Abstract
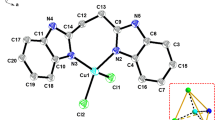
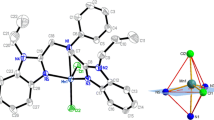
A V-shaped ligand, 1,3-bis(1-methylbenzimidazol-2-yl)-2-thiapropane (L), and its copper(II) and manganese(II) picrate complexes have been synthesized and characterized. The compositions of the complexes are [Cu(L)2](pic)2·2DMF (1) and [Mn(L)(pic)2] (2), respectively. The crystal structure of complex 1 reveals a distorted tetrahedral geometry provided by four N donors from two ligands. Complex 2 is six coordinated, with a distorted octahedral geometry. Experimental studies of the DNA-binding properties indicated that the free ligand and both complexes bind to DNA via the intercalation mode, and the order of the binding affinity is L > 1 > 2. Antioxidant tests in vitro show that the Cu(II) complex possesses significant antioxidant activity against superoxide and hydroxyl radicals, with better scavenging effects than mannitol and vitamin C.








Similar content being viewed by others
References
Guckian KM, Morales JC, Kool ET (1998) J Org Chem 63:9652
Morales JC, Kool ET (1998) Nat Struct Biol 5:950
Wu HL, Wang KT, Kou F, Jia F, Liu B, Yuan JK, Bai Y (2011) J Coord Chem 64:2676
Wu HL, Huang XC, Yuan JK, Kou F, Jia F, Liu B, Wang KT (2010) Eur J Med Chem 45:5324
White AW, Almassy R, Calvert AH, Curtin NJ, Griffin RJ, Hostomsky Z, Maegley K, Newell DR, Srinivasan S, Golding BT (2000) J Med Chem 43:4084
Horton DA, Bourne GT, Smythe ML (2003) Chem Rev 103:893
Pan GL, Bai YC, Wang H, Kong J, Shi FR, Zhang YH, Wang XL, Wu HL (2013) Zeitschrift Für Naturforschung B 68:257
Soderlind KJ, Gorodetsky B, Singh AK, Bachur NR, Miller GG, Lown JW (1999) Anticancer Drug Des 14:19
Wilhelmsson LM, Westerlund F, Lincoln P, Norden B (2002) J Am Chem Soc 124:12092
Dandlier PJ, Holmlin RE, Barton JK (1997) Science 274:1465
Sathyaraj G, Nair BU (2010) Eur J Med Chem 45:284
Manju S, Arun V (2010) J Coord Chem 63:307
Boca M, Boca R, Kickelbick G, Linert W, Svoboda I (2002) Inorg Chim Acta 338:36
Yang ZY, Wang Y, Wang Y (2007) Bioorg Med Chem Lett 17:2096
Wu HL, Shi FR, Wang XL, Zhang YH, Bai YC, Kong J, Wang CP (2014) Transit Met Chem 39:261
Xi PX, Xu ZH, Liu XH, Chen FH, Zeng ZZ, Zhang XW, Liu Y (2009) J Fluoresc 19:63
Qi GF, Yang ZY, Qin DD, Wang BD, Li TR (2008) Chem Pharm Bull 56:452
Lakowicz JR, Webber G (1973) Biochemistry 12:4161
Wu HL, Kou F, Jia F, Liu B, Yuan JK, Bai Y (2011) J Coord Chem 64:3041
Tan CP, Liu J, Chen LM, Shi S, Ji LN (2008) J Inorg Biochem 102:1644
Guo ZY, Xing RE, Liu S, Yu HH, Wang PB, Li CP, Li PC (2005) Med Chem Lett 15:4600
Leonard SS, Keil D, Mehlmanb T, Proper S, Shi X, Harris GK (2006) J Ethnopharmacol 103:288
Le XY, Liao SR, Liu XP, Feng XL (2006) J Coord Chem 59:985
Dagdigian JV, Reed CA (1979) Inorg Chem 18:2623
Bruker (2006) APEX2, SAINT. Bruker AXS Inc., Madison
Sheldrick GM (2004) SADABS, program for empirical absorption correction of area detector data. University of Göttingen, Göttingen
Sheldrick GM (2008) Acta Crystal A 64:112
Geary WJ (1971) Coord Chem Rev 7:81
Aghatabay NM, Tulu M, Mahmiani Y, Somer M, Dulger B (2008) Struct Chem 19:71
Su CY, Kang BS, Du CX, Yang QC (2000) Inorg Chem 39:4843
Wu HL, Wang KT, Yun RR, Huang XC (2009) Synth React Inorg Met-Org Chem 39:629
Wu HL, Sun T, Li K, Xu Y, Yun RR, Sun Q (2009) Z Anorg Allg Chem 635:146
Baldini M, Belicchi-Ferrari M, Bisceglie F, Dall’Aglio PP, Pelosi G, Pinelli S, Tarasconi P (2004) Inorg Chem 43:7170
Chen AY, Yu C, Gatto B, Liu LF (1993) Proc Natl Acad Sci USA 90:8131
Dimitrakopoulou A, Dendrinou-Samara C, Pantazaki AA, Alexiou M, Nordlander E, Kessissoglou DP (2008) J Inorg Biochem 102:618
Mukherjee S, Basu C, Chowdhury S, Chattopadhyay AP, Ghorai A, Ghosh U, Stoeckli-Evans H (2010) Inorg Chim Acta 363:2752
Baguley BC, LeBret M (1984) Biochemistry 23:937
Tsai K, Hsu TG, Hsu KM, Cheng H, Liu TY, Hsu CF, Kong CW (2001) Free Radic Biol Med 31:1465
Udilova N, Kozlov AV, Bieberschulte W, Frei K, Ehrenberger K, Nohl H (2003) Biochem Pharmacol 65:59
Li TR, Yang ZY, Wang BD, Qin DD (2008) Eur J Med Chem 43:1688
Acknowledgments
The present research was supported by the National Natural Science Foundation of China (Grant No. 21367017), the Fundamental Research Funds for the Gansu Province Universities (212086), Natural Science Foundation of Gansu Province (Grant No. 1212RJZA037), and ‘Qing Lan’ Talent Engineering Funds for Lanzhou Jiaotong University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, H., Kong, J., Yang, Z. et al. Copper(II) and manganese(II) picrate complexes with the V-shaped ligand 1,3-bis(1-methylbenzimidazol-2-yl)-2-thiapropane: preparation, structure, DNA-binding properties and antioxidant activities. Transition Met Chem 39, 951–960 (2014). https://doi.org/10.1007/s11243-014-9880-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-014-9880-3