Abstract
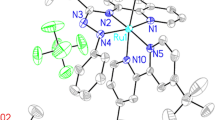
Eight ruthenium complexes having thioalkyl-azoimine (SNN) ligands of two general types have been synthesized: (a) [Ru(L)(bipy)Cl](PF6) where {bipy = bipyridine, L = (2-SR)C6H4N=NC(COCH3)=NC6H4X, R = Ph (X = H, L1, 1; X = CH3, L2, 2; X = F, L3, 3); R = Me, (X = H, L4, 4)} and (b) [Ru(L1)(N–N)Cl](PF6) {N–N = 4,4-dimethyl-2,2′-bipyridine (dmbipy) (5), (1,10-phenanthroline) phen (6), 3,4,7,8-tetramethyl-1,10-phenanthroline (tetrmphen) (7), 5-5-chloro-1,10-phenanthroline (Clphen) (8). The crystal structures for 1 and 6 as well as for L2 are reported; the crystal structures of 1 and 6 show that L1 is bound to the ruthenium center as κ3[S,N,N′]. The complexes have been characterized by IR, UV/Vis and NMR spectroscopy as well as electrochemical (CV) techniques. The effect of groups X, the substituent R and N–N bidentate ligands on the electronic properties of the resulting mononuclear ruthenium complexes was investigated. The catalytic activity of these complexes was tested in the liquid-phase hydrogenation of acetophenone.












Similar content being viewed by others
References
Egdal RK, Hazell A, Larsen FB, Mckenzie CJ, Scarrow RC (2003) J Am Chem Soc 125:32
Sellmann D, Lauderbach F, Geipel F, Heinemann FW, Moll M (2004) Angew Chem 116:3203
Kruger HJ, Peng G, Holm RH (1991) Inorg Chem 30:734
Koley AP, Purohit S, Prasad LS, Ghosh S, Manoharan PT (1992) Inorg Chem 31:305
Koley AP, Nirmala R, Prasad LS, Ghosh S, Manoharan PT (1992) Inorg Chem 31:1764
Raja M, Gowri N, Ramesh R (2010) Polyhedron 29:1175
Hossain M, Chattopadhyay SK, Ghosh S (1997) Polyhedron 16:4313
Basuli F, Peng SM, Bhattacharya S (1997) Inorg Chem 36:5645
Jana M, Pramanik A, Kundu S, Sarkar D, Jana S, Mondal TK (2013) Inorg Chim Acta 394:583
Jana M, Pramanik A, Sarkar D, Biswas S, Mondal TK (2013) J Mol Struct 1047:73
Sarkar SK, Jana MS, Mondal TK, Sinha C (2014) Appl Organometal Chem 28:641
Al-Noaimi M, Crutchley RJ, AlDamen M, Rawashdeh A, Khanfar MA, Seppelt K (2011) Polyhedron 30:2075
Al-Noaimi M, El-khateeb M, Haddad S, Saadeh H (2010) Transit Met Chem 35:877
Gennett T, Milner DF, Weaver MJ (1985) J Phys Chem 89:2787
Agilent (2011) CrysAlis PRO. Agilent Technologies, Yarnton
SHELXTL (2002) (XCIF, XL, XP, XPREP, XS), version 6.10. Bruker AXS Inc., Madison, WI
Gaussian 09, Revision A.1, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian Inc., Wallingford
Hay PJ, Wadt WR (1985) J Chem Phys 82:270
Wadt WR, Hay PJ (1985) J Chem Phys 82:284
Andrae D, Haeussermann U, Dolg M, Stoll H, Preuss H (1990) Theoret Chim Acta 77:23
Bauernschmitt R, Ahlrichs R (1996) Chem Phys Lett 256:454
Casida MK, Jamorski C, Casida KC, Salahub DR (1998) J Chem Phys 108:4439
Stratmann RE, Scuseria GE, Frisch MJ (1998) J Chem Phys 109:8218
Cossi M, Rega N, Scalmani G, Barone V (2003) J Comput Chem 24:669
O’Boyle NM, Tenderholt AL, Langner KM (2008) J Comput Chem 29:839
Dinda J, Bag K, Sinha C, Mostafa G, Lu T (2003) Polyhedron 22:1367
Al-Noaimi M, Abdel-Jalil R, Haddad S, Al-Far R, Sunjuk M, Crutchley RJ (2006) Inorg Chim Acta 359:2395
Weiss T, Böhme U, Walfort B, Rheinwald G, Lang H (2005) Organometallics 24:2577
Akita M, Takahashi Y, Hikichi S, Moro-oka Y (2001) Inorg Chem 40:169
Al-Noaimi M, Hammoudeh A, Awwadi F, Bader R, Mahmoud A (2018) Inorg Chim Acta 471:186
Li K, Niu J, Yang M, Li Z, Wu L, Hao X, Song M (2015) Organometallics 34(7):1170
Abdur-Rashid K, Clapham S, Hadzovic A, Harvey J, Lough A, Morris R (2002) J Am Chem Soc 124:15104
Acknowledgements
Financial support from the Scientific Research Support Fund (Bas/2/3/2014) and Hashemite University, Jordan, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Al-Noaimi, M., Awwadi, F.F., Hammoudeh, A. et al. Mixed thioalkyl-azoimine (SNN′)/α-diimine–ruthenium complexes: synthesis, characterization, DFT calculations, crystal structure and application as pre-catalysts for hydrogenation of acetophenone. Transit Met Chem 44, 355–367 (2019). https://doi.org/10.1007/s11243-018-00302-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-018-00302-2