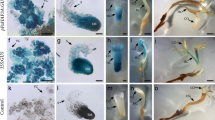
Abstract
Birch (Betula platyphylla Suk.) is a deciduous tree with medicinal and ornamental value. During the process of genetic transformation, somatic embryos do not easily develop into transgenic plants, which is a limitation in genetic breeding. The Arabidopsis thaliana WUSCHEL (AtWUS) gene, which is a transcription factor, plays an important role in maintaining and regulating stem cell characteristics, which determines whether the stem cell population is differentiated. To explore methods for inducing somatic embryogenesis (SE) in birch, we overexpressed the AtWUS gene and transferred it to birch. The expression of AtWUS increased the SE rate from 101.4 to 717.1%. The expression of the AtWUS gene led to the downregulation of BpWUS gene expression in both calli and globular embryos as well as bud meristems. The expression of a few genes, i.e., BpLEC1 (LEAFY COTYLEDON 1), BpLEC2 (LEAFY COTYLEDON 2) and BpFUS3 (FUSCA 3), was upregulated during both embryogenesis and bud meristem development. However, BpABI3 (ABSCISIC ACID INSENSITIVE 3) gene expression was upregulated only in calli embryos, while BpSTM (SHOOT MERISTEMLESS) and BpCUC2 (CUP-SHAPED COTYLEDON 2) gene expression was upregulated only in bud meristems. This result indicated that overexpression of the AtWUS gene promoted SE by increasing the expression of SE-related genes. In conclusion, this study focused on the role of the AtWUS gene in birch SE and the molecular mechanism by which SE was promoted.
Key message
This work indicates that overexpression of the WUSCHEL gene from Arabidopsis thaliana in birch can promote somatic embryogenesis and increase the development of lateral branches and buds.







Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SAM:
-
Shoot apical meristem
- 6-BA:
-
6-Benzylaminopurine;
- NAA:
-
Naphthalene acetic acid
- IBA:
-
Indole-3-butyric acid
- CIM:
-
Callus induction medium
- SIM:
-
Shoot induction medium
- ABA:
-
Abscisic acid
- GA:
-
Gibberellic acid
- ABI3:
-
Abscisic acid insensitive 3
- AGL15:
-
Agamous-like 15
- BBM:
-
Baby boom
- FUS3:
-
Fusca 3
- LEC1:
-
Leafy cotyledon 1
- LEC2:
-
Leafy cotyledon 2
- SE:
-
Somatic embryogenesis
- WUS:
-
Wuschel
- STM:
-
Shoot meristemless
- CLV3:
-
Clavata3
- CUC1:
-
Cup-shaped cotyledon 1
- CUC2:
-
Cup-shaped cotyledon 2
- PIN1:
-
Pin-formed 1
References
Aida M (1997) Genes involved in organ separation in Arabidopsis: an Analysis of the cup-shaped cotyledon mutant. The Plant Cell Online 9:841–857
Arroyo-Herrera A, Ku Gonzalez A, Canche Moo R, Quiroz-Figueroa FR, Loyola-Vargas VM, Rodriguez-Zapata LC, Burgeff D’Hondt C, Suárez-Solís VM, Castaño E (2008) Expression of WUSCHEL in Coffea canephora causes ectopic morphogenesis and increases somatic embryogenesis. Plant Cell, Tissue Organ Cult 94:171–180
Barton MK, Poethig RS (1993) Formation of the shoot apical meristem in arabidopsis thaliana: an analysis of development in the wild type and in the shoot meristemless mutant. Development 119:823–831
Baurle I (2005) Regulation of WUSCHEL transcription in the stem cell niche of the Arabidopsis shoot meristem. The Plant Cell Online 119:823–831
Brand U, Fletcher JC, Hobe M, Meyerowitz EM, Simon R (2000) Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 289:617–619
Braybrook SA, Harada JJ (2008) LECs go crazy in embryo development. Trends Plant Sci 13:624–630
Carles CC, Fletcher JC (2003) Shoot apical meristem maintenance: the art of a dynamic balance. Trends Plant Sci 8:394–401
Chatfield SP, Capron R, Severino A, Penttila PA, Alfred S, Nahal H, Provart NJ (2013) Incipient stem cell niche conversion in tissue culture: using a systems approach to probe early events in WUSCHEL-dependent conversion of lateral root primordia into shoot meristems. Plant J 73:798–813
Elahi N, Duncan RW, Stasolla C (2016) Effects of altered expression of LEAFY COTYLEDON1 and FUSCA3 on microspore-derived embryogenesis of Brassica napus L. J Genet Eng Biotechnol 14:19–30
Endrizzi K, Moussian B, Haecker A, Levin JZ, Laux T (1996) The SHOOT MERISTEMLESS gene is required for maintenance of undifferentiated cells in Arabidopsis shoot and floral meristems and acts at a different regulatory level than the meristem genes WUSCHEL and ZWILLE. Plant J 10:967–979
Feher A (2015) Somatic embryogenesis - stress-induced remodeling of plant cell fate. Biochim Biophys Acta 1849:385–402
Fister AS, Landherr L, Perryman M, Zhang Y, Guiltinan MJ, Maximova SN (2018) Glucocorticoid receptor-regulated TcLEC2 expression triggers somatic embryogenesis in Theobroma cacao leaf tissue. PLoS One 13:e0207666
Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426:147–153
Gaj MD, Zhang S, Harada JJ, Lemaux PG (2005) Leafy cotyledon genes are essential for induction of somatic embryogenesis of Arabidopsis. Planta 222:977–988
Gallois JL, Woodward C, Reddy GV, Sablowski R (2002) Combined SHOOT MERISTEMLESS and WUSCHEL trigger ectopic organogenesis in Arabidopsis. Development 129:3207–3217
Gallois JL, Nora FR, Mizukami Y, Sablowski R (2004) WUSCHEL induces shoot stem cell activity and developmental plasticity in the root meristem. Genes Dev 18:375–380
Gambino G, Perrone I, Gribaudo I (2008) A rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem Anal 19:520–525
Gazzarrini S, Tsuchiya Y, Lumba S, Okamoto M, McCourt P (2004) The transcription factor FUSCA3 controls developmental timing in Arabidopsis through the hormones gibberellin and abscisic acid. Dev Cell 7:373–385
Guo F, Liu C, Xia H, Bi Y, Zhao C, Zhao S, Hou L, Li F, Wang X (2013) Induced expression of AtLEC1 and AtLEC2 differentially promotes somatic embryogenesis in transgenic tobacco plants. PLoS One 8:e71714
Horstman A, Li M, Heidmann I, Weemen M, Chen B, Muino JM, Angenent GC, Boutilier K (2017) The BABY BOOM Transcription Factor Activates the LEC1-ABI3-FUS3-LEC2 Network to Induce Somatic Embryogenesis. Plant Physiol 175:848–857
Ikeda-Iwai M, Satoh S, Kamada H (2002) Establishment of a reproducible tissue culture system for the induction of Arabidopsis somatic embryos. J Exp Bot 53:1575–1580
Jha P, Ochatt SJ, Kumar V (2020) WUSCHEL: a master regulator in plant growth signaling. Plant Cell Rep 39:431–444
Jia H, Suzuki M, Mccarty DR (2014) Regulation of the seed to seedling developmental phase transition by the LAFL and VAL transcription factor networks. Wiley Interdiscip Rev Dev Biol 3:135–145
Kadri A, Grenier De March G, Guerineau F, Cosson V, Ratet P (2021) WUSCHEL overexpression promotes callogenesis and somatic embryogenesis in Medicago truncatula Gaertn. Plants (basel) 10:715
Kieffer M (2006) Analysis of the transcription factor WUSCHEL and its functional homologue in antirrhinum reveals a potential mechanism for their roles in meristem maintenance. The Plant Cell Online 18:560–573
Kim JY, Adhikari PB, Ahn CH, Kim DH, Chang Kim Y, Han JY, Kondeti S, Choi YE (2019) High frequency somatic embryogenesis and plant regeneration of interspecific ginseng hybrid between Panax ginseng and Panax quinquefolius. J Ginseng Res 43:38–48
Laux T, Mayer KF, Berger J, Jürgens G (1996) The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development (cambridge, England) 122:87–96
Lenhard M, Jurgens G, Laux T (2002) The WUSCHEL and SHOOTMERISTEMLESS genes fulfil complementary roles in Arabidopsis shoot meristem regulation. Development 129:3195–3206
Li K, Wang J, Liu C, Li C, Qiu J, Zhao C, Xia H, Ma C, Wang X, Li P (2019) Expression of AtLEC2 and AtIPTs promotes embryogenic callus formation and shoot regeneration in tobacco. BMC Plant Biol 19:314
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta C(T)) method. Methods 25:402–408
Long JA, Moan EI, Medford JI, Barton MK (1996) A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379:66–69
Martinez MT, San-Jose MDC, Arrillaga I, Cano V, Morcillo M, Cernadas MJ, Corredoira E (2019) Holm Oak somatic embryogenesis: current status and future perspectives. Front Plant Sci 10:239
Mendez-Hernandez HA, Ledezma-Rodriguez M, Avilez-Montalvo RN, Juarez-Gomez YL, Skeete A, Avilez-Montalvo J, De-la-Pena C, Loyola-Vargas VM (2019) Signaling overview of plant somatic embryogenesis. Front Plant Sci 10:77
Moon J, Hake S (2011) How a leaf gets its shape. Curr Opin Plant Biol 14:24–30
Murashige T, Skoog F (1962) A Revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:42–44
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4326
Nakagawa T, Suzuki T, Murata S, Nakamura S, Hino T, Maeo K, Tabata R, Kawai T, Tanaka K, Niwa Y, Watanabe Y, Nakamura K, Kimura T, Ishiguro S (2007) Improved gateway binary vectors: high-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci Biotechnol Biochem 8:2095–2100
Negin B, Shemer O, Sorek Y, Eshed Williams L (2017) Shoot stem cell specification in roots by the WUSCHEL transcription factor. PLoS One 12:e0176093
Parcy F (1994) Regulation of gene expression programs during Arabidopsis seed development: roles of the ABI3 locus and of endogenous abscisic acid. The Plant Cell Online 6:1567–1582
Qu C, Bian X, Han R, Jiang J, Yu Q (2020) Liu G (2020) Expression of BpPIN is associated with IAA levels and the formation of lobed leaves in Betula pendula ‘Dalecartica.’ J For Res 31(1):87–97
Reddy GV (2005) Stem-cell homeostasis and growth dynamics can be uncoupled in the Arabidopsis shoot apex. Science 310:663–667
Reddy GV (2008) Live-imaging stem-cell homeostasis in the Arabidopsis shoot apex. Curr Opin Plant Biol 11:88–93
Roscoe TT, Guilleminot J, Bessoule JJ, Berger F, Devic M (2015) Complementation of seed maturation phenotypes by ectopic expression of ABSCISIC ACID INSENSITIVE3, FUSCA3 and LEAFY COTYLEDON2 in arabidopsis. Plant Cell Physiol 56:1215–1228
Rose RJ (2019) Somatic embryogenesis in the medicago truncatula model: cellular and molecular mechanisms. Front Plant Sci 10:267
Salaun C, Lepiniec L, Dubreucq B (2021) Genetic and molecular control of somatic embryogenesis. Plants (basel) 10:1467
Schoof H, Lenhard M, Haecker A, Mayer KFX, Jürgens G, Laux T (2000) The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100:635–644
Solorzano-Cascante P, Sanchez-Chiang N, Jimenez VM (2018) Explant type, culture system, 6-Benzyladenine, meta-topolin and encapsulation affect indirect somatic embryogenesis and regeneration in Carica papaya L. Front Plant Sci 9:1769
Su YH, Zhao XY, Liu YB, Zhang CL, O’Neill SD, Zhang XS (2009) Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. Plant J 59:448–460
Takada S, Hibara K, Ishida T, Tasaka M (2001) The CUP-SHAPED COTYLEDON1 gene of Arabidopsis regulates shoot apical meristem formation. Development 128:1127–1135
Tian R, Paul P, Joshi S, Perry SE (2020) Genetic activity during early plant embryogenesis. Biochem J 477:3743–3767
To A (2006) A network of local and redundant gene regulation governs Arabidopsis seed maturation. The Plant Cell Online 18:1642–1651
Wisniewska J, Xu J, Brewer PB, Blilou L, Scheres B, Friml J (2006) Polar PIN localization directs auxin flow in plants. Science 312:883
Wojcik AM, Wojcikowska B, Gaj MD (2020) Current perspectives on the auxin-mediated genetic network that controls the induction of somatic embryogenesis in plants. Int J Mol Sci 21:1333
Xiao Y, Chen Y, Ding Y, Wu J, Wang P, Yu Y, Wei X, Wang Y, Zhang C, Li F, Ge X (2018) Effects of GhWUS from upland cotton (Gossypium hirsutum L.) on somatic embryogenesis and shoot regeneration. Plant Sci 270:157–165
Yadav RK, Tavakkoli M, Reddy GV (2010) WUSCHEL mediates stem cell homeostasis by regulating stem cell number and patterns of cell division and differentiation of stem cell progenitors. Development (cambridge, England) 137:3581–3589
Yang J, Yang D, Lü W, Zhang X, Ma M, Liu G, Jiang J, Li C (2021) Somatic embryogenesis and plant regeneration in Betula platyphalla. J Forestry Res 32:937–944
Zeng F, Qian J, Luo W, Zhan Y, Xin Y, Yang C (2010) Stability of transgenes in long-term micropropagation of plants of transgenic birch (Betula platyphylla). Biotechnol Lett 32:151–156
Zheng W, Zhang X, Yang Z, Wu J, Li F, Duan L, Liu C, Lu L, Zhang C, Li F (2014) AtWuschel promotes formation of the embryogenic callus in Gossypium hirsutum. PLoS One 9:e87502
Zuo J, Niu QW, Frugis G, Chua NH (2002) The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J 30:349–359
Acknowledgements
The author thanks the Fundamental Research Fund of Heilongjiang Province.
Funding
This work was supported by the Science Foundation of Heilongjiang Province, China (No. C2018002), Modern Agricultural Industrial Technology System Funding of Shandong Province, China (No. SDAIT-04–03), Agricultural Variety Improvement Project of Shandong Province, China (No. 662–2316109), and The Fundamental Research Funds for the Central Universities (No. 2572020DY15).
Author information
Authors and Affiliations
Contributions
All the authors read and approved the final manuscript. HL, LS and QJX designed the experiments and wrote the manuscript. HL, YTH, WZW and ZYC analyzed these data. Others participated in the experiments. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Paloma Moncaleán.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lou, H., Huang, Y., Wang, W. et al. Overexpression of the AtWUSCHEL gene promotes somatic embryogenesis and lateral branch formation in birch (Betula platyphylla Suk.). Plant Cell Tiss Organ Cult 150, 371–383 (2022). https://doi.org/10.1007/s11240-022-02290-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-022-02290-9