Abstract
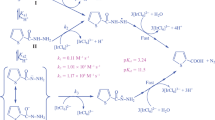
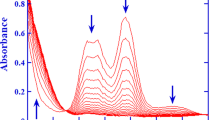
A kinetic analysis of the oxidation of semicarbazide (SEM) by the single-electron oxidant [IrCl6]2− has been carried out by stopped-flow spectrometric techniques. The reaction proved to be first order each in [IrCl6 2−] and [SEM]tot, leading to overall second-order kinetics. The variation in the observed second-order rate constant k′ with pH was explored over the pH range of 0–7.11. Spectrophotometric titration revealed a stoichiometry of Δ[IrCl6 2−]/Δ[SEM]tot = 4:1 for the redox reaction. On the basis of the rate law, the redox stoichiometry, and the rapid scan spectra, a reaction mechanism is proposed which involves parallel attacks of [IrCl6]2− on both H2NCONHNH3 + and H2NCONHNH2 as rate-determining steps, followed by several rapid reactions. The rate expression, derived from the reaction mechanism, was utilized to simulate the k′–pH profile yielding a virtually perfect fit and indicating that the reaction path involving H2NCONHNH3 + does not make a significant contribution to the overall rate. The reaction between [IrCl6]2− and H2NCONHNH2 was further studied as a function of both temperature and ionic strength. From the temperature dependence, activation parameters were obtained as: ∆H ‡2 = 34.9 ± 1.5 kJ mol−1 and ∆S ‡2 = −78 ± 5 J K−1 mol−1. The observed ionic strength dependence suggests that the rate-determining step is between [IrCl6]2− and a neutral species of SEM. Hence, both the temperature and ionic strength dependency studies are in good agreement with the proposed reaction mechanism, in which the rate-determining step involves an outer sphere electron transfer.







Similar content being viewed by others
References
Bogolubsky AV, Moroz YS, Mykhailiuk PK, Dmytriv YV, Pipko SE, Babichenko LN, Konovets AI, Tolmachev A (2015) RSC Adv 5:1063
Podyminogin MA, Lukhtanov EA, Reed MV (2001) Nucleic Acids Res 29:5090
Vazquez J, Albericio F (2006) Tetrahedron Lett 47:1657
Deinderfer P, Davis R, Zentel R (2007) Soft Matter 3:1308
Mercader J, Iffia-Soltesz Z, Bour S, Carpene C (2011) J Obes ID 475786:1
Pereira AS, Donato JL, De Nucei G (2004) Food Addit Contam 21:63
de la Calle MB, Anklam E (2005) Anal Bioanal Chem 382:968
Szilagyi S, de la Calle MB (2006) Eur Food Res Technol 224:141
Szilagyi S, de la Calle MB (2006) Anal Chim Acta 572:113
Casella IG, Contursi M (2015) Sens Actuators B Chem 209:25
Stadler RH, Verzegnassi L, Seefelder W, Racault L (2015) Food Addit Contam Part A 32:1842
Takahashi M, Yoshida M, Inoue K, Morikawa T, Nishikawa A, Ogawa K (2014) Food Chem Toxicol 73:84
Hirakawa K, Midorikawa K, Oikawa S, Kawanishi S (2003) Mutat Res 536:91
Marlier JF, Fogle EJ, Cleland WW (2008) Biochemistry 47:11158
Ratnam S, Anipindi NR (2012) Transit Met Chem 37:453
Pelizzetti E, Mentasti E, Baiocchi C (1976) J Phys Chem 80:2979
Pelizzetti E, Mentasti E, Pramauro E (1978) Inorg Chem 17:1181
Stanbury DM (1984) Inorg Chem 23:2879
Hubbard CD, Gerhard A, van Eldik R (2001) Dalton Trans 1069
Hu Y, Stanbury DM (2016) Inorg Chem 55:7797
Kimura M, Yamamoto M, Yamabe S (1982) J Chem Soc Dalton Trans 423
Zhang J, Guo Y, Lu T, Shi H, Dai T, Shi T (2015) Transit Met Chem 40:281
Remco M, Rode BM (1995) J Mol Struct Theochem 339:125
Sorensen PE, Bruhn K, Lindelov F (1974) Acta Chem Scand A 28:162
Johnson MD, Hornstein BJ (1994) Inorg Chim Acta 225:145
Pethybridge AD, Prue JE (1972) Prog Inorg Chem 17:327
Mehrotra RN, Kirschenbaum LJ (1989) Inorg Chem 28:4327
Wu L, Schwederski BE, Margerum DW (1990) Inorg Chem 29:3578
Shi T, Elding LI (1998) Inorg Chim Acta 282:55
Acknowledgments
Financial support of this work by grants from the Natural Science Foundation of Hebei Province (B2015201073), from the You Bo Program of Hebei University (YB201403), from the Natural Science Foundation of Hebei University (2014-11), and from the National Natural Science Foundation of China (81273128 to H.S.), is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Nan, C., Dong, J., Jiao, X. et al. Stopped-flow kinetic analysis of the oxidation of semicarbazide by hexachloroiridate(IV). Transit Met Chem 42, 9–15 (2017). https://doi.org/10.1007/s11243-016-0100-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-016-0100-1