Abstract
Pinus massoniana is a critical afforestation and ecological tree species in China. However, the continued existence of this pine is severely threatened by pine wilt disease. Somatic embryogenesis serves as a highly efficient clonal propagation approach. Although significant progress has been made in somatic embryogensis research on P. massoniana, resulting in the successful regeneration of plants, the limited embryogenic potential of improved cell lines and loss of embryogenic properties resulting from prolonged proliferation have posed obstacles to the industrialization of SE production. In this study, we investigated the effect of phytosulfokine on embryo development of cell lines from P. massoniana which lead to a cascade of physicochemical changes. Eight embryogenic cell lines of P. massoniana were used to observe phenotype and cytological changes. Physiological factors and the contents of nutrients and endogenous hormones were measured before and after phytosulfokine addition. We found that PSK promoted a change in the embryogenic mass of P. massoniana, leading to their development from pro-embryogenic mass (PEM)I to PEMII or PEMIII stages of pro-embryos. In addition, PSK accumulated soluble sugar, protein, and starch, and maintained redox homeostasis during cell line proliferation by reducing H2O2 levels. Our findings increase our understanding of how PSK affects somatic embryogensis in P. massoniana, thereby providing a valuable tool for establishing efficient somatic embryogensis systems in conifer species.
Key message
This study aimed to evaluate the effect of exogenously supplemented PSK on the proliferation and embryo development of different cell lines from P. massoniana.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Somatic embryogenesis (SE) is a popular biotechnological process, which plays an important role in the efficient study of high-value plantation seedlings, thereby improving forest productivity and sustainable development (Pullman et al. 2003). Notably, SE system utilization has emerged as a prominent area of research for establishing a genetic transformation platform and applying gene editing technology to plant modification (Wang et al. 2023). From the late 1980s to early 1990s, rapid advancements in molecular and cell biology and a high demand for seedlings prompted the emergence of unprecedented research on SE in conifer species, leading to abundant research findings. According to local comprehensive statistics, over 50 conifer species, including Picea asperata (Krogstrup et al. 1988), Abies fabri (Salaj et al. 2020), Torreya grandis (Gong et al. 2018), Pinus taeda (Hu et al. 2022), and Larix gmelinii (Liu et al. 2020), achieved large-scale production and SE application.
Notably, P. massoniana is a fast-growing afforestation tree species native to China, accounting for 8.09% of the country’s forests; this species supports the development of numerous industries, such as wood, paper making, and forestry chemicals (Liu et al. 2022). Studies on P. massoniana SE began in 1995, with four regenerated plants were obtained successfully with mature P. massoniana zygotic embryos as explants (Huang et al. 1995). Subsequently, Yang et al. (Wang et al. 2020) initially established the SE system for immature embryos of P. massoniana, and Chen et al. (Chen et al. 2019) optimized the P. massoniana SE and regeneration system against pine wood nematode disease. This study led to optimization of the embryogenic mass induction system of P. massoniana, and establishment of an efficient tissue culture system based on P. massoniana somatic embryo seedlings (Wang and Yao 2020). Although SE research on P. massoniana has made some breakthroughs, the SE system of P. massoniana remains largely unexplored. For example, the mechanism of SE system optimization by adjusting the hormone ratio, the embryo development of cell lines during culture, and reasons for the loss of embryogenic properties caused by long-term proliferation are unclear.
Phytosulfokine (PSK) is a short peptide active substance, which has plant growth regulatory substance properties (Chen et al. 2005), and acts as an intercellular signal peptide and autocrine growth factor involved in several developmental processes in vivo, such as the response to abiotic and biotic stress, tissue differentiation, and sexual reproduction (Mestinšek Mubi et al. 2024). Furthermore, PSK treatment has the potential to enhance mass growth in rice (Oryza sativa) (Yang et al. 1999) and Arabidopsis thaliana (Matsubayashi et al. 2006). In addition, PSK has been used in studies on SE in conifer species such as Cryptomeria japonica (Igasaki et al. 2003), Larix leptolepis (Umehara et al. 2005), and Cunninghamia lanceolata (Hao et al. 2023). Notably, PSK can accelerate embryonic cell development in C. japonica and strongly stimulate somatic embryo formation (Igasaki et al. 2003). In a study on C. lanceolata (Hao et al. 2023), PSK helped maintain reactive oxygen species (ROS) homeostasis by reducing early SE-induced peroxidase activity and promoted SE induction by reducing H2O2 levels. Taken together, these studies revealed the biological function of PSK and provided new inspiration for our research on P. massoniana embryogenesis.
This study aimed to evaluate the effect of exogenously added PSK on the proliferation and embryo development of different cell lines from P. massoniana, and analyze its influence on their nutrient content, antioxidant enzyme activity, endogenous hormone content, H2O2 content, and other indicators during the proliferation process. Our study aimed to elucidate the role of PSK in embryogenic cell lines proliferation and contribute to developing a valuable tool for establishing efficient SE systems in P. massoniana.
Materials and methods
Materials
P. massoniana cones were collected on July 15, 2022, at Yongxiu Forest Seed Farm in Nanchang, Jiangxi, China. The seeds were subsequently stored at a temperature of 4 °C in a refrigerator for 5 days. Seeds were washed with running water for 30 min, surface-sterilized with 75% (v/v) ethanol for 30 s, treated with 0.1% (w/v) HgCl2 for 8–10 min, and then rinsed five times in sterile distilled water.
For the study, a total of eight stable embryogenic cell lines were generated from three P. massoniana families. Among these, family No. 1 contributed to the generation of five embryogenic cell lines (YI-5, Y1-6, Y1-12, Y1-27, and Y1-29), all exhibiting maternal homology. Additionally, family No. 3 yielded two cell lines (Y3-4 and Y3-5) and family No. 7 contributed the Y7-20 cell line.
Medium and culture conditions
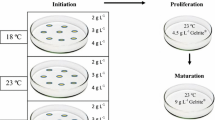
After disinfection, seed coats were removed as previously described (Pullman et al. 2003). Subsequently, seeds were placed in Gupta and Durzan medium (DCR) (Gupta & Durzan 1985), supplemented with 2.0 mg/L 2,4-dichlorphenoxyacetic (2, 4-D), 1.0 mg/L 6-benzylaminpurine (6-BA), 0.5 mg/L 1-Naphthaleneacetic (NAA), 3% sucrose, 0.5 g/L casein hydrolysate (CH), 0.5 g/L L-glutamine, 0.25 g/L 2-Morpholinoethanesulphonic acid (MES), and 4 g/L gellan gum (Phytagel™, Sigma-Aldrich, St. Louis, Mo., USA). The pH of the medium was adjusted to 5.8–6.0 using KOH or HCl after the addition of all of the ingredients except the gelling agent. The gelling agent was added before autoclaving at 121 °C for 20 min (Zhou et al. 2017), and then cultured at 23 ± 1 °C in the dark.
Approximately 2 weeks later, the embryogenic mass began to appear. When the embryogenic mass (cell line) reached the size of pea grains after approximately one month, it was subcultured into the proliferation medium (basal LP medium (Teasdale et al. 1987) supplemented with 0.5 mg/L 6-BA, 2.0 mg/L NAA, 0.5 mg/L kinetin (KT), 3% sucrose, 0.5 g/L CH, 0.5 g/L L-glutamine, 0.25 g/L MES, and 4 g/L gellan gum). The pH and sterilization method were the same as above. The embryogenic mass was cultured at 23 ± 1 °C in the dark, and subcultured once every 2 weeks. Subsequent experiments were performed after 8 weeks of proliferation.
Morphological analysis
Embryogenic mass were collected on day 7 of proliferation culture, and the morphology was observed using a stereo microscope (Olympus Co., GRGHT-BDEL, Tokyo, Japan). A 0.1 g embryogenic mass was taken and stained with acetocarmine and Evans blue dyes (Leonardo Jo et al. 2014); this process was repeated three times.
Exogenous phytosulfokine treatment experiment
The effects of different PSK concentrations on the embryogenic potential and proliferation efficiency of embryogenic mass were investigated using Y1-5 and Y1-6 cell lines. The Y1-5 and Y1-6 cell lines in the same growth states were selected, and the fresh weight of embryogenic mass was weighed before being placed on the proliferation medium with different PSK concentrations (0 mg/L, 0.5 mg/L, 1.0 mg/L, 1.5 mg/L, and 2.0 mg/L) for culture. Three replicates were set for each treatment. After 14 days, the fresh weight of the proliferated embryogenic mass was weighed to calculate the proliferation rate under different PSK treatments. Additionally, the mass samples from each treatment were stained and pressed to observe changes in embryo development under different PSK concentrations.
We found that 0.5 mg/L PSK treatment significantly promoted embryo development (Fig. 2). Therefore, we selected 0.5 mg/L PSK for use in subsequent experiments. Eight embryogenic cell lines were placed on the proliferation medium with 0.5 mg/L PSK to observe the effects of PSK treatment on the different cell lines, including embryo development, endogenous hormone content, and changes in physiological and biochemical indices.
Biochemical analysis
Eight embryogenic cell lines were measured after 2 weeks of culture with 0 mg/L and 0.5 mg/L PSK added into the proliferation medium. Determination of soluble protein, starch, soluble sugar, and amino acid (AA), malondialdehyde (MDA), and H2O2 contents and superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and polyphenol oxidase (PPO) activities was performed using respective assay (Comin biotechnology Co., Ltd. Suzhou, China). Zeatin-riboside (ZR), gibberellin (GA3), abscisic acid (ABA), and indole-3-acetic acid (IAA) contents were measured by high-performance liquid chromatography. Samples weighing 0.2 g were prepared using the extract and the process was repeated three times. All methods were performed according to manufacturer’s instructions(Comin biotechnology Co., Ltd. Suzhou, China).
Data processing
ANOVA and Duncan multiple comparisons were performed on the data using SPSS 27.0 (SPSS Inc., Chicago, USA). All figures were typeset using Adobe Photoshop CC 2019 (Adobe Systems Software Ireland Ltd, California, USA). The proliferation efficiency of embryogenic mass was calculated according to the following formula:
where embryogenic mass’ weight before proliferation refers to the weight on day 0 when the mass were placed in the proliferation medium, and mass weight after proliferation refers to that on day 14.
Results
Morphological differences among P. massoniana embryogenic cell lines
Morphological differences among the embryogenic cell lines were mainly reflected in the color, texture, and structure of the mass. As shown in Fig. 1, all the eight embryogenic cell lines were embryonal with viscosity, high water content, light yellow to snow white coloration, and surface structures that were convex to varying degrees. Among them, cell lines Y1-5 (Fig. 1A), Y1-6 (Fig. 1B), Y1-12 (Fig. 1C), and Y7-20 (Fig. 1H) had noticeable structures. Furthermore, Y3-4 (Fig. 1F) and Y3-5 (Fig. 1G) were flat and mixed with yellow-brown cells.
Effects of PSK on the proliferation and embryogenesis of P. massoniana embryogenic cell lines
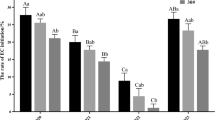
Cell lines Y1-5 and Y1-6 were selected and treated with different PSK concentrations (0, 0.5, 1.0, 1.5, 2.0 mg/L) in one proliferation cycle. The results showed that PSK (0–2 mg/L) treatment had no significant effect on P. massoniana cell line proliferation efficiency (Tables 1). The proliferation efficiency of Y1-5 cell line initially increased and subsequently decreased with increasing PSK concentration. Conversely, the proliferation efficiency of Y1-6 cell line remained stable, followed by a slight decrease with increasing PSK concentration. Regardless, these results demonstrate that PSK promotes embryo development in P. massoniana cell lines (Fig. 2). After adding PSK to Y1-5 and Y1-6 cell lines, the pro-embryos were in the PEMIII stage instead of PMEI or PMEII.
Effects of 0.5 mg/L PSK treatment on P. massoniana embryogenic cell lines
We observed that treatment of the embryogenic cell lines with 0.5 mg/L PSK significantly promoted embryo development (Fig. 2). To investigate the potential of PSK in enhancing embryogenic potential of other cell lines, we selected six additional cell lines for PSK treatment. Considering the high price of PSK, we opted to add 0.5 mg/L PSK into the proliferation medium. Subsequently, the embryogenic suspensor cells mass structure, endogenous hormone content, as well as physiological and biochemical indices of the eight embryogenic cell lines before and after treatment were measured.
Changes in embryo development
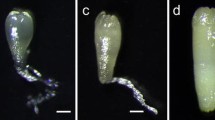
Embryogenic potential of the P. massoniana embryogenic cell lines treated with PSK were distinct, and the embryo development was promoted in all the eight embryogenic cell lines (Fig. 3). Irrespective of embryo head size or suspensor structure of the cells, the promotive effect was distinct. Before PSK treatment, all the eight embryogenic cell lines showed an embryo development; however, some differences were observed. Among them, the embryo development of the Y1-12 (Fig. 3. C-1) and Y7-20 (Fig. 3. H-1) cell lines was relatively complete, with bullet-head structure and more clustered and suspensor cells. The embryo development of the Y1-6 (Fig. 3. B-1) and Y1-29 (Fig. 3. E-1) cell lines was poorly developed, with looser head cells and fewer, less compact suspensor cells.
After 0.5 mg/L PSK treatment, the embryo development of the eight embryogenic cell lines changed significantly. The head cells became larger, structure was dense, and suspensor cells were extended. In the Y1-6 (Fig. 3. B-1) and Y1-29 (Fig. 3. E-1) cell lines, which were originally poorly developed in pro-embryos, the embryonic changes were particularly distinct after 0.5 mg/L PSK treatment (Fig. 3. B-2 and Fig. 3. E-2). The head cells were assembled into bullet shapes and suspensor cells were slender. The pro-embryos were in the PMEIII stage instead of PMEI or PMEII.
Variations in endogenous hormone concentrations
Endogenous hormones are important signals in plant SE and play an important role in its different stages. The results of this study showed that endogenous hormone content of the eight P. massoniana cell lines responded differently to PSK, with both IAA and ZR contents increased; however, changes in the ABA and GA3 contents were inconsistent in different cell lines before and after PSK treatment (Fig. 4).
Without PSK treatment, significant differences were observed among the eight P. massoniana cell lines in the contents of four endogenous hormones (P < 0.05). The Y3-5 cell line displayed the highest ZR and IAA contents, with 0.50 µg/g and 20.92 ng/L, respectively. The highest ABA content (0.68 µg/g) was observed in the Y1-12 cell line. The Y1-6 and Y1-29 cell lines had the highest and lowest GA3 contents, respectively. The Y1-6 and Y1-29 cell lines belonged to the same family, indicating that the GA3 content of cell lines from the same family also differed significantly according to the specific cell genotype.
After 0.5 mg/L PSK treatment, changes in the four endogenous hormones of the eight tested P. massoniana embryogenic cell lines varied. The ZR content of different P. massoniana embryogenic cell lines showed varying responses to PSK; Y1-12 and Y3-5 cell lines showed significant increases in ZR content, whereas the other embryogenic cell lines either showed slightly decreased ZR content or remained the same (Fig. 4A). The IAA content of most of P. massoniana cell lines increased significantly after PSK treatment, except for Y7-20, which showed a small decrease (Fig. 4B). In addition, the ABA content among P. massoniana cell lines exhibited variations, with a decrease observed in five cell lines and a slight increase in three cell lines (Fig. 4C). Following PSK treatment, the GA3 content showed a significant increase of approximately 300%, which was particularly prominent in the Y1-27 cell line (Fig. 4D).
Physiological differences
The activity of antioxidant enzymes, PPO activity, H2O2 content, and MDA content can reflect relevant plant metabolic information during SE. Our results showed that PSK reduced the content of antioxidant enzymes (SOD, CAT, and POD), PPO, and H2O2 and increased the MDA content.
The physiological indices of antioxidant enzymes and MDA and H2O2 contents differed significantly among the eight tested P. massoniana embryogenic cell lines (P < 0.01). Particularly, the SOD, CAT, and POD activities of the Y1-12 cell line were significantly higher than those of the Y3-5 cell line (Fig. 5), which may be attributed to faster proliferation and physiological and biochemical reactions (Fig. S1). After PSK treatment, the physiological indices of antioxidant enzymes and MDA and H2O2 contents significantly varied among the cell lines (P < 0.01), and SOD, CAT, POD, and PPO activities and the H2O2 content of the eight embryogenic cell lines showed a decreasing trend, whereas MDA content showed an increase (Fig. 5).
Variations in nutrient (starch, protein, and soluble sugar) and AA contents
Nutrients provide energy for cell proliferation and embryo development. The nutrient and AA contents of the eight embryogenic cell lines displayed strongly significant differences (P < 0.01). The starch content of cell lines Y1-6 and Y3-5 was significantly lower than that of the other six cell lines (Fig. 6A). The protein (Fig. 6B), soluble sugar (Fig. 6C), and AA contents (Fig. 6D) of the Y1-12 cell line were all lower than those of the other seven embryogenic cell lines. Furthermore, the Y7-20 cell line had low AA content and the highest protein and soluble sugar contents among the eight embryogenic cell lines.
After 0.5 mg/L PSK treatment, the starch, protein, and soluble sugar contents of the eight tested P. massoniana embryogenic cell lines increased to different degrees (Fig. 6), and the nutrient contents were significantly different among the cell lines (P < 0.05). However, AA content decreased to a certain extent in most embryogenic cell lines, although they increased in the Y1-12 and Y1-27 cell lines. The results indicated that PSK enhanced the accumulation of storage materials and facilitated the utilization of AAs in P. massoniana cell lines, and the accumulation of nutrients could potentially facilitate the embryogenic improvement of P. massoniana cell lines.
Discussion
Effects of PSK on P. massoniana cell line proliferation and embryogenesis
Embryogenic differences between P. massoniana embryogenic cell lines are mainly genotypic. Among different genotypes of the same family, embryogenic differences are distinct, a finding congruent with that of previous studies [Cheng et al. 2021]. Combining the phenotypic characteristics of embryogenic cell lines and differences in the structure and quantity of embryo development can provide us with a preliminary selection of better embryogenic research materials. Treatment of P. massoniana embryogenic cell lines with different PSK concentrations for one proliferation cycle did not significantly affect proliferation efficiency; this was inconsistent with a previous finding that PSK promoted suspended cell proliferation in Chinese fir (Hao et al. 2023). These contrasting results may be because the culture medium in which PSK was added in this study was a solid medium; therefore, direct contact with medium was only a small part of the material. In contrast, previous conclusions were based on cells suspended in fluid medium, thereby having a contact area with the medium greater than that of cells in this study. In our study, the proliferation efficiency of the Y1-5 cell line increased linearly with PSK concentration, indicating that PSK was still conducive to P. massoniana cell line proliferation. Further significant results may require increasing the number of subgenerations and more embryogenic cell lines.
Treatment with PSK at different concentrations significantly affected the structure of embryotic suspensor cells in P. massoniana cell lines (Fig. 2). However, several experiments in this study were conducted based on the addition of 0.5 mg/L PSK because we observed that PSK at this concentration could better promote embryo development in P. massoniana cell lines without affecting proliferation efficiency (Fig. 3). In addition, adding 0.5 mg/L PSK was able to achieve the research purpose (considering the high purchase price of PSK). We found that PSK promoted embryogenic mass, consistent with similar findings from previous studies. For example, PSK can accelerate the development process of embryonic cells and strongly stimulate pro-embryo formation (Igasaki et al. 2003). In Chinese fir, PSK could promote proliferation efficiency and maturation during SE (Hao et al. 2023). Consistent with previous conclusions, our study confirms that PSK promotes the embryo development of P. massoniana embryogenic cell lines.
Effects of PSK on endogenous hormone content of P. massoniana
Endogenous hormone is an important determinant of plant embryogenesis, which affects all stages of embryo development (Kim et al. 2018). Studies have shown that the mass of different plants and the same plant in different culture periods are often different, which is associated with variations in endogenous hormones and other endogenous factors. In this study, ZR, IAA, ABA and GA3 contents were determined and analyzed in eight P. massoniana embryogenic cell lines before and after PSK treatment. The results showed significant differences in endogenous hormone content among all embryogenic cell lines. Furthermore, PSK had a greater effect on IAA content, whereas the contents of other endogenous hormones were relatively stable.
Notably, IAA is an important regulator of plant growth and development, affecting plant organ development and morphogenesis. Vondrakova et al. (Vondráková et al. 2011) measured endogenous IAA content during embryo maturation (with or without auxin culture) of two Abies alba species and found that embryo development supplemented with exogenous auxin was promoted. Aderkas et al. (Aderkas et al. 2001) found similar changes in IAA levels during Larix gmelinii SE, and IAA levels increased with pro-embryos development. In addition, IAA level changes during the zygote embryo development of Douglas Fir (Pseudotsuga menziesii) (Chiwocha & Aderkas 2002) and Pinus elliottii (Cheng et al. 2021) were similar. A similar pattern of IAA change was observed in liquid embryonic culture of Picea abies (Vágner et al. 1998). Conversely, a study on of pro-embryo maturation in Picea morrisonicola found that the endogenous IAA content of the T2 cell line (which can produce mature embryos) increased rapidly from the proliferation to maturity stage, whereas that of the T4 cell line (which cannot produce mature embryos) showed the opposite change (Liao et al. 2008). Therefore, these studies indicate that endogenous auxin content during somatic embryo development varies greatly by species and genotype. In our study, the endogenous IAA content of the eight P. massoniana embryogenic cell lines significantly increased in the proliferation medium supplemented with PSK, and embryogenic potential was enhanced. These results indicate that IAA might affect the embryogenic potential of P. massoniana embryogenic cell lines.
Notably, ABA is an important plant hormone associated with seed maturation, plant aging, and stress resistance (Carneros et al. 2017). Studies have shown that ABA is involved in the regulation of plant development and promoting the growth and differentiation of embryogenic mass (Pullman et al. 2008). Furthermore, the ABA content of somatic embryos of conifers showed an increasing trend during development. In SE of C. lanceolata, endogenous ABA content increases with embryo development and decreases with embryo maturity (Zhou et al. 2017). Additionally, when brassinolide promoted embryo development of different genotypes from Pinus koraiensis, some genotypes showed increased ABA content and embryogenesis, whereas others showed increased embryogenesis and decreased ABA content (Grzyb et al. 2017a, b). Similarly in the present study, we observed that the embryogenic potential of P. massoniana embryogenic cell lines were promoted; however, the endogenous ABA content of different cell lines changed to varying degrees after PSK treatment. The ABA content of five cell lines decreased after PSK treatment, whereas the ABA content of three lines increased. This may indicate that little relationship exists between ABA content and the embryos of P. massoniana cell lines during proliferation. Previous studies mainly focused on ABA content changes during mature culture, which does not conflict with our conclusion.
PSK maintained redox homeostasis of P. massoniana embryogenic cell lines
The initiation and differentiation of SE is regulated by embryogenic mass redox balance. The production of ROS under biological and abiotic stress conditions causes oxidative stress damage, which can be removed by plants through a series of enzymatic and non-enzymatic detoxification systems (Sunita et al. 2015). Notably, ROS levels are regulated by antioxidant enzymes such as SOD, POD, and CAT, which help maintain the dynamic balance of ROS in cells and reduce the potential damage to cells (Nie et al. 2024). Furthermore, SOD is the first line of defense against ROS (Chiwocha et al. 2002; Cui et al. 1999), which converts superoxide anions into H2O2. During SE of Larix gmelinii, the SOD transcription level was lowest on day 0, reached its maximum on day 3, and subsequently decreased from days 5 to 45 (Zhang et al. 2010). The POD enzyme is responsible for converting H2O2 into water and oxygen (Scandalios et al. 1997), and CAT can weaken the inhibitory effect of H2O2 on SE (Liu et al. 2019). In the SE process in banana, SOD activity increased significantly during embryogenic mass to spherical embryo development, and gradually decreased during later stages of somatic embryo development. Furthermore, POD and CAT activities significantly decreased during embryogenic cells to embryo development, which may lead to an increase in H2O2hydrogen peroxide levels in cultured embryogenic cells, and subsequently promote banana pro-embryo formation (Ma et al. 2012). After PSK addition, SOD, POD, and CAT activities and the H2O2 content of the embryogenic mass of P. massoniana decreased after 14 days of culture. This result may be due to PSK promoting the cellular antioxidant enzyme system to convert O2- into H2O and O2, thereby maintaining the redox homeostasis of the cellular environment.
Notably, MDA is formed when cell membranes are peroxidized, and plants tend to produce large amounts of MDA under stress, which is often used as an indicator of the extent of cell damage (Zhao et al. 1993). The MDA content of P. massoniana embryogenic cell lines increased after PSK addition, which indicated that PSK had a certain stress on embryogenic mass but not that PSK was harmful to P. massoniana embryogenic cell lines. Relevant studies have shown that increasing a certain level of oxidative stress is necessary to promote embryonic cell formation (Pasternak et al. 2005; Gallego et al. 2014; Zhou et al. 2016). Additionally, this study demonstrated that PSK may increase the oxidative stress of P. massoniana embryogenic cell lines at a certain level, thereby promoting embryo development. Numerous studies have been conducted on the browning reaction produced by PPO (Raj et al. 2006; Ngadze et al. 2012; Araji et al. 2014; Francesca et al. 2017). However, the role of PPO in plant physiology is unclear, although it may play an important role in defense signaling through ROS production (Castaera et al. 1996). Notably, PPO activity decreased in the eight P. massoniana embryogenic cell lines after PSK addition, which indicated that PSK alleviated cell line browning and possibly reduced ROS accumulation.
Effects of PSK on the contents of stored substances of P. massoniana embryogenic cell lines
In the process of plant growth and development, soluble sugars, protein, and starch are the energy supply substances necessary for plant growth and metabolism, which can reflect the overall carbon supply of plants (Zhang et al. 2023). Soluble sugar acts as a direct material and energy supplier for cell expansion and embryo development (Dobrenel et al. 2016; Jiang et al. 2014). Starch serves as a reserve carbohydrate for plants, which can provide energy for anabolic processes during seed development (Zeeman et al. 2010). As an important storage substance, soluble proteins have important research significance in SE (Wang et al. 2018). Relevant studies have shown that proteins affect embryo development and morphogenesis (Cangahuala-Inocente et al. 2013). Additionally, the embryogenic mass of Pyrus communis was high in solubility and protein, but low in starch (Ameri et al. 2020). Peng et al. (Peng et al. 2022; Gao et al. 2023) suggested that high starch content in the embryogenic mass was the basis for successful SE. In this study, the contents of soluble sugar, protein, and starch in the Y7-20 cell line with higher embryogenic potential (better embryo development) were significantly higher than those in the cell lines with lower embryogenic potential. These results suggest that embryo development requires a higher storage material base, which is consistent with conclusions from previous research. In P. pinaster, Tereso et al. (2007) described a similar localization of starch accumulation at the somatic embryo, suggesting that storage protein concentration could be a marker of embryo quality. Comparison of the three mass of Korean pines revealed that embryogenic mass has a higher storage substance content (Peng et al. 2020). In a study of white Pinus bungeana embryos, the soluble protein content of the embryogenic mass was significantly higher than that of the non-embryogenic mass (Li et al. 2008). Following PSK treatment of P. massoniana embryogenic cell lines, we observed that the storage substances of all the eight embryogenic cell lines increased following one proliferation cycle. Therefore, PSK promoted nutrient accumulation in P. massoniana embryogenic cell lines, which may be consistent with embryogenesis enhancement. The AA content of the P. massoniana cell lines decreased following PSK treatment, likely because PSK promoted cell growth and triggered AA consumption.
Overall, PSK can promote the embryo development of P. massoniana cell lines and can induce a series of physicochemical changes, including those to the nutrient content, antioxidant enzymes, and endogenous hormones. Among them, the IAA content of P. massoniana embryogenic cell lines increased, potentially explaining the observed improvements in embryogenic potential. Additionally, embryo development of P. massoniana cell lines was accompanied by nutrient accumulation. The findings of our study can enhance our comprehension of PSK in SE.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Abbreviations
- SE:
-
Somatic embryogenesis
- PSK:
-
Phytosulfokine
- ROS:
-
Reactive oxygen species
- DCR:
-
Durzan medium
- 2, 4-D:
-
2,4-dichlorphenoxyacetic
- DCR:
-
Durzan medium
- 6-BA:
-
6-benzylaminpurine
- NAA:
-
Naphthaleneacetic
- CH:
-
Casein hydrolysate
- MES:
-
2-Morpholinoethanesulphonic acid
- AA:
-
Amino acid
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- POD:
-
Peroxidase
- PPO:
-
Polyphenol oxidase
- ZR:
-
Zeatin-riboside
- GA3 :
-
Gibberelline
- ABA:
-
Abscisic acid
- IAA:
-
Indole-3-acetic acid
- PEM:
-
Pro-embryogenic mass
References
Aderkas Pv, Lelu M-A, Label P (2001) Plant growth regulator levels during maturation of larch somatic embryos. Plant Physiol Biochem 39:495–502. https://doi.org/10.1016/s0981-9428(01)01271-2
Ameri A, Davarynejad GH, Moshtaghi N, Tehranifar A (2020) The role of Carbohydrates on the induction of somatic embryogenesis and the biochemical state of the embryogenic mass in Pyrus communis L. Cv. ‘Dar Gazi’. Erwerbs-Obstbau 62:411–419. https://doi.org/10.1007/s10341-020-00518-6
Araji S, Grammer TA, Gertzen R, Anderson SD, Mikulic-Petkovsek M, Veberic R, Phu ML, Solar A, Leslie CA, Dandekar AM (2014) Novel roles for the polyphenol oxidase enzyme in secondary metabolism and the regulation of cell death in walnut. Plant Physiol 164:1191–1203
Cangahuala-Inocente GC, Silveira V, Caprestano CA, Floh EIS, Guerra MP (2013) ‘Dynamics of physiological and biochemical changes during somatic embryogenesis of Acca sellowiana’. In Vitro Cellular & Developmental Biology - Plant 50: 166– 75. https://doi.org/10.1007/s11627-013-9563-3
Carneros E, Toribio M, Celestino C (2017) Effect of ABA, the auxin antagonist PCIB and partial desiccation on stone pine somatic embryo maturation. Plant Cell Tissue Organ Cult (PCTOC) 131:445–458. https://doi.org/10.1007/s11240-017-1296-2
Castaera P, Steffens JC, W., M., and, Tingey (1996) Biological performance of Colorado potato beetle larvae on potato genotypes with differing levels of polyphenol oxidase. J Chem Ecol 22:91–101
Chen X, Yang Y, Chen J, Cui D (2005) A new peptide Plant Growth Regulation Substance, Phytosulfokine (PSK). Plant Physiol J 41:69–673
Chen T, Ye J, Wu X, Shen L, Zhu L (2019) Somatic embryogenesis and plantlet regeneration of disease-resistant Pinus massoniana Lamb. J Nanjing Forestry Univ (Natural Sci Edition) 43:1–8
Cheng Z, Yi M, Song C, Cheng Q, Huang R, Deng Z, Zhang Y, Zhang L (2021) Optimization of embryogenic mass induction conditions for somatic embryogenesis of Pinus elliottii. Acta Agriculturae Universitatis Jiangxiensis 43:1054–1064
Chiwocha S, Aderkas PV (2002) Endogenous levels of free and conjugated forms of auxin, cytokinins and abscisic acid during seed development in Douglas Fir. Plant Growth Regul 36:191–200
Cui K, Xing G, Liu X, Xing G, Wang Y (1999) Effect of hydrogen peroxide on somatic embryogenesis of Lycium barbarum L. Plant Sci 146:9–16
Dobrenel T, Caldana C, Hanson J, Robaglia C, Vincentz M, Veit B, Meyer C (2016) TOR Signaling and Nutrient Sensing. Annu Rev Plant Biol 67:261–285. https://doi.org/10.1146/annurev-arplant-043014-114648
Francesca T, Antonella P, Giacomo M, Pasquale T, Monica M, Stefano P, Cinzia M (2017) Polyphenol oxidases in crops: biochemical, physiological and genetic aspects. Int J Mol Sci 18:1–16
Gallego P, Martin L, Blazquez A, Guerra H, Villalobos N (2014) Involvement of peroxidase activity in developing somatic embryos of Medicago arborea L. Identification of an isozyme peroxidase as biochemical marker of somatic embryogenesis. J Plant Physiol 171:78–84. https://doi.org/10.1016/j.jplph.2013.09.017
Gao F, Wang R, Shi Y, Shen H, Yang L (2023) Reactive oxygen metabolism in the proliferation of Korean pine embryogenic mass cells promoted by exogenous GSH. Sci Rep 13:2218. https://doi.org/10.1038/s41598-023-28387-5
Gong L, Hu H, Hu Y, Yu W, Wu J, Huang J, Zhang Q (2018) Immature embryo development and embryogenic frequency in Torreya grandis ‘Merrillii’. J Zhejiang A&F Univ 35:861–867
Grzyb M, Kalandyk A, Waligórski P, Mikuła A (2017b) The content of endogenous hormones and sugars in the process of early somatic embryogenesis in the tree fern Cyathea delgadii Sternb. Plant Cell Tissue Organ Cult (PCTOC) 129:387–397. https://doi.org/10.1007/s11240-017-1185-8
Grzyb M, Kalandyk A, Mikuła A (2017a) Effect of TIBA, fluridone and salicylic acid on somatic embryogenesis and endogenous hormone and sugar contents in the tree fern Cyathea delgadii Sternb. Acta Physiol Plant 40:1–11. https://doi.org/10.1007/s11738-017-2577-4
Gupta PK, Durzan DJ (1985) Shoot multiplication from mature trees of Douglas-fir (Pseudotsuga menziesii) and sugar pine (Pinus lambertiana). Plant Cell Rep 4:177–179. https://doi.org/10.1007/BF00269282
Hao Z, Shi J, Wu H, Yan Y, Xing K, Zheng R, Shi J, Chen J (2023a) Phytosulfokine contributes to suspension culture of Cunninghamia lanceolata through its impact on redox homeostasis. BMC Plant Biol 23:1–12. https://doi.org/10.1186/s12870-023-04496-1
Hao Z, Wu H, Zheng R, Li R, Zhu Z, Chen Y, Lu Y, Cheng T, Shi J, Chen J (2023b) The plant peptide hormone phytosulfokine promotes somatic embryogenesis by maintaining redox homeostasis in Cunninghamia lanceolata. Plant J 113:716–733. https://doi.org/10.1111/tpj.16077
Hu S, Yang C, Gu Z, Du Q, Xiao P, Li H (2022) Optimization of somatic embryogenesis for Pinus taeda. For Res 35:9–17
Huang J, Wei Z, Xu Z (1995) Somatic embryogenesis and plantlet regeneration from callus of mature zygotic embryos of masson pine. Acta Bot Sinica 37:289–294
Igasaki T, Akashi N, Ujino-Ihara T, Matsubayashi Y, Sakagami Y, Shinohara K (2003) Phytosulfokine stimulates somatic embryogenesis in Cryptomeria japonica. Plant Cell Physiol 44:1412–1416
Jiang R, Fangren P, Tan P (2014) Somatic embryogenesis and the physiological and biochemical characteristics in Catalpa fargesii Bur. f.duclouxii(Dode) Gilmour. J Forestry Eng 28:25–29
Kim DH, Kang KW, Sivanesan I (2018) Influence of auxins on somatic embryogenesis in Haworthia retusa. Duval’ Biol 74:25–33. https://doi.org/10.2478/s11756-018-0151-1
Krogstrup P, Eriksen EN, Mller JD, Roulund H (1988) Somatic embryogenesis in Sitka spruce (Picea sitchensis (Bong.) Carr). Plant Cell Rep 7:594–597
Leonardo Jo, Andre LWD, Santos, Caroline A, Bueno HRB, Eny IS, Floh (2014) Proteomic analysis and polyamines, ethylene and reactive oxygen species levels of Araucaria angustifolia (Brazilian pine) embryogenic cultures with different embryogenic potential. Tree Physiol 34:94–104
Li Q, Zhang C, Qin P (2008) Physiological and biochemical characteristics of embryogenic mass and non-embryogenic mass in Pinus bungeana. J Northwest A&F Univ (Nat Sci Ed) 36:151–155. https://doi.org/10.13207/j.cnki.jnwafu.2008.08.026
Liao YK, Liao C-K, Ho YL (2008) Maturation of somatic embryos in two embryogenic cultures of Picea Morrisonicola Hayata as affected by alternation of endogenous IAA content. Planr Cell Tissue Organ Cult 93:257–268. https://doi.org/10.1007/s11240-008-9371-3
Liu J, Ou X, Wang J (2019) Effects of Exogenous Hydrogen Peroxide on Photosynthesis and reactive Oxygen Metabolism in leaves of Avena nuda L. Seedlings under Lanthanum stress. J Chin Soc Rare Earths 37:248–258
Liu J, Liu Y, Liu K, Chi Y, Huo Z, Huo Y, You X (2020) Optimization of the regeneration system from somatic embryogenesis in Larix olgensis. Chin Bull Bot 55:605–612
Liu B, Xie Y, Yin H, Zhou Z, Liu Q (2022) Identification and defensive characterization of PmCYP720B11v2 from Pinus massoniana. Int J Mol Sci 23:1–17. https://doi.org/10.3390/ijms23126640
Ma L, Xie L, Lin G, Jiang S, Chen H, Li H, Takáč T, Šamaj J, Xu C (2012) Histological changes and differences in activities of some antioxidant enzymes and hydrogen peroxide content during somatic embryogenesis of Musa AAA Cv. Yueyoukang 1. Sci Hort 144:87–92. https://doi.org/10.1016/j.scienta.2012.06.039
Matsubayashi Y, Ogawa M, Kihara H, Niwa M, Sakagami Y (2006) Disruption and overexpression of Arabidopsis Phytosulfokine receptor gene affects Cellular Longevity and potential for growth. Plant Physiol 142:45
Mestinšek Mubi Š, Kunej U, Vogrinčič V, Jakše J, Murovec J (2024) The effect of phytosulfokine alpha on haploid embryogenesis and gene expression of Brassica napus microspore cultures. Front Plant Sci 15:1336519
Ngadze E, Icishahayo D, Coutinho TA, dW V, Jacquie E (2012) Role of Polyphenol Oxidase, Peroxidase, Phenylalanine Ammonia Lyase, Chlorogenic Acid, and total Soluble Phenols in Resistance of Potatoes to Soft Rot. Plant Dis 96:186–192
Nie S, Yan Y, Wang Y, Liu S, Guo W, Yang L, Shen H (2024) Proper doses of brassinolide enhance somatic embryogenesis in different competent Korean pine cell lines during embryogenic mass differentiation. Front Plant Sci 15:1–19. https://doi.org/10.3389/fpls.2024.1330103
Pasternak, Taras P, Geert C, Roland J, Marcel AK (2005) Complementary interactions between oxidative stress and auxins control plant growth responses at plant, organ, and cellular level. J Exp Bot 58:991–1000
Peng C, Gao F, Wang H, Shen H, Yang L (2020) ‘Physiological and Biochemical Traits in Korean Pine Somatic Embryogenesis’. Forests 11. https://doi.org/10.3390/f11050577
Peng C, Gao F, Wang H, Tretyakova IN, Nosov AM, Shen H, Yang L (2022) Morphological and Physiological Indicators for Screening Cell Lines with high potential for somatic embryo maturation at an early stage of somatic embryogenesis in Pinus Koraiensis. Plants 11:1–17. https://doi.org/10.3390/plants11141867
Pullman GS, Mike B (2008) Identification and quantitative analysis of stage-specific carbohydrates in loblolly pine (Pinus taeda) zygotic embryo and female gametophyte tissues. Tree Physiol 28:985–996
Pullman GS, Johnson S, Peter G, Cairney J, Xu N (2003) Improving loblolly pine somatic embryo maturation: comparison of somatic and zygotic embryo morphology, germination, and gene expression. Plant Cell Rep 21:747–758
Raj SN, Sarosh BR, Shetty HS (2006) Induction and accumulation of polyphenol oxidase activities as implicated in development of resistance against pearl millet downy mildew disease. Funct Plant Biol 33:563–571. https://doi.org/10.1071/fp06003
Salaj T, Klubicov K, Panis B, Swennen R, Salaj J (2020) Physiological and structural aspects of in Vitro somatic embryogenesis in Abies alba. Mill’ Forests 11:1210
Scandalios JG, Guan LM, Polidoros AN (1997) Catalases in plants: gene structure, properties, regulation, and expression. Cold Spring Harbor Monogr Archive 1:343–406
Sunita JS, Shekhawat GS (2015) Micropropagation of Chlorophytum borivilianum: in vitro Clonal Fidelity test and antioxidant enzymatic study. Am J Biology Life Sci 3:36–42
Teasdale RD, Woolhouse DHW (1987) Mineral nutrient requirements of a Loblolly Pine (Pinus taeda) cell suspension culture: evaluation of a medium formulated from seed Composition Data. Plant Physiol 82:942–945
Tereso S, Zoglauer K, Milhinhos A, Miguel C, Oliveira MM (2007) Zygotic and somatic embryo morphogensis in Pinus pinaster: comparative histological and histochemical study. Tree Physiol 27:661–669
Umehara M, Ogita S, Sasamoto H, Eun CH, Matsubayashi Y (2005) Two stimulatory effects of the peptidyl growth factor phytosulfokine during somatic embryogenesis in Japanese larch (Larix leptolepis Gordon). Plant Sci 169:901–907
Vágner M, Vondráková Z, Strnadová Z, EJ, Macháčková I (1998) Endogenous levels of plant growth hormones during early stages of somatic embryogenesis of Picea abies. Adv Hort Sci 12:11–18
Vondráková Z, Eliášová K, Fischerová L, Vágner M (2011) The role of auxins in somatic embryogenesis of Abies alba. Open Life Sci 6:587–596. https://doi.org/10.2478/s11535-011-0035-7
Wang Y, Yao R (2020) Establishment of an effective protocol for cultivation of tissue cultured seedlings in Pinus massoniana superior provenance. J BEIJING FORESTRY Univ 42:43–51
Wang H, Wei H, Liu J, Hou Y, Liang H (2018) Physiological and biochemical differences and isoenzymes analysis of embryogenic mass and non-embryogenic mass in Cercidiphyllum japonicum. Genomics Appl Biology 37:4449–4454
Wang J, Yang M, Zheng L, Li P, Chen GW, Shuqi, Shan Y (2020) Establishment and optimization on initiation of embryogenic masses in Pinus massoniana. J Cent South Univ Forestry Technol 40:73–84
Wang S, Wang G, Li H, Li F, Wang J (2023) Agrobacterium tumefaciens-mediated transformation of embryogenic mass and CRISPR/Cas9-mediated genome editing in ‘Feizixiao’ litchi. Hortic Plant J 9:947–957. https://doi.org/10.1016/j.hpj.2023.01.011
Yang H, Matsubayashi Y, Nakamura K, Sakagami Y (1999) ‘Oryza sativa PSK gene encodes a precursor of phytosulfokine-alpha, a sulfated peptide growth factor found in plants’. Proceedings of the National Academy of Sciences
Zeeman SC, Kossmann J, Smith AM (2010) Starch: its metabolism, evolution, and biotechnological modification in plants. Annu Rev Plant Biol 61:209–234. https://doi.org/10.1146/annurev-arplant-042809-112301
Zhang SG, Han SY, Yang WH, Wei HL, Zhang M, Qi LW (2010) ‘Changes in H2O2 content and antioxidant enzyme gene expression during the somatic embryogenesis of Larix leptolepis’. Plant Cell, Tissue and Organ Culture (PCTOC) 100: 21–29
Zhang H, Jia H, Cui Y, He L, Haoyan, Zou B, Wang S (2023) Xiaohua ‘Linkages of soil CO2 emission with plant functional traits in young subtropical plantations’. Chinese Journal of Ecology 34: 2898– 906
Zhao KF, Zou Q, Li DQ, Harris PJC (1993) The effect of salt and water stress on membrane lipid peroxidation in Leaf cells of halophyte and non-halophyte. J Integr Plant Biol 35:519–525
Zhou T, Yang X, Guo K, Deng J, Xu J, Gao W, Lindsey K, Zhang X (2016) ROS homeostasis regulates somatic embryogenesis via the regulation of auxin Signaling in cotton. Mol Cell Proteom 15:2108–2124. https://doi.org/10.1074/mcp.M115.049338
Zhou X, Zheng R, Liu G, Xu Y, Zhou Y, Laux T, Zhen Y, Harding SA, Shi J, Chen J (2017) Desiccation treatment and endogenous IAA levels are key factors influencing high frequency somatic embryogenesis in Cunninghamia lanceolata (Lamb.) Hook. Front Plant Sci 8:1–15. https://doi.org/10.3389/fpls.2017.02054
Acknowledgements
The authors would like to thank the Jiangxi Provincial Forestry Science and Technology Extension and Publicity and Education Center of Yongxiu Linfeng Comprehensive Science and Technology Demonstration Base for supplying pine cones. The authors also thank Xie Yunyun, Guo Mengting, and Feng Qiao for assisting with field work, data processing, and manuscript composition.
Funding
This study was supported by the Major Project of Open Research Fund of Guangxi Key Laboratory of Superior Timber Trees Resource Cultivation (Grant number 2019-B-03-01), Sub-Project of STI 2030-Major Projects (Grant number 2022ZD0401601-2), and Forestry Science and Technology Innovation Special Project of Jiangxi Forestry Bureau (Grant number 2021. No. 13).
Author information
Authors and Affiliations
Contributions
Qunfeng Luo, Shan Hu, and Chunxia Yang conceived and designed the project. Guang Zhou., Qian Liu, and Qiang Du collected the materials. Zhaolei Deng., Shan Hu, and Chunxia Yang analyzed the data and drew the diagram. Shan Hu and Chunxia Yang wrote the manuscript. Qunfeng Luo supervised the experiments. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Sergio J. Ochatt.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Luo, Q., Hu, S., Deng, Z. et al. Plant peptide hormone phytosulfokine promotes embryo development of mass in Pinus massoniana. Plant Cell Tiss Organ Cult 158, 58 (2024). https://doi.org/10.1007/s11240-024-02857-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11240-024-02857-8