Abstract
Carbon dioxide injection into deep saline aquifers is governed by a number of physico-chemical processes including mineral dissolution and precipitation, multiphase fluid flow, and capillary trapping. These processes can be coupled; however, the impact of fluid–rock reaction on the multiphase flow properties is difficult to study and is not simply correlated with variations in porosity. We observed the impact of rock mineral dissolution on multiphase flow properties in two carbonate rocks with distinct pore structures. Observations of steady-state \(\hbox {N}_2\)–water relative permeability and residual trapping were obtained, along with mercury injection capillary pressure characteristics. These tests alternated with eight stages in which 0.5% of the mineral volume was uniformly dissolved into solution from the rock cores using an aqueous solution with a temperature-controlled acid. Variations in the multiphase flow properties did not relate simply to changes in porosity, but corresponded to the changes in the underlying pore structure. In the Ketton carbonate, dissolution resulted in an increase in the fraction of pore volume made up by the smallest pores and a decrease in the fraction made up by the largest pores. This resulted in an increase in the relative permeability to the nonwetting phase, a decrease in the relative permeability to the wetting phase, and a modest, but systematic decrease in residual trapping. In the Estaillades carbonate, dissolution resulted in an increase in the fraction of pore volume made up by pores in the central range of the initial pore size distribution, and a corresponding decrease in the fraction made up by both the smallest and largest pores. This resulted in a decrease in the relative permeability to both the wetting and nonwetting fluid phases and no discernible impact on the residual trapping. In summary, the impact of rock matrix dissolution will be strongly dependent on the impact of that dissolution on the underlying pore structure of the rock. However, if the variation in pore structure can be observed or estimated with modelling, then it should be possible to estimate the impacts on multiphase flow properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The movement of \(\hbox {CO}_2\) injected into the subsurface involves an interplay of geochemical, geomechanical, flow, and transport processes (Depaolo and Cole 2013). Understanding the coupling between these processes is essential for accurate modelling of the migration and trapping of the \(\hbox {CO}_2\).
One potentially significant coupling is geochemical reactions that induce changes in fluid flow properties. The injected \(\hbox {CO}_2\) will dissolve into the formation brine and lead to a decrease in pH. Field observations have confirmed this, with pH generally observed between 5.7 and neutral, with values as low as 3.2 observed in the case of the Frio project (Knauss et al. 2005; Hovorka et al. 2006; Mito et al. 2008). In carbonate rock reservoirs, this will under-saturate the aqueous phase with respect to carbonate minerals and induce the dissolution of calcite, dolomite, and magnesite. Sufficient dissolution will result in structural alterations to the pore structure of the rock that control fluid flow. This includes the porosity, absolute and relative permeability, residual trapping, and mechanical properties (Vanorio et al. 2011; Bemer and Lombard 2010).
The impact of mineral dissolution on fluid flow has been investigated qualitatively in a number of contexts. Initial studies were in application to acid stimulation and \(\hbox {CO}_2\)-enhanced oil recovery (Hoefner and Fogler 1988; Fredd and Fogler 2011). The focus has been on categorising patterns of mineral dissolution, e.g. into wormhole regimes or more uniform dissolution. The occurrence of one pattern or another has been found to depend on the spatial and temporal scales, fluid flow velocity, and the kinetics of mineral–fluid chemical reactions (Oleg et al. 2009). The dimensionless Peclet (Pe) and Damkohler (Da) numbers have been used to define these regimes (Schechter and Gidley 1969; Daccord et al. 1989; Golfier et al. 2002). Recently, these observations have been shown to apply to \(\hbox {CO}_2\)–brine systems relevant to carbon storage, including two-phase flow systems (Mangane et al. 2013; Luquot et al. 2014; Menke et al. 2015; Luhmann et al. 2017; Celia 2017). However, the implications of the occurrence of these dissolution patterns for modelling changes in flow properties are not addressed by these studies.
A number of studies have investigated the impact of rock matrix dissolution on single-phase flow properties, porosity and absolute permeability (Mangane et al. 2013; Luquot et al. 2014; Menke et al. 2015; Noiriel et al. 2009; Qajar et al. 2013). The studies used aqueous fluids with a pH range from 3.1 to 4.4, relevant to the region within a few metres of a \(\hbox {CO}_2\) injection well. These studies found that absolute permeability increased with increasing porosity. At higher pH, the studies of (Mangane et al. 2013; Qajar et al. 2013; Luquot et al. 2014; Luhmann et al. 2017) found that absolute permeability could also decrease even as porosity increased. This was hypothesised to have resulted from pore clogging from fine particles that had been dislodged in the dissolution process.
Less well understood are the impacts of rock dissolution on multiphase flow properties, relative permeability, capillary pressure, and residual trapping characteristics, key to modelling field-scale \(\hbox {CO}_2\) plume migration (Krevor and Pini 2019; Krevor et al. 2015). These are continuum properties that are used to represent the aggregate impact of pore structure on multiphase fluid flows (Bear 2013). Measurements of these properties are conventionally performed on homogenous samples at size scales larger than what is considered a representative elementary volume (Egermann et al. 2005; McPhee et al. 2015). A key challenge in evaluating the impact of changes in pore structure on multiphase flow properties is inducing those changes systematically and in a way that the changes are distributed homogenously throughout the rock sample. In principle, the Da and Pe number scaling can be used in the alteration of rock samples while flowing reactive fluids through the core. In practice, we have found that the injection of acidic fluids into carbonate rocks results in non-uniform dissolution of the rock core, even at high fluid flow velocities.
In this work, we used an alternative approach. Following from (Egermann et al. 2006), a temperature-controlled acid was used to dissolve rock matrix homogenously throughout the core samples. This type of dissolution may not be representative of a reservoir scenario where both reaction and transport controls will combine in the resulting impact on the pore structure. However, this approach allowed us to systematically evaluate the impact of pore structure changes, induced by reaction, on the multiphase flow properties. We evaluated the multiphase flow properties of carbonate rocks with distinct pore structures over a sequence of steps in which mineral dissolution was induced homogenously throughout the rock cores. By observing the variation in the relative permeability and residual trapping with alterations to the pore size distribution of the rocks, we were able to directly attribute the observed variations in flow properties to the variations in pore structure.
2 Experimental Methods
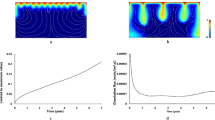
Two carbonate rocks were obtained from quarries, selected for their distinct pore structures. The Ketton carbonate was an ooidal limestone with a bimodal pore structure. The Estaillades carbonate was a bioclastic limestone with a wide range in pore size and structure. High-resolution X-ray images of the rocks obtained with a micro-CT scanner are shown in Fig. 1, and corresponding pore size distributions measured with mercury porosimetry are shown in Fig. 2. Note that X-ray imaging used in quantifying fluid saturation was obtained at much lower resolution with a conventional medical CT X-ray scanner.
Routine properties of the rock samples are summarised in Table 1. The porosity was measured using X-ray imaging, and absolute permeability was measured with distilled water in a core flood using standard techniques described in Niu et al. (2015). Both rocks are composed of more than 99% calcite (Lai et al. 2015). Capillary pressure characteristic curves were characterised using mercury injection capillary pressure measurements (AutoPore IV 9500, Micromeritics). These observations were reported as a pore throat radius, \(r_\mathrm{{p}}\), distribution using the Young–Laplace relationship, \(r_\mathrm{{p}}={2\sigma \cos \theta }/{P_\mathrm{{c}}}\), with contact angle and interfacial tension estimated to be \(\theta =130\,^{\circ }\) and \(\sigma =485\) mN/m.
Uniform dissolution was induced in the rock samples making use of a temperature-controlled acid. This was following from (Egermann et al. 2006) where the approach was used to evaluate the impact of rock dissolution on single-phase flow properties. A solution under the commercial name Acidgen FG3\(^{{\textregistered }}\) (a trademark of Cleansorb Ltd.) was used for this study. It is an in-situ organic acid production system. Water-soluble organic compounds (an ester and an enzyme) hydrolyse at elevated temperatures to produce formic or acetic acid. It is primarily used in the oil industry for obtaining formation coverage in acidising for permeability enhancement of natural fracture networks (Harris et al. 2001; Buijse et al. 2003).
The solution was diluted with water as mixture of \(10\%\)w/v. In a test of the system, we monitored the pH of the solution as the temperature was increased from 19 to \(65\,^{\circ }\)C over 180 min, as shown in Fig. 3, and the pH from 3.25–1.3, with no significant lag relative to the timescales of the temperature change. Because the solution was weakly acidic at low temperature, a 2-cm-long sacrificial core sample was placed upstream of the experimental rock samples to buffer reactions while the core was initially saturated with the reactive solution.
The rock samples were dissolved in stages alternating with tests characterising the multiphase flow properties—drainage relative permeability and residual trapping. A single dissolution stage consisted of the following: first, the rock core sample was saturated with the acid solution at room temperature. This was then heated for \(>10\) h at \(65\,^{\circ }\)C to induce the acid production. The cores were subsequently cooled and cleaned with the injection of 10 pore volumes of deionised water, and dried for the start of the flow property measurement. During each stage, around \(0.5\%\) of the mass of the rock was dissolved.
The measurements of relative permeability and residual trapping were performed using a conventional steady-state procedure with detailed descriptions of the equipment and procedures provided in Reynolds and Krevor (2015), Niu et al. (2015). Deionised water and nitrogen (\(\hbox {N}_2\), with purity \(99\%\), BOC, Ltd, UK) were used as the wetting and nonwetting phases, respectively. The experiments were performed at 100 bars of pore fluid pressure and \(20\,^{\circ }\hbox {C}\). The interfacial tension between \(\hbox {N}_2\) and water was estimated to be 67 mN/m (Yan et al. 2001). The viscosities of the fluids were estimated to be \(\mu _{\mathrm{{N}}_2} = 0.02\) cp (Jacobsen et al. 1986) and \(\mu _\mathrm{{w}}=0.88\) cp (Wagner and Pruss 2002) at the experimental conditions. The use of nitrogen ensured that there was no alteration of the rock structure during the tests and that there were no concerns about cleaning residual fluid from the rocks. The total fluid injection rate was held constant, \(Q_\mathrm{{T}} = Q_\mathrm{{N}_2} + Q_\mathrm{{w}} = 20\) ml \(\hbox {min}^{-1}\), corresponding to a capillary number \(N_\mathrm{{c}}=u\mu _{\mathrm{{N}}_2}/\sigma = 10^{-6}\).
The relative permeability was estimated from the observations making use of the 1D history matching software SENDRA\(^{\textregistered }\), allowing for the correction of capillary end effects (Sendra 2012). The Chierici correlation of relative permeability was found to be the most suitable model for the history matching of the experimental data (Chierici 1984). Steady-state pressure time series and saturation profiles were input as fitting targets. No parameters from the Chierici model were imposed in the fitting.
Residual trapping observations followed the procedure developed in Niu et al. (2015). This was a technique to rapidly parametrise the residual trapping characteristic curve by making use of the capillary end effect and X-ray imaging to measure the saturation. The residual trapping core floods followed immediately after the relative permeability tests. The residual trapping properties of both rocks were described as the relationship between the initial and residual gas saturations, also called initial–residual curves, and were fitted by the Land model (Land 1968), Eq. 1,
where \(S_{\mathrm{{g,max}}}\) was the initial \(\hbox {N}_2\) saturation at the end of the drainage process and C was the Land trapping coefficient. The greater the value of C, the less the trapping.
Mercury injection capillary pressure measurements are destructive process. They were performed on samples drilled from the same rock blocks as the cores undergoing the flow tests. A parallel set of dissolution steps were performed on these samples, after which a mercury porosimetry test would be applied.
For the Estaillades carbonate, typically two dissolution stages would be performed prior to a flow test measurement, whereas properties were measured after each dissolution stage for the Ketton carbonate. Table 2 shows the detailed measurement schedule. Note that no measurements were made for either rock after the seventh dissolution stage.
3 Results and Discussion
3.1 Porosity, Absolute Permeability, and Pore Size Distribution
The variation of porosity for the rocks after each dissolution stage is shown in Fig. 4. The porosity increased uniformly along the length of each of the rock samples, even while the samples themselves were heterogeneous. Two-dimensional porosity maps of a central slice of the rock cores are shown in Fig. 5. Imagery of porosity in the Estaillades carbonate showed that there was compaction in the downstream region of the rock samples after the fourth and sixth dissolution stages. These parts of the rock core were removed prior to the measurement of flow properties.
The variation in measured permeability with increasing core-averaged porosity is shown in Fig. 6. Permeability in the Estaillades carbonate increased from 190 to 220 mD through the first six dissolution stages, corresponding to an increase in 0.02 porosity units. Subsequent to the sixth dissolution stage, the permeability decreased due to rock compaction. In contrast, there was no systematic change in permeability in the Ketton carbonate, which varied between 2.2 and 3 D. However, most measured values were below the initial value of 2.8 D. Uncertainty in the measurement of permeability was estimated using standard error propagation and was primarily driven by variation in the pressure measurement. The uncertainty was greater for the measurements with the Ketton carbonate than for the Estaillades, due to the low differential pressure across the rock.
Non-systematic or decreasing permeability through reaction-induced porosity increases is indicative of rock compaction and the migration of small mineral grains released during the reaction process (Noiriel et al. 2005; Mangane et al. 2013; Luquot et al. 2014). Studies have also observed monotonically increasing permeability variation with increasing porosity through dissolution; however, these variations generally occur over much larger variations in porosity (Mangane et al. 2013; Luquot et al. 2014; Menke et al. 2015).
The variation in the pore size distribution of the rocks is shown in Fig. 7. In the Ketton carbonate, the fraction of intergranular pores, with throat sizes \(r_\mathrm{{p}}>3.16\, \upmu \)m, decreased with increasing dissolution, while the fraction of intragranular pores with throat sizes \(r_\mathrm{{p}}<0.1\,\upmu \)m increased. During the dissolution of the Estaillades, the fractions of both the smallest pores, with throats \(r_\mathrm{{p}}< 0.418 \,\upmu \)m, and the largest pores, with throats \(r_\mathrm{{p}}> 3.618\, \upmu \)m, decreased. This was concurrent with an increase in the fraction of pores of intermediate sizes, \(0.418< r_\mathrm{{p}} < 3.618\, \upmu \)m.
3.2 Relative Permeability
The relative permeability curves for both rock samples are shown in Fig. 8. Data points are shown corresponding to the values of average saturation obtained at each fractional flow step of the core flood, with the relative permeability inferred from the history-matched best fit to all of the data. The main error source of uncertainty in the data was propagated from the absolute permeability. Tabular data are provided in Tables 3, 4, 5 and 6 in “Appendix 1”.
The initial–residual characteristic of the Ketton (top) and Estaillades (bottom) carbonates at each dissolution stages. Curves show best fits of the Land Trapping model, Eq. 1
In the Ketton carbonate, the gas relative permeability increased and the water relative permeability decreased with increasing porosity. The mineral dissolution resulted in an increasing fraction of the smallest pores and a decreasing fraction of the largest pores with increasing total porosity (Fig. 7). While the fraction of the largest pores decreased, their volume did not. It is seen in Fig. 7 that the volume increase in the smallest pores occurs at the large size end of this group of pores. This change in structure opened up pathways for the nonwetting phase to flow where it was previously excluded, thus increasing the relative permeability. These same pathways were previously only available to the wetting phase, and as a result of the infiltration of the nonwetting phase, there was a corresponding decrease in the wetting phase relative permeability. A similar effect was estimated using numerical simulations in Jiang and Tsuji (2014).
In the Estaillades carbonate, both the gas and water relative permeability decreased with increasing porosity. There was a modest increase in the relative permeability of both phases with the final dissolution stage, after compaction had occurred. The mineral dissolution had resulted in a decrease in the fraction of both the smallest and largest groups of pores in the rock, with the middle range of pores taking up an increasing fraction of the pore volume (Fig. 7). As a result, wetting and nonwetting phases were less efficiently partitioned into smaller and larger pore networks. With an increasing fraction of fluid moving through the same networks of pores, the relative permeabilities to both phases were decreased.
3.3 Residual Trapping
Residual trapping was characterised through the construction of initial–residual characteristic curves shown in Fig. 9. This measurement does not depend on observations of pressure or fluid flow, and uncertainty was driven by random noise in the CT image reconstruction (Niu et al. 2015). In these experiments, the maximum uncertainty associated with the saturation was \(\pm \, 0.006\).
In the Ketton carbonate, there was a modest, but systematic decrease in residual trapping with increasing porosity (Fig. 10). In the case of the Ketton, the mineral dissolution preferentially targeted small pores, increasing the average size of the small pore size distribution (Fig. 7). This likely contributed to wetting layer flow. Trapping is promoted by flow of a wetting phase through layers on the pore walls of rocks. As these layers swell with increasing wetting phase pressure, they will promote snap-off and isolation of isolated blobs of the nonwetting phase. The loss of water flux along these layers may be the source of the modest decrease in trapping. In contrast, residual trapping did not significantly change with increasing porosity in the Estaillades carbonate, as shown in Fig. 10.
4 Conclusions
We have evaluated the impact of rock mineral dissolution on the pore structure and multiphase flow properties, relative permeability and residual trapping, of two carbonate rocks with distinct pore structures. Variations in the multiphase flow properties were not correlated simply with changes in porosity. In contrast, the changes were well correlated with the changes in the underlying pore structure. In the Ketton carbonate, dissolution resulted in an increase in the fraction of pore volume made up by the smallest pores and a corresponding decrease in the fraction made up by the largest pores. This resulted in a systematic increase in the relative permeability to the nonwetting phase and decrease in relative permeability of the wetting phase. There was also a modest, but systematic decrease in residual trapping, possibly because of a reduction in wetting layer flow in small pores. In the Estaillades carbonate, dissolution resulted in an increase in the fraction of pore volume made up by pores in the central range of the initial pore size distribution, and a corresponding decrease in the fraction made up by both the smallest and largest pores. This resulted in a decrease in the relative permeability to both the wetting and nonwetting fluid phases and no discernible impact on the residual trapping. The impact of rock matrix dissolution will be strongly dependent on the impact of that dissolution on the underlying pore structure of the rock. However, if the variation in pore structure can be observed or estimated with modelling, then it should be possible to estimate the impacts on multiphase flow properties.
References
Bear, J.: Dynamics of Fluids in Porous Media. Dover Publications, New York (2013)
Bemer, E., Lombard, J.M.: From injectivity to integrity studies of \(\text{CO}_2\) geological storage: chemical alteration effects on carbonates petrophysical and geomechanical properties. Oil Gas Sci. Technol.-Rev. IFP 65(3), 445–459 (2010)
Buijse, M., de Boer, P., Breukel, B., Klos, M., Burgos, G.: Organic acids in carbonate acidizing. In: Paper SPE 82211 Presented at SPE European Formation Damage Conference, The Hague, Netherlands, 13–14 May (2003)
Celia, M.A.: Geological storage of captured carbon dioxide as a large-scale carbon mitigation option. Water Resour. Res. 53, (2017). https://doi.org/10.1002/2017WR020841
Chierici, G.L.: Novel relations for drainage and imbibition relative permeabilities. Soc. Pet. Eng. 24(3), 275–276 (1984)
Daccord, G., Touboul, E., Lenormand, R.: Carbonate acidizing: toward a quantitative model of the wormholing phenomenon. SPE Prod. Eng. 4, 63–68 (1989)
Depaolo, D.J., Cole, D.R.: Geochemistry of geologic carbon sequestration: an overview. Rev. Mineral. Geochem. 77, 1–14 (2013)
Egermann, P., Bazin, B., Vizika, O.: An experimental investigation of reaction-transport phenomena during \(\text{ CO }_2\) injection. In: Paper SPE 93674 Presented at the 14th SPE Middle East Oil & Gas Show and Conference held in Bahrain International Exhibition Centre, Bahrain, 12–15 March (2005)
Egermann, P., Bemer, E., Zinszner, B.: An experimental investigation of the rock properties evolution associated to differential levels of \(\text{ CO }_2\) injection like alternation processes. In: Paper SCA2006-34 Presented at the International Symposium of the Society of Core Analysts held in Trondheim, Norway 12–16 September (2006)
Fredd, C.N., Fogler, H.S.: Influence of transport and reaction on wormhole formation in porous media. AIChE J. 44, 1933–1949 (2011)
Golfier, F., Zarcone, C., Bazin, B., Lenormand, R., Lasseux, D., Quintard, M.: On the ability of a Darcy-scale model to capture wormhole formation during the dissolution of a porous medium. J. Fluid Mech. 457(213), C245 (2002)
Harris, R.E., McKay, I.D., Mbala, J.M., Schaaf, R.P.: Stimulation of a producing horizontal well using enzymes that generate acid in-situ—case history”. In: Paper SPE 68911 Presented at the European Formation Damage Conference, The Hague, Netherlands, 21–22 May (2001)
Hoefner, M., Fogler, H.S.: Pore evolution and channel formation during flow and reaction in porous media. AIChE J. 34, 45–54 (1988)
Hovorka, S.D., Benson, S.M., Doughty, C., Freifeld, B.M., Sakurai, S., Daley, T.M., Kharaka, Y.K., Holtz, M.H., Trautz, R.C., Nance, H.S., Myer, L.R., Knauss, K.G.: Measuring permanence of \(\text{ CO }_2\) storage in saline formations: the Frio experiment. Environ. Geosci. 13(2), 105–121 (2006)
Jacobsen, R.T., Stewart, R.B., Jahangiri, M.: Thermodynamic properties of nitrogen from the freezing line to 2000 K at pressures to 1000 MPa. J. Phys. Chem. Ref. Data 15(2), 735–909 (1986)
Jiang, F., Tsuji, T.: Changes in pore geometry and relative permeability caused by carbonate precipitation in porous media. Phys. Rev. E 90, 053306 (2014)
Knauss, K.G., Johnson, J.W., Steefel, C.I.: Evaluation of the impact of \(\text{ CO }_2\), co-contaminant gas, aqueous fluid and reservoir rock interactions on the geologic sequestration of \(\text{ CO }_2\). Chem. Geol. 217, 339–350 (2005)
Krevor, S., Blunt, M.J., Benson, S.M., Pentland, C.H., Reynolds, C., Al-Menhali, A., Niu, B.: Capillary trapping for geologic carbon dioxide storage: from pore scale physics to field scale implications. Int. J. Greenh. Gas Control 40, 221–237 (2015)
Krevor, S., Pini, R.: Chapter 7 Laboratory studies to understand the controls on flow and transport for \(\text{ CO }_2\) storage. In: Newell, P., Ilgen, A.G. (eds.) Science of Carbon Storage in Deep Saline Formations. Elsevier, Amsterdam (2019)
Lai, P., Moulton, K., Krevor, S.: Pore-scale heterogeneity in the mineral distribution and reactive surface area of porous rocks. Chem. Geol. 411, 260–273 (2015)
Land, C.S.: Calculation of imbibition relative permeability for two- and three-phase flow from rock properties. Soc. Pet. Eng. J. 8(2), 149–156 (1968)
Luhmann, A.J., Tutolo, B.M., Bagley, B.C., Mildner, D.F.R., Seyfried, J.R., Saar, W.E., Martin, O.: Permeability, porosity, and mineral surface area changes in basalt cores induced by reactive transport of \(\text{ CO }_2\)-rich brine. Water Resour. Res. 53(3), 1908–1927 (2017)
Luquot, L., Rodriguez, O., Gouze, P.: Experimental characterization of porosity structure and transport property changes in limestone undergoing different dissolution regimes. Transp. Porous Media 101(3), 507–532 (2014)
Mangane, P.O., Gouze, L., Gouze, P., Luquot, L.: Permeability impairment of a limestone reservoir triggered by heterogeneous dissolution and particle migration during \(\text{ CO }_2\)-rich injection. Geophys. Res. Lett. 40(17), 4614–4619 (2013)
McPhee, C., Reed, J., Zubizarreta, I.: Core Analysis: A Best Practice Guide, vol. 64. Elsevier, Amsterdam (2015)
Menke, H.P., Bijeljic, B., Andrew, M.G., Blunt, M.J.: Dynamic three-dimensional pore-scale imaging of reaction in a carbonate at reservoir conditions. Environ. Sci. Technol. 49(7), 4407–4414 (2015)
Mito, S., Xue, Z., Ohsumi, T.: Case study of geochemical reactions at the Nagaoka \(\text{ CO }_2\) injection site. Jpn. Int. J. Greenh. Gas Control 2, 309–318 (2008)
Niu, B., Al-Menhali, A., Krevor, S.C.: The impact of reservoir conditions on the residual trapping of carbon dioxide in Berea sandstone. Water Resour. Res. 51(4), 2009–2029 (2015)
Noiriel, C., Bernard, D., Gouze, P., Thibault, X.: Hydraulic properties and microgeometry evolution accompanying limestone dissolution by acidic water. Oil Gas Sci. Technol.-Rev. IFP 60(1), 177–192 (2005)
Noiriel, C., Luquot, L., Mad, B., Raimbault, L., Gouze, P., Van der Lee, J.: Changes in reactive surface area during limestone dissolution: an experimental and modelling study. Chem. Geol. 265(1–2), 160–170 (2009)
Oleg, C.P., Golubev, S.V., Schott, J., Castillo, A.: Calcite, dolomite and magnesite dissolution kinetics in aqueous solutions at acid to circumneutral pH, 25 to \(150^{circ}\text{ C }\) and 1 to 55 atm \(\text{ pCO }_2\): New constraints on \(\text{ CO }_2\) sequestration in sedimentary basins. Chem. Geol. 265, 20–32 (2009)
Qajar, J., Francois, N., Arns, C.H.: Microtomographic characterization of dissolution-induced local porosity changes including fines migration in carbonate rock. SPE J. 18(03), 545–562 (2013)
Reynolds, C.A., Krevor, S.: Characterizing flow behavior for gas injection: relative permeability of \(\text{ CO }_2\)-brine and N2-water in heterogeneous rocks. Water Resour. Res. 51(12), 9464–9489 (2015)
Schechter, R.S., Gidley, J.L.: The change in pore size distribution from surface reaction in porous media. AIChE J. 15, 339–350 (1969)
Sendra: Sendra User Guide Windows Version 2012.1 (2012)
Vanorio, T., Nur, A., Ebert, Y.: Rock physics analysis and time-lapse rock imaging of geochemical effects due to the injection of \(\text{ CO }_2\) into reservoir rocks. Geophysics 76(5), O23–O33 (2011)
Wagner, W., Pruss, A.: The IAPWS formulation 1995 for the thermodynamic properties of ordinary water substance for general and scientific use. J. Phys. Chem. Ref. Data 31(2), 387–535 (2002)
Yan, W., Zhao, G.Y., Chen, G.J., Guo, T.M.: Interfacial tension of (methane + nitrogen) + water and (carbon dioxide + nitrogen) + water systems. J. Chem. Eng. Data 46(6), 1544–1548 (2001)
Acknowledgements
The authors gratefully acknowledge funding support for this work from the Qatar Carbonates and Carbon Storage Research Centre provided jointly by Shell, Qatar Petroleum and the Qatar Science and Technology Park. Vincenzo Cunsolo carried out many of the mercury porosimetry tests used in this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix A: Relative Permeability Tabular Data
Appendix A: Relative Permeability Tabular Data
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Niu, B., Krevor, S. The Impact of Mineral Dissolution on Drainage Relative Permeability and Residual Trapping in Two Carbonate Rocks. Transp Porous Med 131, 363–380 (2020). https://doi.org/10.1007/s11242-019-01345-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11242-019-01345-4