Abstract
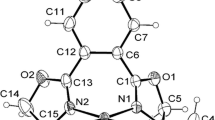
The ortho-substituted (E)-1-((2-methoxyphenyl)diazenyl)naphthalen-2-ol and the meta-substituted (E)-1-((3-methoxyphenyl)diazenyl)naphthalen-2-ol were, respectively, used in the synthesis of two new complexes, bis[1-(2-methoxyphenylazo)-2-naphthoxy]palladium(II) and bis[1-(3-methoxyphenylazo)-2-naphthoxy]palladium(II), noted (I) and (II), respectively. (I) and (II) were characterized by physicochemical and spectroscopic methods, and their molecular structures were determined by X-ray crystallography. Both complexes display a square-planar geometry, which is reproduced by full geometry optimizations at the DFT/B3LYP level. Calculations were also performed on the free ligands (in their precursor form), as well as their para-substituted isomer (E)-1-((4-methoxyphenyl)diazenyl)naphthalen-2-ol and its hypothetical complex bis[1-(4-methoxyphenylazo)-2-naphthoxy]palladium(II) (compound (III). Calculations were also performed on the free p-phenylazo-2-naphthol ligand (p-MoxyPhNap), in order to understand their bonding and to analyze their electronic structure. TD-DFT calculations were also performed on the three complexes to simulate their absorption spectra from and compare to the experimental UV–Vis data of (I) and (II). The main peaks in the spectrum of (I) are assigned to mixed LMCT/LLCT and π–π * (ILCT) transition, while the unique major peak afforded by (II) is assigned to MLCT and LLCT transitions.










Similar content being viewed by others
References
Dharmalingam V, Ramasamy AK, Balasuramanian V (2011) Synthesis and EPR studies of copper metal complexes of dyes derived from remazol red B, procino yellow, fast green FCF, brilliant cresyl blue with copper acetate monohydrate. E-J Chem 8:S211–S224
Khedr AM, Gaber M, Abd El-Zaher EH (2011) Synthesis, structural characterization, and antimicrobial activities of Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) complexes of triazole-based azodyes. Chin J Chem 29:1124
Kirkan B, Gup R (2008) Synthesis of new Azo dyes and copper(II) complexes derived from Barbituric acid and 4-Aminobenzoyl hydrazone. Turk J Chem 32:9–17
Sekar N (1999) Ecofriendly metal complex dyes an update. Colourage 46:63–65
Thomas AM, Nethaji M, Chakravarty ARJ (2004) Different modes of DNA cleavage activity of dihydroxo-bridged dicopper (II) complexes having phenanthroline bases. Inorg Biochem 98:1087–1097
Reed JE, Arnal AA, Neidle S, Vilar R (2006) Stabilization of G-quadruplex DNA and inhibition of telomerase activity by square-planar nickel (II) complexes. J Am ChemSoc 128:5992–5993
Selvakumar B, Rajendiran V, Uma Maheswari P, Stoeckli-Evans H, Palaniandavar M (2006) Structures, spectra, and DNA-binding properties of mixed ligand copper(II) complexes of iminodiacetic acid: the novel role of diimine co-ligands on DNA conformation and hydrolytic and oxidative double strand DNA cleavage. J Inorg Biochem 100:316–330
Patra AK, Nethaji M, Chakravarty AR (2007) J Synthesis, crystal structure, DNA binding and photo-induced DNA cleavage activity of (S-methyl-L-cysteine)copper(II) complexes of heterocyclic bases. Inorg Biochem 101:233–244
Chen GJ, Qiao X, Qiao PQ, Xu GJ, Xu JY, Tian JL, Gu W, Liu X, Yan SP (2010) Synthesis, DNA binding, photo-induced DNA cleavage, cytotoxicity and apoptosis studies of copper(II) complexes. J Inorg Biochem 105:119–126
Cvek B, Milacic V, Taraba J, Dou QP (2008) Ni (II), Cu (II), and Zn (II) diethyldithiocarbamate complexes show various activities against the proteasome in breast cancer cells. J Med Chem 51:6256–6258
Abe T, Mano S, Yamaya Y, Tomotake A (1999) Thermal dye transfer printing with chelate compounds. J Imag Sci Tech 43:339–344
Meyers GA, Michaels FM, Reeves RL, Trotter P (1985) Kinetics and mechanism of chelation of nickel(II) by a tridentate alpha.-[(2-hydroxyphenyl)azo]-alpha.-acetoacetonitrile and an alpha.-(8-quinolylazo)-.alpha.-acetoacetonitrile dye. J InorgChem 24:731–738
Graves HM, Johnston LG, Reiser A (1988) The effect of metallization on singlet oxygen formation by azo dyes. J PhotochemPhotobiolA 43:183–192
Liu JC-I, Bailar JC Jr (1998) The structures and properties of lakes of some azo dyes. InorgChimActa 145:181–184
Woodward C, Freiser H (1973) Sulphonated azo-dyes as extractive metallochromic reagents. Talanta 20:417–420
Shibata S, Furukawa M, Toei K (1973) Syntheses and spectrophotometric studies of azo dyes containing m-dimethylaminophenol as analytical reagents. Anal ChimActa 66:397–409
Pilipenko AT, Savransky LI (1987) Selectivity and sensitivity of metal determination by co-ordination compounds. Talanta 34:77–86
Szurdoki F, Ren D, Walt DR (2000) A combinatorial approach to discover new chelators for optical metal ion sensing. Anal Chem 72:5250–5257
Wang S, Shen S, Xu H (2000) Synthesis, spectroscopic and thermal properties of a series of azo metal chelate dyes. Dyes Pigments 44:195–198
Park HY, Lee NH, Je JT, Min KS, Huh YJ, Kim E-R (2001) Synthesis and Characterization of X-azo Dyes (X=Ni, Cu, Zn) for Digital Versatile Disc-Recordable (DVD-R). MolCrystLiqCryst 371:305–308
Park H, Kim E-R, Kim DJ, Lee H (2002) Synthesis of metal-azo dyes and their optical and thermal properties as recording materials for DVD-R. Bull ChemSocJpn 75:2067–2070
Pratihar JL, Mandal P, Lin CH, Lai CK, Mal D (2017) Azo-amide palladium(II) complexes: synthesis, characterization and application in C-C cross-coupling reactions. Polyhedron. https://doi.org/10.1016/j.poly.2017.06.055
Munusamy S, Muniyappan P, Galmari V (2019) Synthesis and structural characterization of palladium(II) 2-(arylazo)naphtholate complexes and their catalytic activity in Suzuki and Sonogashira coupling reactions. J CoordChem 72:1910–1921
Jana S, Chandan RN, Manna K, Mondal TK (2020) Synthesis, characterization, X-ray structure and DNA binding study of palladium(II) complex with new thioether containing ONS donor ligand. J ChemSci 132:64–72
Pratihar P, Mondal TK, Patra AK, Sinha C (2009) trans-Dichloro-bis-(arylazoimidazole)palladium(II) azo-N to make free azo (-NdN-) function is important to reveal photochromic activity. Inorg Chem 48:2760–2769
Sen C, Roy S, Mondal TK, Ghosh R, Mondal JA, Palit DK, Sinha C (2015) Palladium(II)-iodo-{1-alkyl-2-(arylazo)imidazole} complexes: Synthesis, structure, dynamics of photochromism and DFT computation. Polyhedron 85:900–911
Salmen R, Malterud KE, Pedersen BF (1988) Acta Chem. Scand A 42:493
Gilli P, Bertolasi V, Pretto L, Antonov L, Gilli G (2005) J Am Chem Soc 127:4943
Sheldrick GM (2008) A shrt history of SHELX. Acta Cryst A64:112–122
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Cryst C71:3–8
Farrugia LJ (2012) WinGX and ORTEP for Windows: an update. J Appl Cryst 45:849–854
ADF2016.01 (2016) Version Theoretical Chemistry Vrije Universiteit Amsterdam, The Netherlands, SCM
Baerends EJ, Ellis DE, Ros P (1973) Self-consistent molecular Hartree-Fock-Slater calculations I. Comput Proc Chem Phys 2:41
teVelde G, Baerends EJ (1992) Numerical integration for polyatomic systems. J Comput Phys 99:84
Fonseca Guerra C, Snijders JG, teVelde G, Baerends EJ (1998) Towards an order-N DFT method. The ChimAcc 99:391
Bickelhaupt FM, Baerends EJ (2000) Kohn-Sham density functional theory: predicting and understanding chemistry. Rev ComputChem 15:1
teVelde G, Bickelhaupt FM, Fonseca Guerra C, van Gisbergen SJA, Baerends EJ, Snijders JG, Ziegler T (2001) Chemistry with ADF. J ComputChem 22:931
Becke AD (1993) Density functional thermochemistry.III. The role of exact exchange. J Chem Phys 98:5648
Lee C, Yang W, Parr RG (1998) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785
vanLenthe E, Baerends EJ, Snijders JG (1993) Relativistic regular two-component Hamiltonians. J Chem Phys 99:4597–4610
vanLenthe E, Baerends EJ, Snijders JG (1994) Relativistic total energy using regular approximations. J Chem Phys 101:9783–9792
vanLenthe E, Baerends EJ, Snijders JG (1999) Geometry optimizations in the zero order regular approximation for relativistic effects. J Chem Phys 110:8943–8953
Fan L, Ziegler T (1992) Application of density functional theory to infrared absorption intensity calculations on main group molecules. J Chem Phys 96:9005
Fan L, Ziegler T (1992) Application of density functional theory to infrared absorption intensity calculations on transition-metal carbonyls. J Chem Phys 96:6937
Runge E, Gross EKU (1984) Density-functional theory for time-dependent systems. Phys Rev Lett 52:997–1000
Klamt A, Schüümann G (1993) COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J ChemSoc Perkin Trans 2:799–805
Weinhold F, Landis CR (2005) Valency and bonding: a natural bond orbital donor–acceptor perspective. Cambridge University Press, Cambridge
Weinhold F, Glendening ED (2001) NBO 5.0 program manual: natural bond orbital analysis programs. University of Wisconsin, Madison, Theoretical Chemistry Institute and Department of Chemistry
Chetioui S, Rouag DA, Djukic JP, Bochet CG, Touzani R, Bailly C, Crochet A, Fromm KM (2016) Crystal structures of a copper(II) and the isotypic nickel(II) and palladium(II) complexes of the ligand (E)-1-[(2,4,6-tribromophenyl)diazenyl]naphthalen-2-ol. Acta Cryst E 72:1093
Lin ML, Tsai CY, Li CY, Huang BH, Ko BT (2010) Bis{1-[(E)-(2-methyl-phen-yl)diazen-yl]-2-naphtho-lato}palladium(II). Acta Cryst E 66:m1022
MerzougM ZB (2014) Coordination diversity of the phenazine ligand in binuclear transition metal sandwich complexes: theoretical investigation. J Organomet Chem 770:69–78
KorichiH ZouchouneF, ZendaouiSM ZouchouneB, Saillard JY (2010) The coordination chemistry of azulene: a comprehensive DFT investigation. Organometallics 29:1693–1706
ZendaouiSM ZouchouneB (2013) Molecular properties and electronic structure of phenazine ligand in binuclear molybdenum and manganese metal complexes: a density functional theory study. Polyhedron 51:123–131
Bouchakri N, Benmachiche A, Zouchoune B (2011) Bonding analysis and electronic structure of transition metalbenzoquinoline complexes: a theoretical study. Polyhedron 30:2644–2653
ZouchouneB MerzougM (2019) BensalemN. Struct Chem. https://doi.org/10.1007/s11224-019-01322-z
Ababsa S, Farah S, Zouchoune B, Benhamada N (2010) Theoretical investigation of the coordination of dibenzazepine to transition-metal complexes. Polyhedron 29:2722–2730
Mkpenie VN, Essien EE (2015) Solvent and methyl group effects on the electronic spectral properties of azo-2-naphtol dye. Am ChemSci J 8:1–8
Zouchoune B, Mansouri L (2019) Electronic structure and UV–Vis spectra simulation of square planar Bis (1-(4-methylphenylazo)-2-naphtol)-Transition metal complexes [M(L)2]x (M= Ni, Pd, Pt, Cu, Ag, and x = − 1, 0, + 1): DFT and TD-DFT study. StructChem 30:691–701
Mansouri L, Zouchoune B (2015) Substitution effects and electronic properties of the azo dye (1-phenylazo-2-naphthol) species: a TD-DFT electronic spectra investigation. Can J Chem 93:509–517
Funding
The authors received financial support from the Algerian MESRS (Ministère de l’Enseignement Supérieur et de la RechercheScientifique) and DGRSDT (Direction Générale de la Recherche Scientifique et du Développement Technologique).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contribution of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
This paper is dedicated to Dr. Jean-René Hamon at the occasion of his 65th birthday.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chetioui, S., Zouchoune, B., Merazig, H. et al. Synthesis, spectroscopic characterization, crystal structure and theoretical investigation of two azo-palladium (II) complexes derived from substituted (1-phenylazo)-2-naphtol. Transit Met Chem 46, 91–101 (2021). https://doi.org/10.1007/s11243-020-00425-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-020-00425-5