Abstract
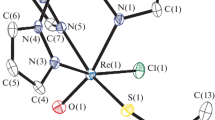
Rhenium(V)-nitridocyano complexes with 2,2′bipyridine (bipy), 1,10-phenanthroline (phen) and 2,9-dimethyl-1,10-phenanthroline (Me2phen) are isolated for the first time and characterized by IR and X-ray crystallography as (AsPh4)[ReN(CN)3(2,2′bipy)]·2H2O·0.5bipy, 2{(AsPh4)[ReN(CN)3(phen)]·0.5H2O·0.54EtOH·0.46MeOH and (PPh4)[ReN(CN)3(Me2phen]·H2O·Me2phen. The rhenium anions in all three structures have a distorted octahedral geometry with the Re(V) atom coordinated by a terminal nitrido ligand, three carbon atoms of the cyanide ligands and bidentate ligands that are bonded bidentally via two pyridine nitrogen atoms. The bidentate ligands coordinate to the Re(V) metal centre via two nitrogen atoms and form five-membered chelate rings with bite angles of 71.37(9)°, 72.7(2)° and 70.7(3)° for the bipy, phen and Me2phen complexes, respectively. Principal dimensions are: bipy: Re≡N = 1.651(3), Re–N(11) = 2.144(1), Re–N(12) = 2.374(2), Re–C(1) = 2.025(4), Re–C(2) = 2.142(4) and Re–C(3) 2.080(3) Å; phen: Re≡N = 1.674(5), Re–N(11) = 2.164(5) Å, Re–N(12) = 2.374(5) Å, Re–C(1) = 2.112(6), Re–C(2) = 2.082(6) and Re–C(3) 2.102(6) Å; Me2phen: Re≡N = 1.634(9), Re–N(11) = 2.190(7) Å, Re–N(22) = 2.454(8), Re–C(1) = 2.074(9) Å, Re–C(2) = 2.095(10) Å and Re–C(3) 2.094(10) Å. One of the major differences between the complexes of phen and Me2phen is that the latter exhibits a fluxional behaviour, while the phenanthroline plane in the phen complex is almost coplanar with the coordination plane. This pronounced distortion in the Me2phen complex is illustrated by the dihedral angle of 117°(2) between the plane of the chelating moiety and that of the square-planar coordination plane.




Similar content being viewed by others
References
Abram U (2004) Comprehensive coordination chemistry II, vol V. Elsevier, Amsterdam, p 271 (and references cited therein)
Sattelberger AP, Bryan JC (1994) Comprehensive organometallic chemistry II, vol VI. Elsevier, Amsterdam (and references cited therein)
Steigman I, Eckelman WC (1992) The chemistry of technetium in medicine. National Academy Press, Washington DC
Prats E, Aisa F, Abos MD, Villavieja L, Garcia-Lopez F, Asenjo MJ, Razola P, Banzo J (1999) J Nucl Med 40:296
Liu S, Edwards DS (1999) Chem Rev 99:2235
Volkert W, Deutsch EA (1993) Adv Met Med 1:115
Chen X, Femia FJ, Babich JW, Zubieta J (2001) Inorg Chim Acta 316(1–2):33
World Nuclear Association, Radioisotopes in Medicine (2015) Accessed 5 Mar 2015. http://www.world-nuclear.org/info/Non-Power-Nuclear-Applications/Radioisotopes/Radioisotopes-in-Medicine/
Johnson NP, Lock CJL, Wilkinson G (1964) J Chem Soc 1054
Leipoldt JG, Basson SS, Roodt A, Purcell W (1992) Polyhedron 11(18):2277
Purcell W, Roodt A, Leipoldt JG, Basson SS (1991) Transit Met Chem 18:339
Leipoldt JG, Basson SS, Roodt A, Purcell W (1987) Transit Met Chem 195(3):209
Basson SS, Leipoldt JG, Potgieter IM (1984) Inorg Chim Acta 87:71
Damoense LJ, Purcell W, Leipoldt JG, Basson SS (1994) Transit Met Chem 19(6):619
Mtshali NT, Purcell W, Visser HG, Basson SS (2006) Polyhedron 25(12):2415
Mtshali TN, Purcell W, Visser HG, Basson SS (2007) Acta Cryst Sect E 63:M80
Johnson NJ (1969) Chem Soc A 1843
Bruker (1999) SAINT+. Version 6.02 (includes XPREP and SADABS). Bruker AXS Inc, Madison
Bruker (1999) SHELXTL. Version 5.1 (includes XS, XL, XP, XSHELL). Bruker AXS Inc, Madison
Brandenburg K, Putz H (2005) DIAMOND. Release 3.1b. Crystal Impact GbR, Bonn
Purcell W, Potgieter IM, Damoense LJ, Leipoldt JG (1992) Transit Met Chem 17:387
Purcell W, Visser HG. Unpublished results
Zhang C, Janiak C (2001) Z Anorg Allg Chem 627:1972
Beck W, Klapötke TM, Klüfers P, Kramer G, Rienäcke CM (2001) Z Anorg Allg Chem 627:1669
Zheng Y-Q, Zhou L-X, Lin J-L, Zhang S-W (2001) Ζ Kristallogr 216:357
El-Azzouzi N, Fueso-Ureña F, Illán-Cabeza NA, Moreno-Carretero MN (2010) Polyhedron 29(5):1405
Amim RS, Oliveira MRL, Janczak J, Rubinger MMM, Vieira LMM, Alves LC, Zambolim L (2011) Polyhedron 30(5):683
Acknowledgments
The authors thank the Research Fund of this University and the National Research Foundation of the Republic of South Africa for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Purcell, W., Visser, H.G. Rhenium(V)-nitrido complexes with different nitrogen donor bidentate ligands. Transition Met Chem 40, 899–906 (2015). https://doi.org/10.1007/s11243-015-9986-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-015-9986-2