Abstract
Quantum chemical calculations at the BP86/def2-TZVP and M06/def2-TZVP levels of theory have been carried out to investigate the nature and strength of the Au-dithiolate bond in gold(III) bis(1,2-dithiolate) homoleptic complexes [AuL2]– where L represents various ligands: ethylene-1,2-dithiolate (edt2−), 1,2-bis(methyl)ethylenedithiolate (dmedt2−), 1,2-maleonitrile-1,2-dithiolate (mnt2−), benzene-1,2- dithiolate (bdt2−), 4,5-dimethylbenzene-1,2-dithiolate (dmbdt2−), and 4,5-dicyanobenzene-1,2-dithiolate (dcbdt2−). The study involved calculating the interaction energies between the fragments as well as assessing the deformation energies of both the Au3+ ion and the dithiolate ions. Furthermore, the total interaction energy and the stabilization energy of the complexes were determined and compared. The investigation also included conducting an energy decomposition analysis (EDA) to examine the characteristics of the bonds between Au(III) and bis(dithiolate) in these complexes. The results demonstrated that the complexes containing dithiolates with ‒CN substitutions ([Au(mnt)2]– and [Au(dcbdt)2]–) have smaller values of stabilization and interaction energies compared to other ones. The analysis of Au − (bis)dithiolate bonds revealed that the electrostatic interactions make a more substantial contribution to the total attractive interactions compared to the orbital interactions. Indeed, the dominant role in stabilizing the complexes is played by the electrostatic attractions between the Au3+ and the dithiolate ligands. Moreover, both the Au → Lπ and Au → Lσ backdonations in all studied complexes are very weak.
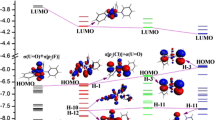
Graphical abstract









Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Kato R (2007) Chem Rev 104:5319–5346
Deplano P, Pilia L, Espa D, Mercuri ML, Serpe A (2010) Coord Chem Rev 254:1434–1447
Bonneval BG, Chinga KIM, Alaryc F, Bui T, Valadea L (2010) Coord Chem Rev 254:1457–1467
Pop F, Avarvari N (2017) Coord Chem Rev 346:20–31
Kusamoto T, Nishihara H (2019) Coord Chem Rev 380:419–439
Pitchaimani J, Ni SF, Dang L (2020) Coord Chem Rev 420:213398
Periyasamy G, Burton NA, Hillier IH, Vincent MA, Disley H, McMaster J, Garner CD (2007) Faraday Discuss 135:469–488
Herman ZS, Kirchner RF, Loew GH, Mueller-Westerhoff UT, Nazzal A, Zerner MC (1982) Inorg Chem 21:46–56
Ray K, George SD, Solomon EI, Wieghardt K, Neese F (2007) Chem Eur J 13:2783–2790
Alarym FJ, Heully L, Scemama A, Bonneval BG, Chane-Ching KI, Caffarel M (2010) Theor Chem Acc 126:243–255
Eisenberg R, Gray HB (2011) Inorg Chem 50:9741–9751
Bushnell EAC, Boyd RJ (2015) J Phys Chem A 119:911–918
Schlimgen AW, Mazziotti DA (2017) J Phys Chem A 121:9377–9384
Lim BS, Fomitchev DV, Holm RH (2001) Inorg Chem 40:4257–4262
Curreli S, Deplano P, Faulmann C, Ienco A, Mealli C, Mercuri ML, Pilia L, Pintus G, Serpe A, Trogu EF (2004) Inorg Chem 43:5069–5079
Ray K, Begum A, Weyhermuller T, Piligkos S, Slageren J, Neese F, Wieghardt K (2005) J Am Chem Soc 127:4403–4415
Herebian D, Wieghardt KE, Neese F (2003) J Am Chem Soc 125:10997–11005
Ray K, Weyhermüller T, Neese F, Wieghardt K (2005) Inorg Chem 44:5345–5360
Bachler V, Olbrich G, Neese F, Wieghardt K (2002) Inorg Chem 41:4179–4193
Petrenko T, Ray K, Wieghardt KE, Neese F (2006) J Am Chem Soc 128:4422–4436
Waters T, Woo HK, Wang XB, Wang LS (2006) J Am Chem Soc 128:4282–4291
Waters T, Wang XB, Woo HK, Wang LS (2006) Inorg Chem 45:5841–5851
Liu X, Hou GL, Wang X, Wang XB (2016) J Phys Chem A 120:2854–2862
Plyusnin VF, Pozdnyakov IP, Grivin VP, Solovyev AI, Lemmetyinen H, Tkachenko NV, Larionov SV (2014) Dalton Trans 43:17766–17774
Kirk ML, McNaughton RL, Helton ME (2003) The Electronic Structure and Spectroscopy of Metallo-Dithiolene Complexes. In: E.I. Stiefel (Ed.), Progress in Inorganic Chemistry, John Wiley & Sons, Inc. Vol. 52.
Pandey KK, Lein M, Frenking G (2003) J Am Chem Soc 125:1660–1668
Krapp A, Pandey KK, Frenking G (2007) J Am Chem Soc 129:7596–7610
Caramori GF, Frenking G (2007) Organometallics 26:5815–5825
Erhardt S, Frenking G (2009) J Organomet Chem 694:1091–1100
Caramori GF, Frenking G (2008) Theor Chem Acc 120:351–361
Prabusankar G, Gemel C, Parameswaran P, Flener C, Frenking G (2009) Fischer RA 48:5526–5529
Gámez JA, Tonner R, Frenking G (2010) Organometallics 29:5676–5680
Bayat M, Hopffgarten M, Salehzadeh S, Frenking G (2011) J Organomet Chem 696:2976–2984
Najafi L, Gholiee Y (2023) J Organomet Chem 1002:122909
Gholiee Y, Salehzadeh S (2023) Inorg Chem Res 7:14–21
Becke AD (1988) Phys Rev A 38:3098–3100
Perdew JD (1986) Phys Rev B 33:8822–8824
Zhao Y, Truhlar DG (2008) Theor Chem Acc 120:215–241
Weigend F, Ahlrichs R (2005) Phys Chem Chem Phys 7:3297–3305
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian Inc., Wallingford CT., 2009.
Frenking G, Frohlich N (2000) Chem Rev 100:717–774
Pan S, Zhao L, Rasika HV, Frenking G (2018) Inorg Chem 57:7780–7791
Velazquez A, Fernandez I, Frenking G, Merino G (2007) Organometallics 26:4731–4736
Salehzadeh S, Maleki F (2016) J Comput Chem 37:2799–2807
Gholiee Y, Salehzadeh S, Khodaveisi S (2019) New J Chem 43:7797–7805
Hokmi S, Salehzadeh S, Gholiee Y (2021) J Comput Chem 42:1354–1363
Nassery-Thekyeh Z, Gholiee Y (2022) Comput Theor Chem 1215:113814
Hokmi S, Salehzadeh S, Gholiee Y (2022) New J Chem 46:2678–2686
Morokuma K (1971) J Chem Phys 55:1236–1244
Ziegler T, Rauk A (1977) Theor Chim Acta 46:1–10
ADF, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands, 2013.
Wang HMJ, Vasam CS, Tsai TYR, Chen SH, Chang AHH, Lin IJB (2005) Organometallics 24:486
Ehlich H, Schier A, Schmidbaur H (2002) Z Naturforsch B 57:890
Madhu V, Das SK (2006) Eur J Inorg Chem 2006:1505–1514
Jørgensen CK (1971) Modern aspects of ligand field theory. North-Holland, American Elsevier, New York, Amsterdam, London
Acknowledgements
The authors are grateful to Malayer University for financial support.
Author information
Authors and Affiliations
Contributions
"H.M. Investigation, Resources, Writing original draft; Y.G. Formal analysis, Investigation, Validation, Supervision. All authors reviewed the manuscript."
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mehri, H., Gholiee, Y. Quantitative assessment of the nature and strength of Au‒dithiolate bond in gold(III) bis(1,2-dithiolate) homoleptic complexes. Transit Met Chem (2024). https://doi.org/10.1007/s11243-024-00579-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11243-024-00579-6