Abstract
This technical note describes the FluidFlower concept, a new laboratory infrastructure for geological carbon storage research. The highly controlled and adjustable system produces a strikingly visual physical ground truth of studied processes for model validation, comparison and forecasting, including detailed physical studies of the behavior and storage mechanisms of carbon dioxide and its derivative forms in relevant geological settings for subsurface carbon storage. The design, instrumentation, structural aspects and methodology are described. Furthermore, we share engineering insights into construction, operation, fluid considerations and fluid resetting in the porous media. The new infrastructure enables researchers to study variability between repeated CO2 injections, making the FluidFlower concept a suitable tool for sensitivity studies on a range of determining carbon storage parameters in varying geological formations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
We develop a laboratory research infrastructure dedicated to geological carbon storage (GCS) with a methodology for repeatable, meter-scale experiments with sufficient precision to allow investigation of any isolated parameter. The two-phase flow of CO2-rich gas and water-rich fluids in complex geological structures, combined with the development of density-driven convective mixing, is difficult to adequately resolve by numerical simulation (Nordbotten et al. 2012; Flemisch et al 2023). As such, there is a strong need for accurate and reproducible experimental data against which numerical simulation tools can be verified. To this end, this technical note details the construction and operation of a laboratory-scale GSC research infrastructure, which we term ‘FluidFlower’ (see Fig. 1), with the following set of characteristics:
-
(A)
Meter-scale, quasi-2D experimental systems containing unconsolidated sands that can be arranged to replicate realistic geological structures such as domes, pinch-outs and faults.
-
(B)
Operational conditions mimicking real GCS operations can be achieved by rate-controlled injection and localized pressure monitoring.
-
(C)
Multiphase flow characteristics such as free gas and CO2 dissolution and concomitant density-driven fingers can clearly be identified visually and quantified by image analysis tools.
-
(D)
Fluids in the pore space of studied geometries can be reset to their initial state, allowing investigations into reproducibility of experimental results as well as variations of operational conditions.
FluidFlower flow cell varieties used in the validation benchmark study. The porous media are built with unconsolidated sands within a vertical, quasi two-dimensional, optically transparent flow cell filled with water. A camera is located on the front side to monitor and record system changes with time lapse imaging. Other instrumentation and most operations such as fluid injections occur at the rear side. To achieve the scale of the experiments, operation occurs at standard sea-level pressure and temperature, still preserving the governing porous media physics, relevant displacement processes and trapping mechanisms for subsurface CO2 storage. The FluidFlower concept serves a dual purpose as research infrastructure for high-quality experimental data, and as a vehicle for public outreach and dissemination. The room scale (right) shown during CO2 injection in a faulted geological geometry motivated by typical North Sea reservoirs. Tabletop version (left) shown containing an idealized folded geometry. The FluidFlower concept produces quantitative meter-scale experimental data for study at both pore and Darcy scale. The data may also be scaled to field scale (Kovscek et al 2023) to provide new insight relevant for subsurface GCS. Virtual flow cells and meterstick
State-of-the-art simulators and experimental methodology build on a century of global oil and gas exploration and production. While this provides a solid scientific and technological foundation, in particular during the injection phase, there remain aspects unique to GCS that require further development. CO2 is physically and chemically very different from crude oil and gaseous organic hydrocarbons: CO2 is highly soluble in water, and its derivative dissolved inorganic carbon (DIC) species both acidify and increase the density of the water. The density increase allows gravitationally induced convective mixing, an essential carbon sequestration mechanism and a dominant cause of post-injection dynamics. Convective mixing has therefore been extensively studied over the past decades (Pau et al. 2010; Riaz et al. 2006; Elenius et al. 2012; Erfani et al. 2022). The aqueous phase density change depends on total DIC concentration, in turn depending on dissolution rates, reactivity, convection and diffusion rate, and latent or induced flow in the reservoir. The acidification allows a range of reactions to occur in different geochemical environments leading to effects such as mineralization or grain dissolution, potentially altering the physical properties of reservoirs.
The technical note is structured as follows: Chapter 2 presents key features of the FluidFlower concept; Chapter 3 describes the FluidFlower laboratory infrastructure, with technical aspects and considerations for the flow cell; Chapter 4 details experimental operation and capabilities; and Chapter 5 provides the rationale for fluids used. The two flow cell varieties presented are further detailed in their own appendices with flow cell specifications, experimental protocols and example data. Appendix A details the room-scale flow cell (FF2), its operation and laboratory setup for the CO2 experiments (Fernø et al. 2023) in the 2022 FluidFlower validation benchmark (VB) study (Flemisch et al. 2023); Appendix B describes the tabletop flow cells (FF3.2) used for methodology development, rapid prototyping and iteration, and smaller meter-scale experiments (Haugen et al. 2023; Saló-Salgado et al. 2023; Keilegavlen et al. 2023; Eikehaug et al. 2023), together with a generalized experiment protocol representative of ongoing research.
2 Key FluidFlower Features
There are four essential features of the FluidFlower concept that enable meter-scale porous media flow experiments for quantitative studies of geological carbon sequestration.
2.1 Physical Repeatability
Repeated CO2 injection experiments performed on the same porous media geometry is a key capability of the FluidFlower concept. Cycling of fluids allows for investigation of isolated experimental parameters and identification of stochastic elements without the uncertainties and workload associated with rebuilding the geometry for each new experiment. The process of resetting fluids between repeated experiments is designed to keep colors and chemical conditions constant during an experiment series for increased reproducibility. Fluid cycling is documented in detail for the experiment series (Fernø et al. 2023) used in the VB study (Flemisch et al. 2023) in appendix section A.6, and a generalized example is presented in section B.4. This technique allows complete tabletop fluid resetting in a few hours and room-scale resetting in a few days.
2.2 Heterogeneous Porous Media
The porous media in the flow cells are constructed by depositing unconsolidated material with known properties to quantify observed processes for model verification, comparison and forecasting. Sand grains should remain within a known and comparatively narrow size distribution, ideally well-rounded grains with minimal shape variation to avoid unwanted packing effects and to minimize uncontrolled microheterogeneities caused by the sedimentation process. The sands should also be chemically inert unless grain dissolution or a similar process is the intended subject of study. The depositing process is designed to mimic natural underwater sedimentation. The above considerations, the construction of porous media geometries and the tools used are further detailed in (Haugen et al. 2023). Settling of unconsolidated sands may be traced using the open-source software DarSIA (Nordbotten et al. 2022, 2023; Both et al. 2023).
2.3 Seeing Fluid Phases
Differentiating between fluid phases is essential to the viability of the method. Both water and gas are normally transparent and provide little optical contrast to one another. For GCS applications, water and CO2 diffuse into each other. Mixes of these two are of particular interest, and its physical effects in a reservoir have been a topic of interest for some time in the simulation community. Aqueous concentration of CO2 and DIC depend on a complex relationship between gas capacity in the formation water, distance from gas phase CO2, diffusion rate, convective mixing, time, chemical environment and the equilibria between the DIC forms and other salts in that environment. To visualize this, we utilize the spontaneous reaction between CO2 and water producing carbonic acid. A range of water-soluble pH-sensitive dyes produce visible changes of color upon acidification, allowing clear distinction of water, gas and water containing dissolved gas. Further fluid details in chapter 5 and VB specifications in appendix A.5.
2.4 Data Collection
Time lapse images of the flow cells are captured at intervals synchronized to experiment time steps. Imperfections in camera optics cause spatial distortion in images, and the curvature of the room-scale flow cell does not communicate well with standard camera optics. Composite grid images of leveling laser lines provide a reference for correction of such lens effects via DarSIA prior to image segmentation. Images captured (by RGB sensors) never fully represent (full spectrum) true colors, and a standard color palette is included in all images as an image processing reference. Temperature, point and ambient pressure, and all fluid injections and production such as passive overflow are measured and logged. Data collection capabilities are exemplified in (Fernø et al. 2023; Saló-Salgado et al. 2023; Haugen et al. 2023; Keilegavlen et al 2023).
3 Infrastructure
The flow cells have vertical transparent plates separating observers and instrumentation from sands and fluids. Key flow cell structural considerations are detailed below.
3.1 Flow Cell Depths
Flow cell depth is a compromise between observational and operational aspects, limiting sand packing boundary and three-dimensional flow effects. The distance between the flowing gas and viewing window should be small so that diffusion allows for early detection of displacement processes in the third dimension, while allowing sufficient depth for manipulation of sands using sand manipulation tools described in (Haugen et al. 2023). Hydrophilic wall surfaces encourage gas flow within the sands rather than along the walls to minimize the effect of artificial grain structuring along the boundary. For grain sizes typically less than 2 mm associated with unconsolidated sands (e.g., Freeze et al. 1979), a depth on the order of 8–10 times the maximum grain size is preferred to mitigate poor packing conditions and preferential flow along the walls (Chapuis 2012).
3.2 Internal Forces and Hydraulic Deformation
Water exerts significant outward static pressure on the flow cell walls. The hydraulic load scales with the squared height of water in the flow cell, increasing with hydrostatic pressure and surface area (approximately eight tons for room-scale version and ¼ ton for tabletop version). Furthermore, the settling of the unconsolidated sands will lead to additional lateral forces. The large span of the walls argues for the accommodation of measurable and safe elastic deformation, rather than to strive for absolute rigidity to avoid potential brittle failure. Hence, transparent plastics has been the material of choice. The perforations of the rear panel weaken the structural integrity of the plate with unknown localization of material stresses, and a safety margin has been applied to all structural dimensions. The room-scale FluidFlower is curved to further increase rigidity with the front in a state of compression and the rear in a tensile state (see Figure 4 in Appendix A).
3.3 Viewing Window Reflections
The reflective surface of the viewing window causes artifacts in optical images. Images are captured by a camera located in the focal point of a curved flow cell, where any horizontal straight line drawn between the camera and the flow cell viewing window is orthogonal to the latter (see Figure 8 in Appendix A), which minimizes reflections compared to imaging of a flat flow cell such as the tabletop version. With maximum overlap between direct and reflected camera field of view, lamps and other objects remain hidden to the camera if not placed directly between the camera and flow cell. This allows illumination with high incident angle and a compact laboratory footprint. Curvature also increases structural rigidity and allows a larger window span without requiring impractically thick walls to withstand internal forces. Film studio standard high-frequency ‘flicker-free’ LED lamps are located outside of the camera field of view.
3.4 Construction Materials and Rear Plate Perforations
Materials in contact with the internal volume should have minimum influence on porous media chemistry and flow behavior. The materials must be resistant to the corrosive nature of the salts and fluctuating pH in the system to allow study of the contained system rather than the container itself. Rear plate perforation limits the instrumentation and tubing required to manipulate and measure the fluids of interest to the rear plate. Hence, the front side remains free of disturbing technical elements. Fluid resetting for repeated experiments occurs through a series of perforations along the lower flow cell boundary where water may be injected to replace the fluids in the system. The rigid flow cell boundary is provided by a double-flange frame for viewing window support. The chassis-like substructures are built to accommodate instrumentation, mobility and ease of operation. They connect to hinges on the flow cell and are constructed to transfer their load to the chassis bottom where adjustable legs are installed, as well as transport wheels for the room-scale version.
See appendix sections A.1 through A.3 and B.1 through B.3 for further details on the flow cell designs.
4 Experimental Operation and Capabilities
This chapter provides a general overview of technical instrumentation that enables the injection and quantitative monitoring of CO2 flow, trapping and dissolution in the geological geometries in the flow cells.
4.1 Water System
There are separate control systems for the aqueous and gaseous phase injections (illustrated in Fig. 2). Water flow is operated with computer-controlled double-piston pumps (Chandler Engineering Quizix Precision used in this work) that connect to the system via gas traps that double as particle traps. Valve manifolds connect gas traps to flow cell ports for controlled injection/production from specific ports, or with a total rate distributed between multiple ports. Manual and/or computer-controlled pneumatic valves may be used. Gravity induced flow has been used instead of piston pumps in experiment series such as (Saló-Salgado et al. 2023), utilizing the hydrostatic pressure obtained by simply placing the water supply above the flow cell.
Conceptual fluid systems: Water (left) and gas (right). Water from supply (A) to computer-controlled cylinder pump (B) and further through a gas trap (C) before it is directed to the system perforations by valve manifolds (D). Displaced water (overflow) € exits an open perforation and is collected by a waste canister (F) sitting on a mass logging scale (G). Gas flows from gas canister (H) with pressure regulator (J), and further through a flow restriction needle valve (K) and a computer-controlled mass flow controller (L) connected to the system perforations or the atmosphere via a three-way valve (M)
4.2 Gas System
Gas flow is regulated by computer-controlled mass flow controllers (MFC, e.g., Bronkhorst El-Flow Prestige), with gas supplied from pressurized gas canisters. Standard pressure regulators connect the gas bottles to the MFCs via a flow restriction needle valve that reduces pressure fluctuations originating from the spring-loaded pressure regulator mechanism. MFC performance must be tested and tuned prior to all experiments to keep fluctuations within listed instrument uncertainty. Aqueous and gaseous operations should ideally be scripted to limit the potential for human error.
4.3 Logging Water Displacement
A constant water level is maintained due to the passive overflow function of the always-open perforations positioned on the top of the flow cell. Water is injected at a constant rate into the free water above the porous media geometry to keep the overflow port wet to eliminate surface tension effects. This approach keeps fluctuations in the hydrostatic pressure to a minimum. By logging overflow rates via interval mass measurement, rates of volumetric displacement of water become detectable and may be coupled to gas phase CO2 volume and its dissolution rate in the system.
4.4 Degassing Water
Aqueous solutions should be degassed by a vacuum pump (e.g., Edwards RV5) prior to injection to minimize the influence of atmospheric gases dissolved in the water on measured variables. Agitation such as magnetic stirring significantly speeds up the equilibration process. Remaining atmospheric gases in the water affect the CO2 dissolution rates, and Henry’s law (further detailed in Chapter 5) implies that when CO2 dissolves into atmosphere-saturated water, non-negligible quantities of nitrogen and oxygen are expelled from water in atmospheric equilibrium. This presents significant challenges for quantitative analysis of time lapse image series and resetting of the porous media if unattended.
4.5 Pressure and Temperature
Pressure transducers connect to chosen perforations for point logging. Typically, fluctuations of interest in the systems occur in the sub-mbar regime and require sensors of adequate precision for any meaningful measurements. Working at sea-level pressure implies atmospheric fluctuations represent significant uncertainty if unattended. Not only may system response disappear in atmospheric noise, but flow experiments using gas phase CO2 are highly dependent on absolute pressure and temperature for both density and dissolution. Temperature is sought to be kept constant during experiment series, yet a gradient has been observed along the height of the flow cells in cold-floor laboratories. Point logging of temperature is collected by dual-purpose pressure transducers (e.g., ESI Technology GS4200-USB).
5 FluidFlower Fluids
Differentiating between fluid phases is essential to the viability of the FluidFlower concept. In pure form, water and gas are both transparent and provide little optical contrast to one another. By introducing water-soluble dyes, the transparent gas becomes clearly distinct from the colored water. The CO2 is injected as a dry gas and will partially dissolve into the formation water. Distinguishing CO2-saturated water is of particular interest for GCS applications.
Dissolved or aqueous CO2 complexes with water and forms carbonic acid. Acidification of the initially neutral to mildly acidic system allows compounds sensitive to pH changes to be an accessible method of aqueous CO2 detection. Common pH indicators are organic compounds that undergo a configurational change when a proton is added or subtracted from the molecule. The configuration change in turn causes a change in the wavelengths of light absorbed, observed as a visible and reversible change of color (Sabnis 2007).
Pure water has a theoretical pH of 7.0, with \(\left[ {{\text{H}}_{3} {\text{O}}^{ + } } \right] = \left[ {{\text{OH}}^{ - } } \right] = 10^{ - 7} {\text{M}}\), but with no buffering capacity it is extremely sensitive to impurities. Atmospheric CO2 diffuses into the water and acidification of freshly deionized water can be measured immediately after air exposure. Hence, pure water in equilibrium with atmospheric gases typically has a pH of approximately 6, compared with a pure CO2 atmosphere (emulating conditions inside the flow cells) at approximately pH 4. Several pH indicator compounds with a transition between pH 6 and 4 exist, typically with transition from a high-absorption color at higher pH toward a lower intensity color (lower pH). Improper match between indicator transition and CO2 saturation pH range complicates precise determination of CO2 concentrations and typically produces unappealing visual impressions.
pH indicators typically have a transition range of \(\Delta\) pH 1–2, regardless of specific pH transition range. With pH being a logarithmic measure of acid or base concentrations centered around 7, transition ranges closer to 7 distinguish a narrower range of concentrations. We have opted to work under the assumption that widening the pH range of the water phase with a diluted strong base has a measurable yet limited effect on CO2 dissolution and the overall system behavior. This allows more intense contrast colors and distinct transition ranges (see Fig. 3), leading to improved signal strength and the possibility of distinguishing multiple concentration levels of CO2.
Bromothymol blue (BTB, transition pH 6.0–7.6) and methyl red (MRe, transition pH 4.4–6.2) have been used extensively in our experiments (solution described in appendix section A.5). In an acidic environment, however, the protonated form of methyl red has a relatively low solubility in water, and high concentrations result in precipitation at pH 6 and below, like that observed in (Fernø et al. 2023; Haugen et al. 2023; Saló-Salgado et al.2023).
The equilibrium concentration of dissolved gas in water is proportional to the system pressure and inversely proportional to temperature and varies between types of gases and combinations thereof. This relation is given by Henry’s law as species specific solubility and remains a topic of experimental study (Henry 1803; Sander et al. 2015). Aqueous gas solubility under a mixed atmosphere is proportional to the partial pressure of the gas species. It follows that when water in equilibrium with an atmosphere containing a combination of gases A is exposed to a different atmosphere dominated by a different gas B, the gaseous partial pressure of A will decrease while its aqueous partial pressure remains high. To re-achieve equilibrium, aqueous concentration of B and gaseous concentration of A must both increase.
When CO2 is injected into water in equilibrium with our N2-rich atmosphere, CO2 displaces N2 (and O2, Ar, etc.) from the aqueous phase if temperature and absolute pressure remains constant. We have opted to reduce the overall concentration of aqueous gases in the water phase by equilibrating or ‘degassing’ the water phase by applying a partial vacuum and for experiments with equipment constraints, by manipulating temperature. Water evaporates at a full vacuum (and cannot alternatively be infinitely hot) and therefore cannot be entirely degassed, but rather exists somewhere on a gas saturation spectrum. The influence of varying degassing is discussed further in Haugen et al. 2023.
6 Concluding Remarks
The FluidFlower concept, as presented in this technical note, represents a new laboratory infrastructure for experimental research to add to the knowledge base for which decisions regarding GCS is made. Details and engineering insights for constructing and operating these highly controlled and adjustable systems are presented for flow cells of different complexity. Both designs have been proven viable and reliable for experiment series lasting up two years and counting. These physical flow cells, the methodology described and the in-house developed open-source software DarSIA (used to analyze high-resolution time lapse images), together make it possible to plan and perform a variety of porous media fluid flow experiments on the meter scale with quantification of key parameters. This provides an opportunity to obtain high-quality experimental data for validation and calibration of numerical simulation models.
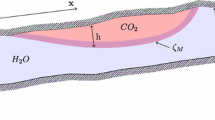
The geological geometries that can be modeled by the FluidFlower are representative of large-scale structures (e.g., faults and folds) and stratigraphic layering (reservoir units, seal units, etc.) observed in subsurface reservoir systems, and the FluidFlower concept allows for studying the impact of these geological geometries on CO2 trapping and flow dynamics. Subsurface CO2 trapping mechanisms that can currently be studied with the FluidFlower concept include; structural and stratigraphic trapping under sealing sand layers; residual trapping is seen in regions with intermediate water saturation and is temporary because of rapid dissolution; dissolution trapping is observed almost instantaneously when the injected CO2 dissolves into the water phase; and convective mixing which occurs when the denser CO2-saturated water migrates downwards, generating gravitational fingers.
Ultimately, our concept allows observation of spatiotemporal interactions of physical processes of multiphase, multicomponent flow during CO2 immobilization in a porous medium at the meter scale, allowing investigations at both pore and Darcy scale. This has high relevance for GCS applications and we encourage the porous media community to explore this experimental method.
References
Both, J.W., Storvik, E., Nordbotten, J.M., Benali, B.: DarSIA v1.0. Zenodo (2023) https://zenodo.org/records/7515016
Britton, H.T.S., Robinson, R.A.: Universal Buffer Solutions and the Dissociation Constant of Veronal. J. Chem. Soc. 1456-1462 (1931). https://doi.org/10.1039/JR9310001456
Chapuis, R.P.: Predicting the saturated hydraulic conductivity of soils: a review. Bull. Eng. Geol. Env. 71, 401–434 (2012). https://doi.org/10.1007/s10064-012-0418-7
Eikehaug, K., Haugen, M., Benali, B., Alcorn, Z.P., Rotevatn, A., Nordbotten, J.M. Fernø, M.A.: Geological carbon storage at the University of Bergen, Norway. In: Médici, E.F., Otero, A.D. (eds) Album of Porous Media. pp 46, Springer, Cham (2023). https://doi.org/10.1007/978-3-031-23800-0_34
Elenius, M.T., Nordbotten, J.M., Kalisch, H.: Effects of a capillary transition zone on the stability of a diffusive boundary layer. IMA J. Appl. Math. 77(6), 771–787 (2012). https://doi.org/10.1093/imamat/hxs054
Erfani, H., Babaei, M., Berg, C.F., Niasar, V.: Scaling CO2 convection in confined aquifers: Effects of dispersion, permeability anisotropy and geochemistry. Adv. Water Resour. Vol 164, 104191, (2022). https://doi.org/10.1016/j.advwatres.2022.104191
Fernø, M.A., M. Haugen, K. Eikehaug, O. Folkvord, B. Benali, J.W Both, E. Storvik, C.W. Nixon, R.L.: Gawthrope and J.M. Nordbotten. Room-scale CO injections in a physical reservoir model with faults. Transp Porous Med (2023). https://doi.org/10.1007/s11242-023-02013-4
Flemisch B., Nordbotten J.M., Fernø M.A., Juanes R., Class H., Delshad M., Doster F., Ennis-King J., Franc J., Geiger S., Gläser D., Green C., Gunning J., Hajibeygi H., Jackson S.J., Jammoul M., Karra S., Li J.,Matthäi S.K., Miller T., Shao Q., Spurin C., Stauffer P., Tchelepi H., Tian X., Viswanathan H., Voskov D., Wang Y., Wapperom M., Wheeler M.F., Wilkins A., Youssef A.A., Zhang Z.: The FluidFlower Validation Benchmark Study for the Storage of CO2. Transp. Porous Med. (2023). https://doi.org/10.1007/s11242-023-01977-7
Freeze R.A., Cherry J.A. Groundwater. Prentice-Hall (1979). ISBN: 0–13–365312–9
Haugen, M., Saló-Salgado, L., Eikehaug, K., Benali, B., Both, J.W., Storvik, E., Folkvord, O., Juanes, R., Nordbotten, J.M., Fernø, M.A. Physical variability in meter-scale laboratory CO2 injections in faulted geometries. Transp. Porous Med. (2023, in review). PrePrint: https://doi.org/10.48550/arXiv.2301.07347
Henry, W.: III: Experiments on the quantity of gases absorbed by water, at different temperatures, and under different pressures. Phil. Trans. R. Soc. 9329–274 (1803). https://doi.org/10.1098/rstl.1803.0004
Keilegavlen E., Fonn E., Johannessen K., Eikehaug K., Both J., Fernø M., Kvamsdal T., Rasheed A., Nordbotten J.M.: PoroTwin: A digital twin for a FluidFlower rig. Transp Porous Med (2023). https://doi.org/10.1007/s11242-023-01992-8
Kovscek, A.R., Nordbotten, J.M., Fernø, M.A.: Scaling up FluidFlower results for carbon dioxide storage in geological media. Transp Porous Med (2023, in review). Preprint: https://doi.org/10.48550/arXiv.2301.09853
Nordbotten, J.M., Flemisch, B., Gasda, S.E., Nilsen, H.M., Fan, Y., Pickup, G.E., Wiese, B., Celia, M.A., Dahle, H.K., Eigestad, G.T., Pruess, K.: Uncertainties in practical simulation of CO2 storage. Int. J. Greenhouse Gas Control (2012). https://doi.org/10.1016/j.ijggc.2012.03.007
Nordbotten, J.M., Fernø, M.A., Flemisch, B., Juanes, R., Jørgensen, M.: Final Benchmark Description: Fluid Flower International Benchmark Study. Zenodo (2022). https://doi.org/10.5281/zenodo.6807102
Nordbotten, J. M., Benali, B., Both, J.W., Brattekås, B., Storvik, E., Fernø, M.A. (2023) DarSIA: An open-source Python toolbox for twoscale image processing of dynamics in porous media. Transp. Porous Med. (2023). https://doi.org/10.1007/s11242-023-02000-9
Pau, G.S.H., Bell, J.B., Pruess, K., Almgren, A.S., Lijewski, M.J., Zhang, K.: High-resolution simulation and characterization of density-driven flow in CO2 storage in saline aquifers. Adv. Water Resour. 33(4), 443–455 (2010). https://doi.org/10.1016/j.advwatres.2010.01.009
Riaz, A., Hesse, M., Tchelepi, H., Orr, F.: Onset of convection in a gravitationally unstable diffusive boundary layer in porous media. J. Fluid Mech. 548, 87–111 (2006). https://doi.org/10.1017/S0022112005007494
Sabnis, R.W.: Handbook of Acid-Base Indicators. CRC Press (2007). https://doi.org/10.1201/9780849382192
Saló-Salgado, L., Haugen, M., Eikehaug, E., Fernø, M.A., Nordbotten, J.M., Juanes, R.: Direct comparison of numerical simulations and experiments of CO injection and migration in geologic media: value of local data and predictability. Transp. Porous Med. (2023). https://doi.org/10.1007/s11242-023-01972-y
Sander, R.: Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmosp. Chem, Phys, 15, 4399–4981 (2015). https://doi.org/10.5194/acp-15-4399-2015
Acknowledgements
KE and MH are partly funded by Centre for Sustainable Subsurface Resources, Research Council of Norway (RCN) project no. 331841. MH is also funded by RCN project no. 280341. BB is funded from RCN project no. 324688. The authors would like to acknowledge engineering interns Ida Louise Mortensen, Mali Ones, Erlend Moen and Johannes Salomonsen for their laboratory and workshop contributions to the experiments. Presented tabletop illustrations use images from experiments by students Ingebrigt Lilleås Midthjell, Håkon Kvanli, Håkon Stavang, Maylin Elizabeth Ordonez Obando and Janner Fernando Galarza Alava. Geology input from and room-scale illustration image from geometry developed in collaboration with Robert Leslie Gawthorpe and Casey William Nixon. Roald Langøen, Charles Thevananth Sebastiampilai, Thomas Husebø, Werner Olsen, Thomas Poulianitis and Rachid Maad have contributed with technical solutions from parts machining to instrument and software prototyping.
Funding
Open access funding provided by University of Bergen (incl Haukeland University Hospital).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix A: Operations and Infrastructure Enabling Repeated CO2 Injections in the FluidFlower Validation Benchmark Study
This appendix provides further details of the room-scale FluidFlower (in-house designation FF2). The flow cell itself is detailed (see Figs. 4–7 and Tables 1–2) in sections A.1 through A.3, while sections A.4 through A.6 present specific details of the Fluid Flower validation benchmark (VB) study (Flemisch et al. 2023) as an example application of the FluidFlower. Section A.4 provides the laboratory layout, emphasizing placement of instrumentation and lighting. Sections A.5 and A.6 provide ‘back-end’ operational information on water phase preparation to further document the five repetitive CO2 injections (detailed in Fernø et al. 2023). used in the VB study for CO2 storage.
1.1 A.1 Layout of the Room-scale FluidFlower Flow Cell
Front, side and top view profiles of the room-scale FluidFlower (FF2) used for the VB study CO2 injection experiments. Instrumentation rails are attached to the rear plate and to the substructure. (Table 1)
1.2 A.2 Structural Components in the Room-Scale FluidFlower
Fig. 5 shows the structural components of the room-scale flow cell, whereas Table 2 provides an overview of the materials used to construct the flow cell.
Exploded assembly of structural room-scale flow cell components. A) A hot-molded monolithic acrylic window rests on a B) ledge inside the C) front frame and a D) sacrificial polycarbonate plate is clamped to its outer radius. The rigid flow cell boundary is provided by steel frames. The front frame is constructed for both conventional flange loading with integrated threads to resist compression and buckling, as well as to resist the keystone-like lateral force of the acrylic plate arising from deformation under load. The outer radius of the E) rear plate needs only resist stretching, not compression or buckling, and is therefore based on a simpler design where top, side and bottom wooden beams are laminated outward and capped with a heavy F) steel band dimensioned to resist inter-bolt deformation. The G) substructure connects to H) hinges on the rear plate assembly of the flow cell. The substructure is designed using a traditional truss approach and allows some twist to distribute forces between the wheels during transport and to dampen shocks. The front frame assembly is attached to the rear assembly by bolts extending through the circumference of the rear plate from the rear frame
1.3 A.3 Perforations in the Room-Scale FluidFlower
The room-scale FF2 has 56 perforations in a grid layout, plus 3 emergency drains at the bottom and 1 permanently open overflow drain at the top (see Fig. 6). Perforations are modified cast 316L steel ¾’’ marine thru-hull fittings with external tensioning nuts to allow a variety of configurations (see Fig. 7). The internal flanges rest on polyurethane in a conical indentation in the plate. The internal perforation volumes contained either solid (plugged) or heat-clamped steel tube perforated polyoxymethylene (POM) fittings with their front-facing circumference sealed off with aquarium silicone. A POM cap secures the insert.
Perforations are located at every second intersection of a 200 ∙ 200 mm square grid along the view side radius of the porous media (arclength at r = 3600 mm), translating to 201 ∙ 200 mm (w ∙ h) grid along the outer flow cell radius (r = 3618 mm), symmetrically distanced from the window boundaries. Three emergency drains are located at the bottom and at there is an always-open port at the top to avoid overfilling. The seven perforations in the lower row have their inner tube extended to the lower flow cell boundary
Cross sections of: A Perforation as configured for sensor wells in the VB study, whereas for injection wells the inner steel tube extended 10 mm into the system. The seven perforations in the lower row have their inner tube extended to the lower flow cell boundary. B Frame cross section 180-mm-long M12 bolts connect rear and front frames. The inner sacrificial polycarbonate glass is clamped in place and the structural acrylic glass rests on a ledge inside the front frame. A in 2:1 scale relative to B. Both oriented with flow cell front toward left
1.4 A.4 Validation Benchmark Study Laboratory Layout
The CO2 experiments were performed in a ground-floor exhibit room within a semi-triangular plywood box (termed ‘blackbox’) to limit temperature variations (Fig. 8). The reflected section of the floor and ceiling, the personnel access point and the wall immediately behind the cameras were all covered with black tapestry. The camera was positioned in the focal point of the curved viewing window, and high-frequency LED lamps were located at each lateral side of the flow cell at half its height. A leveling laser was used to produce the laser grid used for lens correction for image post-processing. A control room was set up in an L shape between the diagonal side of the box and the windows, with all windows blocked to obey the double-blind criteria for the study. Multiple monitors were used for operation, and critical equipment was operated by batteries in case of power outages. The control room included fluid supply systems, with a water distribution manifold connected to the flush ports. A secondary camera provided a live view of the experiment.
Left: VB study laboratory layout and location of flow cell within the ‘blackbox,’ where images were captured through the front window of the A porous media geometry by the B camera. C Lamps were placed in front of the flow cell and temperature was regulated by D panel ovens. Sensors and plumbing were connected to perforations on the E rear rear side of the cell. Fluid cycling and experiments were operated from the F control room dry zone with assorted computers and monitors beside the G fluid supply systems. Liquids were stored and samples were measured in the H wet zone. Equipment repair and modifications were confined to the J garage. K Vacuum pump and air compressor location. L Pressurized air for pneumatic equipment. Right: The curvature of room-scale version allows imaging with minimum reflection. Incident camera field of view overlaps horizontally with reflected field of view, and objects not directly between the camera and experiment remain hidden to the camera
1.5 A.5 Validation Benchmark Study Water Phase Preparation
An alkaline combination of sodium salts of BTB and MRe was used as the pH-sensitive solution termed ‘VB green’ with mixing proportions shown in Table 3. To minimize contamination and degradation, the solutions were prepared using freshly deionized (except C2) water in batches of cans at most 48 h prior to injection (except C1). The VB green was prepared by dissolving and thoroughly mixing 0.80 g NaOH pellets into one liter of water. 200 mL of NaOH solution was watered to 2000 mL before adding 3.88 g of NaBTB and 5.24 g of NaMRe. The mixtures were magnet stirred and repeatedly shaken in closed containers before added to DI water (1000 mL added to 20 L). The mixture was then sealed, shaken vigorously and heated to approximately 30 \(^\circ\) C to avoid gas release inside the flow cell. In solution the components dissociate to the compounds listed in Table 4. A fraction of the sodium ions would combine with CO2 from air exposure, and the pH was therefore measured immediately prior to injection. After the CO2 injection, 0.5 mM NaOH cleaning solution was prepared in batches by dissolving and thoroughly mixing 2.00 g NaOH into 1 L of water before adding 200 mL NaOH to cans containing approximately 20 L before adding water up to the 21 L mark.
Providing a uniform initial condition was challenging, both for a single experiment and over a series of experiments, due to the poor buffering properties of pure water. We exemplify this by considering in detail the initial conditions for the experiments. The water phase injected into the flow cell is known to have had compositional variation between experiments, trending toward less variation at later experiments due to implementation of stricter operational routines. Notably, the VB green in the experiment cycle 1 (C1) was exposed to air for an extended period of time, while the deionization filter used for preparation of VB green used in C2 was deemed faulty. pH in the system was documented prior to experiments listed in Table 5, with values obtained by measuring water samples collected immediately prior to CO2 injections. The trend toward higher pH in the upper section of the system prior to the later experiments corresponds with the reduced volumes of VB green injected during resetting to limit visual impact of MRe precipitation. Reduction of injected VB green is thought to have led to more cleaning solution remaining in system. An expected result of this would be increased CO2 solubility in these regions.
After the experiments were concluded, a series of buffer solutions were injected to provide reference colors for image post-processing, shown in Fig. 9. These images highlight the impact of the sand grains on the visual impression of the pH-sensitive salts and may be contrasted with Fig. 3.
The benchmark geometry was filled with buffer solutions after conclusion of the experiment series to provide color calibration for DarSIA (Nordbotten et al. 2023) image processing. The buffers were prepared using weak acids titrated with sodium hydroxide (Britton and Robinso, 1931) and contained VB green color compounds in images A, B and C. The buffer in image D was prepared without MRe. pH was sampled post-injection to verify buffer homogeneity in the system:A pH 7.96–7.98, 72 L injected. B pH 6.99–7.01, 103 L injected. C pH 6.45–6.49, 99 L injected. D Without MRe, pH 8.00–8.04
1.6 A.6 Validation Benchmark Study Experiment Cycling
The resetting procedure was intended to reproducibly reset the fluids inside the flow cell, so that deviations between the internal flow cell conditions such as local variations in the chemical environment due to different distributions of mixing zones were kept at minimum. Fluid resetting was performed in an injection or ‘flushing’ sequence where the fluid was injected sequentially into the seven ports along the bottom of the flow cell (Fig. 6). This process was standardized for all experiments to keep the diluting effect of mixing zones low and consistent. The condensed protocol presented in this section serves both as an example of experiment cycling and supplementary documentation of system homogeneity between the reported experiments (Fernø et al., 2023).
While they followed a scripted CO2 injection protocol, the experiment series was the first room-scale FluidFlower experiments performed, and methodology consistency was improved upon from a characterization experiment and throughout the experimental series. Additionally, several aspects required adaptation and optimization, as reflected by the variations in the resetting protocol detailed in Table 6. Visual time steps in one full experiment cycle are shown in Fig. 10, with the step numbers in the table and figure corresponding to one another.
Fluid cycling time steps show the resetting process before and after cycle C5, starting and ending with clean system in steps 1 and 32. Steps 1 to 16 show the injection of pH-sensitive solution VB green. Steps 17 a through c show milestone time steps during and after CO2 injection. Steps 18–32 display the cleaning process (notice apparent chromatographic separation of highly mobile blue BTB from orange MRe, visible in steps 18 to 21, with removal of residual MRe requiring an extended cleaning process). Step numbers correspond to those in Table 6 A visual representation of the ports used in the resetting procedure have been superimposed onto the images, where active ports in that time step are displayed as green and inactive ports are orange. Cycles C2 through C5 follow this exact sequence of operations, with some temporal and volumetric variation
Appendix B: Tabletop FluidFlower
This appendix details the tabletop FluidFlower. Different versions exist and have been used for rapid prototyping and methodology development, as well as experiment series such as those detailed in (Haugen et al. 2023; Saló-Salgado et al. 2023, Keilegavlen et al. 2023). In appendix sections B.1 through B.3 we provide the physical characteristics of FF3.2 ‘Bilbo’ (see Figs. 11, 12, 13, 14 and Tables 7 and 8), the tabletop FluidFlower version used in these works.
Section B4 provides ‘back-end’ operational information on fluid preparation and resetting, and includes a generalized example experiment protocol.
2.1 B.1 Layout of a Tabletop FluidFlower
2.2 B.2 Structural Components in a Tabletop FluidFlower
(See Fig. 12). Exploded assembly of flow cell components. Acrylic windows with spacers between them are clamped between the steel frames. Bolts and nuts clamp the circumference of the flow cell. The rear frame connects to the FlexLink substructure, onto which valve manifolds and instrumentation rails are connected. (Table 8)
2.3 B.3 Perforations in a tabletop FluidFlower
Perforations in the tabletop FluidFlowers as configured for the PoroTwin AI controlled experiment series (Keilegavlen et al. 2023). Additional perforations are comparatively easily added between experiment series, and therefore, the tabletop versions to date have been perforated on an as-needed basis
A Perforation cross section as configured for VB and legacy experiments. Perforations are external-gasket standard stainless steel double male parallel NPT ½’’ to 1/8’’ Swagelok connectors, for post-VB configurations with a wedge lodged into the internal volume from the front-facing side so that sands are not extracted from the system during fluid suction. B Frame cross section 60-mm-long M6 bolts with nuts secure all layers in the structure
2.4 B.4 Example Experiment Protocol in a Tabletop FluidFlower
This section contains a generalized example of a protocol (Table 9) for repeated tabletop-scale CO2 injection experiments, similar to those in (Saló-Salgado et al. 2023; Haugen et al. 2023; Eikehaug et al. 2023). The tabletop FF3 flow cell series are dimensioned for convenience in the laboratory, and experiments are typically reset in a few hours. This allows experiments to be performed in substantially faster succession than the room-scale FF2, while requiring much less resources. Ongoing experiment series have seen multiple CO2 injections per week, and water phase only experiments such as (Keilegavlen et al. 2023) have seen complete cycling from and to a clean state in hours. The internal volume of the flow cells allows gravity induced flow to be a practical alternative to costly pumps.
Fluid displacement is performed in a flushing sequence following the same principle as that shown in high detail for FF2 in appendix section A.6. The smaller tabletop FluidFlower allows for a simplified sequence (Fig. 15), and the introduction of wedged perforations (from version FF3.2 and onwards) reduces the risks associated with fluid production. Multiple perforations are used for sequential injection to reduce the required cycled fluid volume, producing minimal waste while promoting fluid homogeneity.
Illustration injection of BTB solution with typical time steps: 1 System cleaned post-experiment. 2 Injection in bottom right corner (green dot) with production in left (blue dot). When the porous media is fully saturated along the bottom, production is stopped to allow the mixing zone to pass left port before 3 injection is moved to left port. Injection continues until 4 system is saturated. Inactive perforations indicated with red dots. The sequence has been simplified (Table 9)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eikehaug, K., Haugen, M., Folkvord, O. et al. Engineering Meter-scale Porous Media Flow Experiments for Quantitative Studies of Geological Carbon Sequestration. Transp Porous Med 151, 1143–1167 (2024). https://doi.org/10.1007/s11242-023-02025-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11242-023-02025-0