:103–108
Srinivas ST, Rao PK (1994) J Catal 148:470–477
Mitchell PCH, Ramirez-Cuesta AJ, Parker SF, Tomkinson J, Thompsett D (2003) J Phys Chem B 107:6838–6845
Lenz DH, Conner WC Jr, Fraissard JP (1989) 117:281–289
Acknowledgements
A partial grant-in-aid via GAIA Grant # 2016000550002 was provided for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, M.H., Park, J.H. & Hong, YS. Characterization of Supported Platinum Nanocrystallites with Different Sizes and These Effects on a Model Reaction. Top Catal 60, 773–781 (2017). https://doi.org/10.1007/s11244-017-0775-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-017-0775-3